One-Pot Environmentally Friendly Synthesis of Nanomaterials Based on Phytate-Coated Fe3O4 Nanoparticles for Efficient Removal of the Radioactive Metal Ions 90Sr, 90Y and (UO2)2+ from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterization of the Nanomaterials
2.3. Quantification of Residual Metal Concentrations Using ICP-OES
2.4. Quantification of Residual 90Sr/90Y Activity
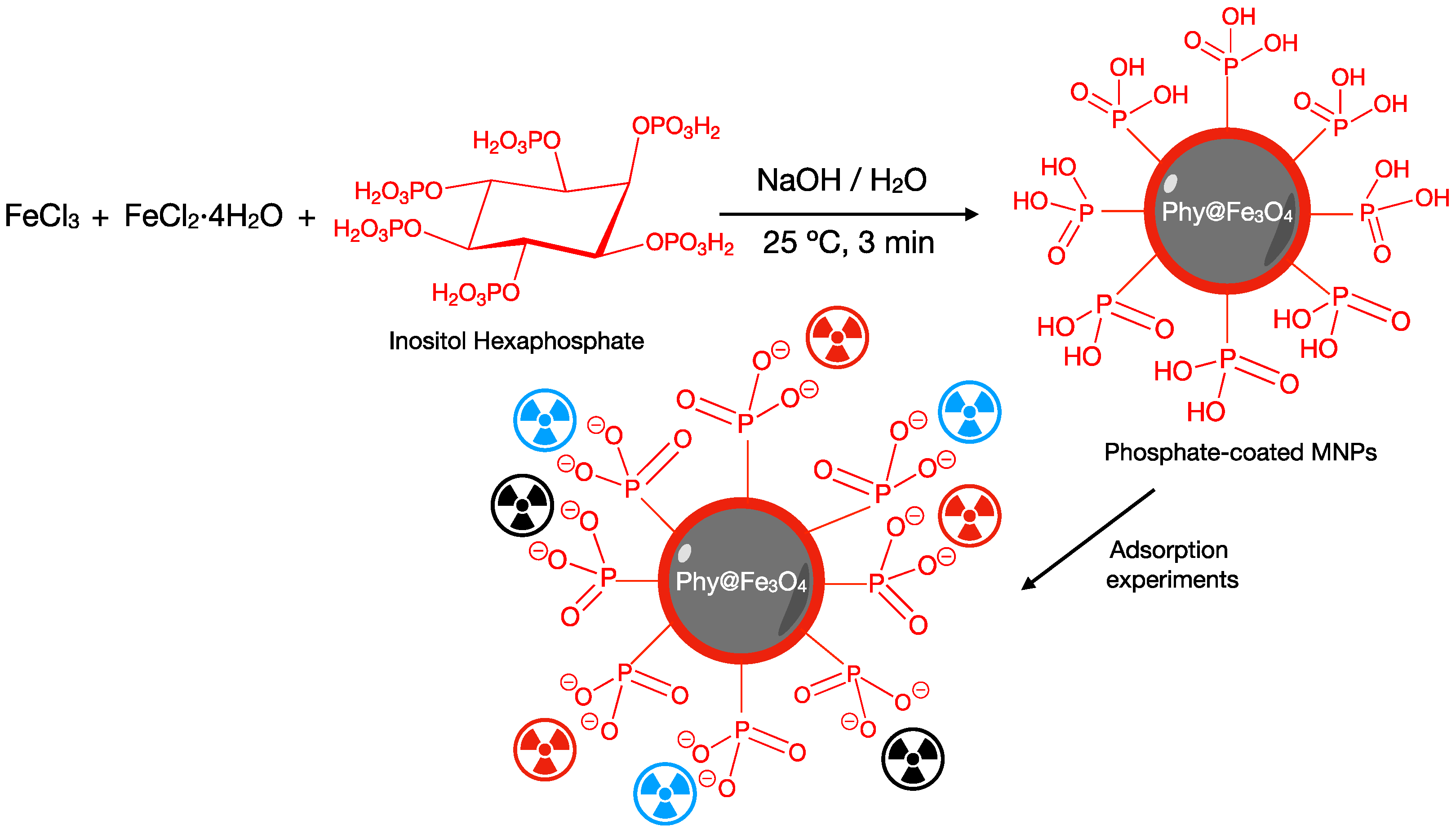
2.5. Synthesis of Fe3O4 and Phy@Fe3O4 Nanoparticles
2.5.1. Synthesis of Fe3O4 Nanoparticles
2.5.2. Synthesis of Phy@Fe3O4 Nanoparticles
3. Results
3.1. Characterization of Magnetite Nanoparticles
3.2. Characterization of Magnetite Nanoparticles Functionalized by Phytate Groups
3.3. Magnesium, Calcium, and Strontium Adsorption on Phytate-Fe3O4 NPs
3.4. Strontium and Yttrium Adsorption on Phytate-Fe3O4 NPs
3.5. Uranium Adsorption on Fe3O4 NPs and Phytate-Fe3O4 NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- USEPA. National Primary Drinking Water Regulation; Radon-222; Proposed Rule; USEPA: Washington, DC, USA, 1999.
