Modulating Direct Growth of Copper Cobaltite Nanostructure on Copper Mesh as a Hierarchical Catalyst of Oxone Activation for Efficient Elimination of Azo Toxicant
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussions
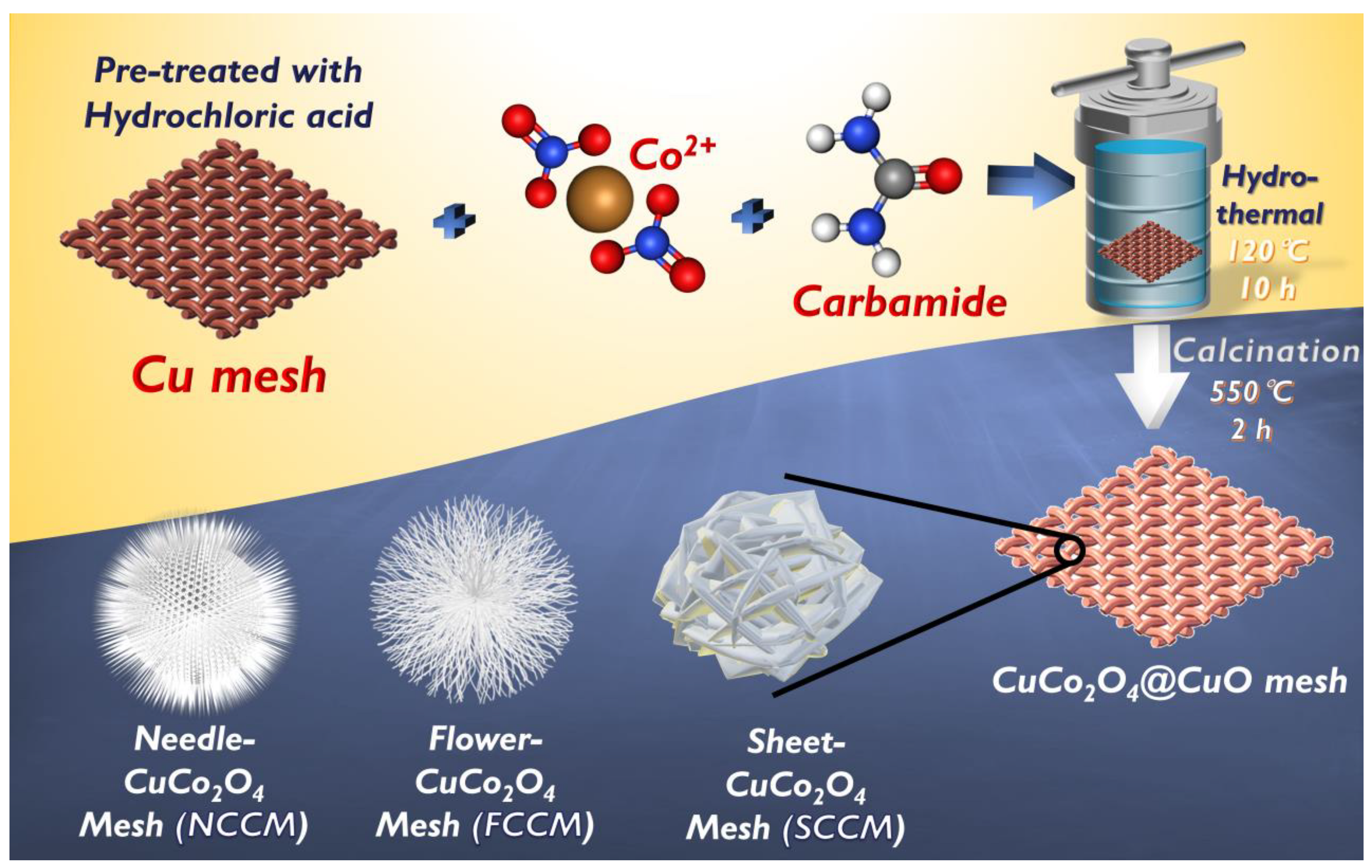
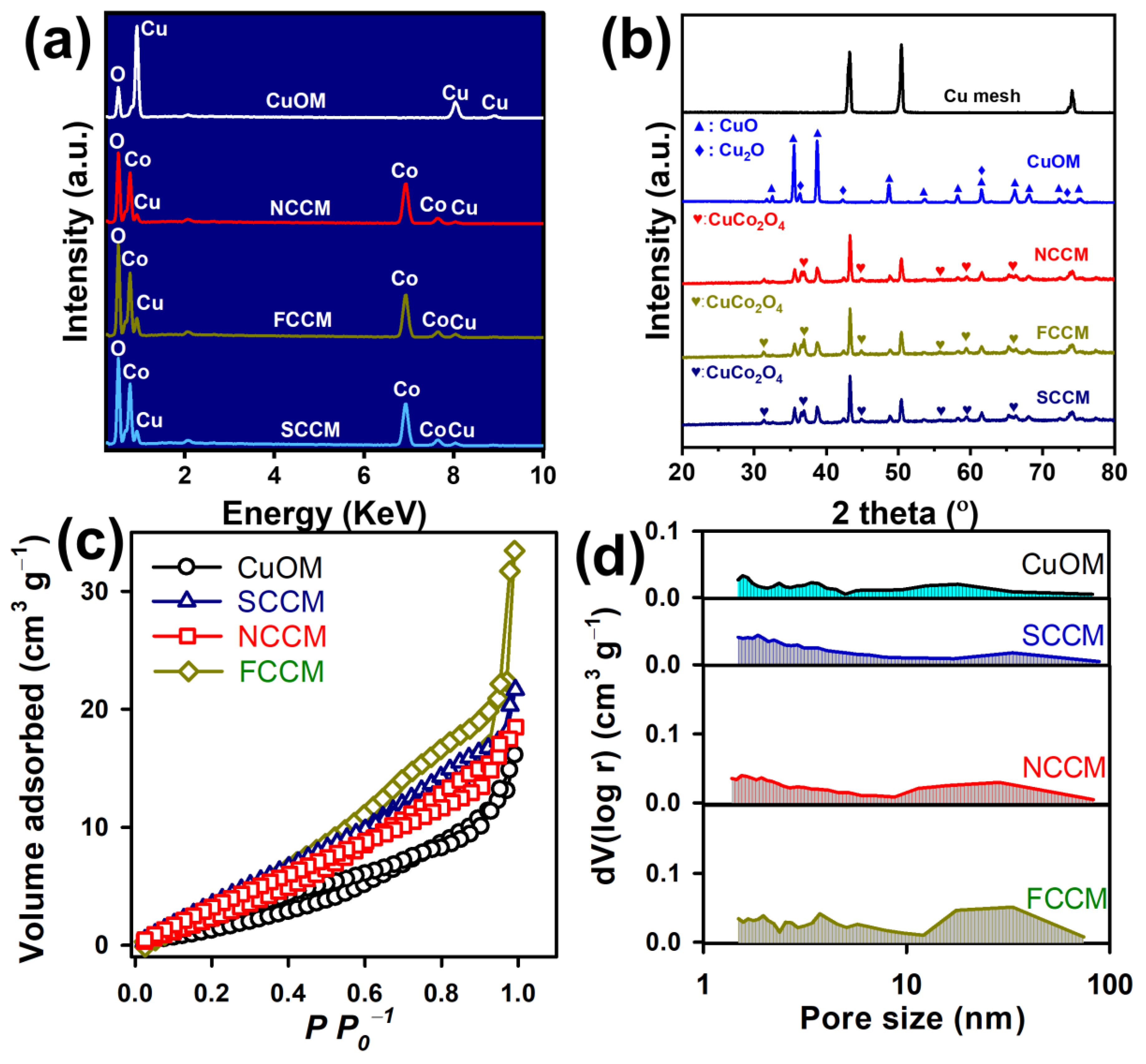
3.1. Characterization of Catalysts
3.1.1. Morphology and Composition
3.1.2. Crystalline Structures
3.1.3. Textural Properties