- Deuber, R.; Heim, T. Yttrium. In Metals and Their Compounds in the Environment: Occurrence, Analysis and Biological Relevance; Marian, E., Ed.; VCH: Weinheim, Germany, 1991; pp. 1299–1308. [Google Scholar]
- USGS. Rare Earths—Scandium, Yttrium, and The Lanthanides. Available online: https://www.usgs.gov/centers/national-minerals-information-center/rare-earths-statistics-and-information (accessed on 15 September 2021).
- Yttrium-90 Selective Internal Radiation Therapy (SIRT). Available online: https://theranostics.sg/yttrium-90-selective-internal-radiation-therapy/ (accessed on 15 September 2021).
- Radovic, M.; Mirkovic, M.; Peric, M.; Vukadinovic, A.; Stankovic, D.; Petrovic, D.; Boskovic, M.; Antic, B.; Markovic, M.; Vranjes-Duric, S. Design and preparation of 90Y-labeled imidodiphosphate- and inositol hexaphosphatecoated magnetic nanoparticles for possible medical applications. J. Mater. Chem. B 2017, 5, 8738–8747. [Google Scholar] [CrossRef] [PubMed]
- Mumma, R.O.; Raupach, D.C.; Sahadewan, K.; Manos, C.G.; Rutzket, M.; Kuntztt, H.T.; Bache, C.A.; Lisk, D.J. National survey of elements and radioactivity in municipal incinerator ashes. Arch. Environ. Contam. Toxicol. 1990, 19, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sneller, F.E.C.; Kalf, D.F.; Weltje, L.; Van Wezel, A.P. Maximum Permissible Concentrations and Negligible Concentrations for Rare Earth Elements (REEs). June 2000. Available online: https://inis.iaea.org/search/searchsinglerecord.aspx?recordsFor=SingleRecord&RN=31053152 (accessed on 15 September 2021).
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Moon, J. The role of vitamin D in toxic metal absorption: A review. J. Am. Coll. Nutr. 1994, 13, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Uskokovic, D.P. (Eds.) Nanotechnologies in Preventative and Regenerative Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Burger, A.A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef] [PubMed]
- Phouthavong, V.; Yan, R.; Nijpanich, S.; Hagio, T.; Ichino, R.; Kong, L.; Li, L. Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods. Materials 2022, 15, 1053. [Google Scholar] [CrossRef]
- Lu, W.; Li, J.; Sheng, Y.; Zhang, X.; You, J.; Chen, L. One-pot synthesis of magnetic iron oxide nanoparticle-multiwalled carbon nanotube composites for enhanced removal of Cr(VI) from aqueous solution. J. Colloid Interface Sci. 2017, 505, 1134–1146. [Google Scholar] [CrossRef]
- Li, J.; Dong, R.; Wang, X.; Xiong, H.; Xu, S.; Shen, D.; Song, X.; Chen, L. One-pot synthesis of magnetic molecularly imprinted microspheres by RAFT precipitation polymerization for the fast and selective removal of 17β-estradiol. RSC Adv. 2015, 5, 10611–10618. [Google Scholar] [CrossRef]
- Fuks, L.; Herdzik-Koniecko, I.; Polkowska-Motrenko, H.; Oszczak, A. Novel procedure for removal of the radioactive metals from aqueous wastes by the magnetic calcium alginate. Int. J. Environ. Sci. Technol. 2018, 15, 2657–2668. [Google Scholar] [CrossRef]
- Duel, P.; Gutiérrez, M.S.; Rodríguez, P.; León, A.; López, K.A.; Morey, J.; Piña, M.N. Removal of Au3+ and Ag+ from aqueous media with magnetic nanoparticles functionalized with squaramide derivatives. RSC Adv. 2018, 8, 36123–36132. [Google Scholar] [CrossRef]
- López, K.A.; Piña, M.N.; Quiñonero, D.; Ballester, P.; Morey, J. Highly efficient coordination of Hg2+ and Pb2+ metals in water with squaramide-coated Fe3O4 nanoparticles. J. Mater. Chem. A 2014, 2, 8796–8803. [Google Scholar] [CrossRef]
- Gutiérrez, M.S.; Duel, P.; Hierro, F.; Morey, J.; Piña, M.N. A very highly efficient magnetic nanomaterial for removal of PAHS from aqueous media. Small 2018, 14, 1702573. [Google Scholar] [CrossRef] [PubMed]
- Piña, M.N.; Rodríguez, P.; Gutiérrez, M.S.; Quiñonero, D.; Morey, J.; Frontera, A. Adsorption and quantification of volatile organic compounds (VOCs) by using hybrid magnetic nanoparticles. Chem. Eur. J. 2018, 24, 12820–12826. [Google Scholar] [CrossRef] [PubMed]
- Daou, T.J.; Grenèche, J.M.; Pourroy, G.; Buathong, S.; Derory, A.; Ulhaq-Bouillet, C.; Donnio, B.; Guillon, D.; Begin-Colin, S. Coupling agent effect on magnetic properties of functionalized magnetite-based nanoparticles. Chem. Mater. 2008, 20, 5869–5875. [Google Scholar] [CrossRef]
- Yu, X.F.; Liu, Y.H.; Zhou, Z.W.; Xiong, G.X.; Cao, X.H.; Li, M.; Zhang, Z.B. Adsorptive removal of U(VI) from aqueous solution by hydrothermal carbon spheres with phosphate group. J. Radioanal. Nucl. Chem. 2014, 300, 1235–1244. [Google Scholar] [CrossRef]
- Bachmaf, S.; Planer-Friedrich, B.; Merkel, B.J. Effect of sulfate, carbonate, and phosphate on the uranium (VI) sorption behavior onto bentonite. Radiochim. Acta 2008, 96, 359–366. [Google Scholar] [CrossRef]
- Aly, M.M.; Hamza, F.M. A review: Studies on uranium removal using different techniques. Overview. J. Dispersion Sci. Technol. 2013, 34, 182–213. [Google Scholar] [CrossRef]
- Calì, E.; Qi, J.; Preedy, O.; Chen, S.; Boldrin, D.; Branford, W.R.; Vandeperrea, L.; Ryan, M.P. Functionalised magnetic nanoparticles for uranium adsorption with ultra-high capacity and selectivity. J. Mater. Chem. A 2018, 6, 3063–3073. [Google Scholar] [CrossRef]
- Park, H.; May, A.; Portilla, L.; Dietrich, H.; Münch, F.; Rejek, T.; Sarcletti, M.; Banspach, L.; Zahn, D.; Halik, M. Magnetite nanoparticles as efficient materials for removal glyphosate from water. Nat. Sustain. 2020, 3, 129–135. [Google Scholar] [CrossRef]
- Torres, J.; Veiga, N.; Gancheff, J.S.; Domínguez, S.; Mederos, A.; Sundberg, M.; Sánchez, A.; Castiglioni, J.; Díaz, A.; Kremer, C. Interaction of myo-inositol hexakisphosphate with alkali and alkaline earth metal ions: Spectroscopic, potentiometric and theoretical studies. J. Mol. Struct. 2008, 874, 77–88. [Google Scholar] [CrossRef]
- Brigando, C.; Mossoyan, J.C.; Favier, F.; Benlian, D. Conformational preferences and protonation sequence of myo-inositol hexaphosphate in aqueous solution; potentiometric and multinuclear magnetic resonance studies. J. Chem. Soc. Dalt. Trans. 1995, 4, 575–578. [Google Scholar] [CrossRef]
- Veiga, N.; Torres, J.; MacHo, I.; Gómez, K.; González, G.; Kremer, C. Coordination, microprotonation equilibria and conformational changes of myo-inositol hexakisphosphate with pertinence to its biological function. Dalton Trans. 2014, 43, 16238–16251. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Chen, B.; Zhou, Z.; Liang, Y.; He, M.; Hu, B. Phytic acid functionalized magnetic adsorbents for facile enrichment of trace rare earth elements in environmental water, digested atmospheric particulates and the extracts followed by inductively coupled plasma mass spectrometry detection. Talanta 2022, 244, 123426. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, Y.; Seddigh, M. Magnetite nanoparticles synthesized by coprecipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Mirabello, G.; Lenders, J.J.M.; Sommerdijk, N.A.J.M. Bioinspired synthesis of magnetite nanoparticles. Chem. Soc. Rev. 2016, 45, 5085–5106. [Google Scholar] [CrossRef]
- Gutiérrez, M.S.; Piña, M.N.; Morey, J. Fast microwave-assisted conjugation of magnetic nanoparticles with carboxylates of biological interest. RSC Adv. 2017, 7, 19385–19390. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Althoff, H.W.; Bartel, H.; Jekel, M. The effect of groundwater composition on uranium(VI) sorption onto bacteriogenic iron oxides. Water Res. 2006, 40, 3646–3652. [Google Scholar] [CrossRef]
- Das, D.; Sureshkumar, M.K.; Koley, S.; Mithal, N.; Pillai, C.G.S. Sorption of uranium on magnetite nanoparticles. J. Radioanal. Nucl. Chem. 2010, 285, 447–454. [Google Scholar] [CrossRef]
- Mokhtari, B.; Pourabdollah, K.; Dallali, D. A review of calixarene applications in nuclear industries. J. Radioanal. Nucl. Chem. 2011, 287, 921–934. [Google Scholar] [CrossRef]
- Jung, J.; Cho, Y.H.; Hahn, P.S. Scavenging of UO22+ using 4-sulfonic calix[6]arene in the presence of goethite. J. Radioanal. Nucl. Chem. 1999, 242, 635–639. [Google Scholar] [CrossRef]
- Shinkai, S.; Koreishi, H.; Ueda, K.; Arimura, T.; Manabe, O. Molecular design of calixarene-based uranophiles which exhibit remarkably high stability and selectivity. J. Am. Chem. Soc. 1987, 109, 6371–6376. [Google Scholar] [CrossRef]
- Carboni, M.; Abney, C.W.; Liu, S.; Lin, W. Highly porous and stable metal–organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. [Google Scholar] [CrossRef]
- Sather, A.C.; Berryman, O.B.; Rebek, J. Selective recognition and extraction of the uranyl ion from aqueous solutions with a recyclable chelating resin. Chem. Sci. 2013, 4, 3601–3605. [Google Scholar] [CrossRef]
- Jin, J.; Huang, X.; Zhou, L.; Peng, J.; Wang, Y. In situ preparation of magnetic chitosan resins functionalized with triethylenetetramine for the adsorption of uranyl (II) ions. J. Radioanal. Nucl. Chem. 2015, 303, 797–806. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, H.; Lin, X.; Yan, K.; Li, M.; Pan, X.; Hu, Y.; Chen, Y.; Luo, X.; Shang, R. Phytic acid-decorated porous organic polymer for uranium extraction under highly acidic conditions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126981. [Google Scholar] [CrossRef]
- Shaikh, Y.; Lai, E.P.C.; Sadi, B.; Li, C. Magnetic nanoparticles impregnated with 18-crown-6 ether: Hybrid material synthesis for binding and detection of radioactive strontium. Nanosci. Technol. 2015, 2, 1–5. [Google Scholar]
- Yin, L.; Kong, X.; Shao, X.; Ji, Y. Synthesis of DtBuCH18C6-coated magnetic metal–organic framework Fe3O4@UiO-66-NH2 for strontium adsorption. J. Environ. Chem. Eng. 2019, 7, 103073. [Google Scholar] [CrossRef]
- Songa, Y.; Dub, Y.; Lva, D.; Yea, G.; Wanga, J. Macrocyclic receptors immobilized to monodisperse porous polymer particles by chemical grafting and physical impregnation for strontium capture: A comparative study. J. Hazard. Mater. 2014, 274, 221–228. [Google Scholar] [CrossRef]
- Xiao, C.; Silver, M.A.; Wang, S. Metal–organic frameworks for radionuclide sequestration from aqueous solution: A brief overview and outlook. J. Chem. Soc. Dalton Trans. 2017, 46, 16381–16386. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Gao, Y.J.; Feng, M.L.; Zhang, B.; Wu, Z.F.; Song, Y.; Huang, X.Y. An easily synthesized microporous framework material for the selective capture of radioactive Cs+ and Sr2+ ions. J. Mater. Chem. A 2018, 6, 3967–3976. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, Y.; Singh, B.K.; Na, K. Selective and rapid capture of Sr2+ with LTA zeolites: Effect of crystal sizes and mesoporosity. Applied Surface Science 2020, 506, 145029. [Google Scholar] [CrossRef]
- Karabayira, E.; Ozdemirb, A.; Senkalc, B.F.; Taskind, O.S. A radioactively durable melamine-styrene based polymer: Highly efficient removal of 90Sr. Appl. Radiat. Isot. 2019, 149, 96–103. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ludwig, R.; Agarwal, S. Strontium(II) sensor based on a modified calix[6]arene in PVC matrix. Anal. Sci. 2005, 21, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.; Lee, J.H.; Kim, J.S. Sr2+ ion selective p-tert- butylthiacalix[4]arene bearing two distal amide units. Bull. Korean. Chem. Soc. 2008, 29, 620–622. [Google Scholar]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochimica Cosmochimica Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
- Zhou, F.; Feng, J.; Xie, X.; Wu, B.; Liu, Q.; Wu, X.; Chi, R. Adsorption of lanthanum (III) and yttrium (III) on kaolinite: Kinetics and adsorption isotherms. Physicochem. Probl. Miner. Process. 2019, 55, 928–939. [Google Scholar]
- Khotimchenko, M.; Kovalev, V.; Khozhaenko, E.; Khotimchenko, R. Removal of yttrium (III) ions from water solutions by alginate compounds. Int. J. Environ. Sci. Technol. 2015, 12, 3107–3116. [Google Scholar] [CrossRef]
- Dubey, S.; Grandhi, S.S. Sorption studies of yttrium (III) ions on nanomaghemite. J. Environ. Chem. Eng. 2016, 4, 4719–4730. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Kopacek, B.; Vegliò, F. Yttrium recovery from primary and secondary sources: A review of main hydrometallurgical processes. Waste Manag. 2014, 34, 1237–1250. [Google Scholar] [CrossRef]
- Galhoum, A.A. Facile synthesis of functionalized polyglycidyl methacrylate-magnetic nanocomposites for enhanced uranium sorption. RSC Adv. 2019, 9, 38783–38796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cui, Z.; Pan, D.; Fan, F.; Tang, J.; Hu, Y.; Xu, Y.; Zhang, P.; Li, P.; Kong, X.Y. An efficient uranium adsorption magnetic platform based on amidoxime-functionalized flower-like Fe3O4@TiO2 core–shell microspheres. ACS Appl. Mater. Interfaces 2021, 13, 17931–17939. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Khajeh, M.; Oveisi, A.R.; Daliran, S.; Ghaffari-Moghaddam, M.; Delarami, H.S. A porous multifunctional and magnetic layered graphene oxide/3D mesoporous MOF nanocomposite for rapid adsorption of uranium(VI) from aqueous solutions. J. Ind. Eng. Chem. 2021, 93, 322–332. [Google Scholar] [CrossRef]
- Amesh, P.; Venkatesan, K.A.; Suneesh, A.S.; Gupta, D.K.; Ravindrand, T.R. Adsorption of uranium by diethylenetriamine functionalized magnetic mesoporous silica. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100583. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Q.; Zhang, H.; Liu, J.; Chen, R.; Li, R.; Li, Z.; Liu, P.; Wang, J. Rapid and efficient uranium(VI) capture by phytic acid/polyaniline/FeOOH composites. J. Colloid Interface Sci. 2018, 511, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Troyer, L.D.; Lee, S.S.; Wu, J.; Kim, C.; Lafferty, B.J.; Catalano, J.G.; Fortner, J.D. Engineering nanoscale ironoxides for uranylsorption and separation: Optimization of particle core size and bilayer surface coatings. ACS Appl. Mater. Interfaces 2017, 9, 13163–13172. [Google Scholar] [CrossRef]
- Lai, Z.; Xuan, Z.; Yu, S.; Zhang, Z.; Cao, Y.; Zhao, Y.; Li, Y.; Luo, J.; Li, X. Synthesis of magnetic-carbon sorbent for removal of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2019, 322, 2079–2089. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Huang, D.; Jin, T.; Qian, Y. Efficient removal of uranium (VI) with a phytic acid-doped polypyrrole/carbon felt electrode using double potential step technique. J. Hazard. Mater. 2022, 433, 128775. [Google Scholar] [CrossRef]
- Lee, S.S.; Li, W.; Kim, C.; Cho, M.; Lafferty, B.J.; Fortner, J.D. Surface functionalized manganese ferrite nanocrystals for enhanced uranium sorption and separation in wáter. J. Mater. Chem. A 2015, 3, 21930–21939. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Ouyang, J.; Wu, X.; Luo, J.; Liu, S.; Gong, X. A facile preparation of dicyclohexano-18-crown-6 ether impregnated titanate nanotubes for strontium removal from acidic solution. Solid State Sci. 2019, 90, 49–55. [Google Scholar] [CrossRef]
- Younjlin, P.; Youngchae, L.; Wonsik, S.; Sangjune. Removal of cobalt, strontium and cesium from radioactive laubdry wasterwater by ammonium molybdophosphate-polyacrylonitrile (AMP-PAN). Chem. Eng. J. 2010, 162, 685–695. [Google Scholar]
- Sakr, A.K.; Cheira, M.F.; Hassanin, M.A.; Mira, H.I.; Mohamed, S.A.; Khandaker, M.U.; Hamid Osman, H.; Eed, E.M.; Sayyed, M.I.; Hanfi, M.Y. Adsorption of yttrium ions on 3-amino-5-hydroxypyrazole impregnated bleaching clay, a novel sorbent material. Appl. Sci. 2021, 11, 10320. [Google Scholar] [CrossRef]








| Test | Initial Radioactivity Bq 1 | % Radioactivity Measured in A 2 | % Radioactivity Measured in B 2 | % Total |
|---|---|---|---|---|
| 1 | 73.43 | 31 | 68 | 99 |
| 2 | 72.75 | 31 | 71 | 102 |
| 3 | 74.00 | 32 | 70 | 102 |
| 4 | 72.19 | 30 | 74 | 104 |
| Average | 73.09 ± 0.79 | 31 ± 0.82 | 70.75 ± 2.5 | 101.75 ± 2.06 |
| Sorbent | Adsorption or Removal Performance | pH/Time | Ref. |
|---|---|---|---|
| URANIUM | |||
| Biorecovery of uranium from aqueous solutions at the expense of phytic acid | qm = 4.1 mg/g 75% | 4.5/12 h | [56] |
| Fe3O4 NPs (50–100 nm) | qm = 5 mg/g | 7/5 h | [34] |
| Phytic acid-decorated porous organic polymer for uranium extraction under highly acidic conditions | qm = 106.7 mg/g | 5/11 h | [41] |
| Iminodiphosphonate functionalized PGMA–Fe3O4 1 | qm = 147 mg/g | 4–5/12 h | [57] |
| Triethylene tetramine functionalized chitosan resin–Fe3O4 | qm = 166.6 mg/g | 5/1 h | [40] |
| Amidoxime functionalized flower-like TiO2 microspheres–Fe3O4 | qm = 313.6 mg/g | 6/12 h | [58] |
| Graphene oxide modified with OPO3H2/mesoporous Zr-MOF–Fe3O4 | qm = 416.7 mg/g | 6.2/3 min | [59] |
| Diethylenetriamine Fe3O4-embedded mesoporous silica | qm = 470 mg/g | 6/5 h | [60] |
| Phytic acid/polyaniline/FeOOH composites | qm = 555.8 mg/g (Removal efficiency = 92%) | 8/5 min | [61] |
| Oleic acid bilayer@Fe3O4 NPs | qm = 635 mg/g | 5.6/24 h | [62] |
| Hydrothermal carbon modified with NaOH–Fe3O4 | qm = 761.2 mg/g | 5.5/200 min | [63] |
| Phytic acid doped polypyrrole/Carbon felt electrode | qm = 1562 mg/g (Removal efficiency = 98%) | 5/8 h | [64] |
| Oleyl phosphate bilayer@MnFe2O4 NPs | qm = 1667 mg/g | 5.6/24 h | [65] |
| Phosphated-Fe3O4 NPs | qm = 1690 mg/g (100%) | 7/1 min | [24] |
| Phy@Fe3O4NPs | qm = 948 mg/g (Removal efficiency = 80%) | 6.5/2 h | This work |
| STRONTIUM | |||
| 4′,4″(5″)-di-tert-butyldicyclohexano-18-crown-6 onto Fe3O4@UiO-66-NH2 | qm = 284 mL/g | 1/90 min | [43] |
| γ-Fe2O3 impregnated with 4′,4″(5″)-di-tert-butyldicyclohexane 18-crown-6 | (Removal efficiency = 66%) | 7/10 min | [42] |
| Dicyclohexano-18-crown-6 ether impregnated titanate nanotubes | qm = 49 mg/g | 1/180 min | [66] |
| MOF material | qm = 43.83 mg/g (Removal efficiency = 99,89%) | 7/12 h | [47] |
| Ammonium molybdophosphate–polyacrylonitrile (AMP–PAN) | qm = 15 mg/g (Removal efficiency = 66%) | 5/24 h | [67] |
| Phy@Fe3O4NPs | qm = 20 mg/g (Removal efficiency = 73%) | 6.5/2 h | This work |
| YTTRIUM | |||
| Nanomaghemite | qm = 13.5 mg/g (Removal efficiency = 94%) | 6.9/50 min | [55] |
| 3-Amino-5-Hydroxypyrazole Impregnated Bleaching Clay | qm = 171.3 mg/g | 6/60 min | [68] |
| Alginate compounds | qm = 99.01 mg/g (sodium alginate) qm = 181.81 mg/g (calcium alginate) | 6/60 min | [54] |
| Kaolinite | qm = 0.029 mmol/g | 5/3.5 h | [53] |
| Phy@Fe3O4NPs | qm = 20 mg/g (Removal efficiency = 100%) | 6.5/2 h | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duel, P.; Piña, M.d.l.N.; Morey, J. One-Pot Environmentally Friendly Synthesis of Nanomaterials Based on Phytate-Coated Fe3O4 Nanoparticles for Efficient Removal of the Radioactive Metal Ions 90Sr, 90Y and (UO2)2+ from Water. Nanomaterials 2022, 12, 4383. https://doi.org/10.3390/nano12244383
Duel P, Piña MdlN, Morey J. One-Pot Environmentally Friendly Synthesis of Nanomaterials Based on Phytate-Coated Fe3O4 Nanoparticles for Efficient Removal of the Radioactive Metal Ions 90Sr, 90Y and (UO2)2+ from Water. Nanomaterials. 2022; 12(24):4383. https://doi.org/10.3390/nano12244383
Chicago/Turabian StyleDuel, Paulino, María de las Nieves Piña, and Jeroni Morey. 2022. "One-Pot Environmentally Friendly Synthesis of Nanomaterials Based on Phytate-Coated Fe3O4 Nanoparticles for Efficient Removal of the Radioactive Metal Ions 90Sr, 90Y and (UO2)2+ from Water" Nanomaterials 12, no. 24: 4383. https://doi.org/10.3390/nano12244383
APA StyleDuel, P., Piña, M. d. l. N., & Morey, J. (2022). One-Pot Environmentally Friendly Synthesis of Nanomaterials Based on Phytate-Coated Fe3O4 Nanoparticles for Efficient Removal of the Radioactive Metal Ions 90Sr, 90Y and (UO2)2+ from Water. Nanomaterials, 12(24), 4383. https://doi.org/10.3390/nano12244383






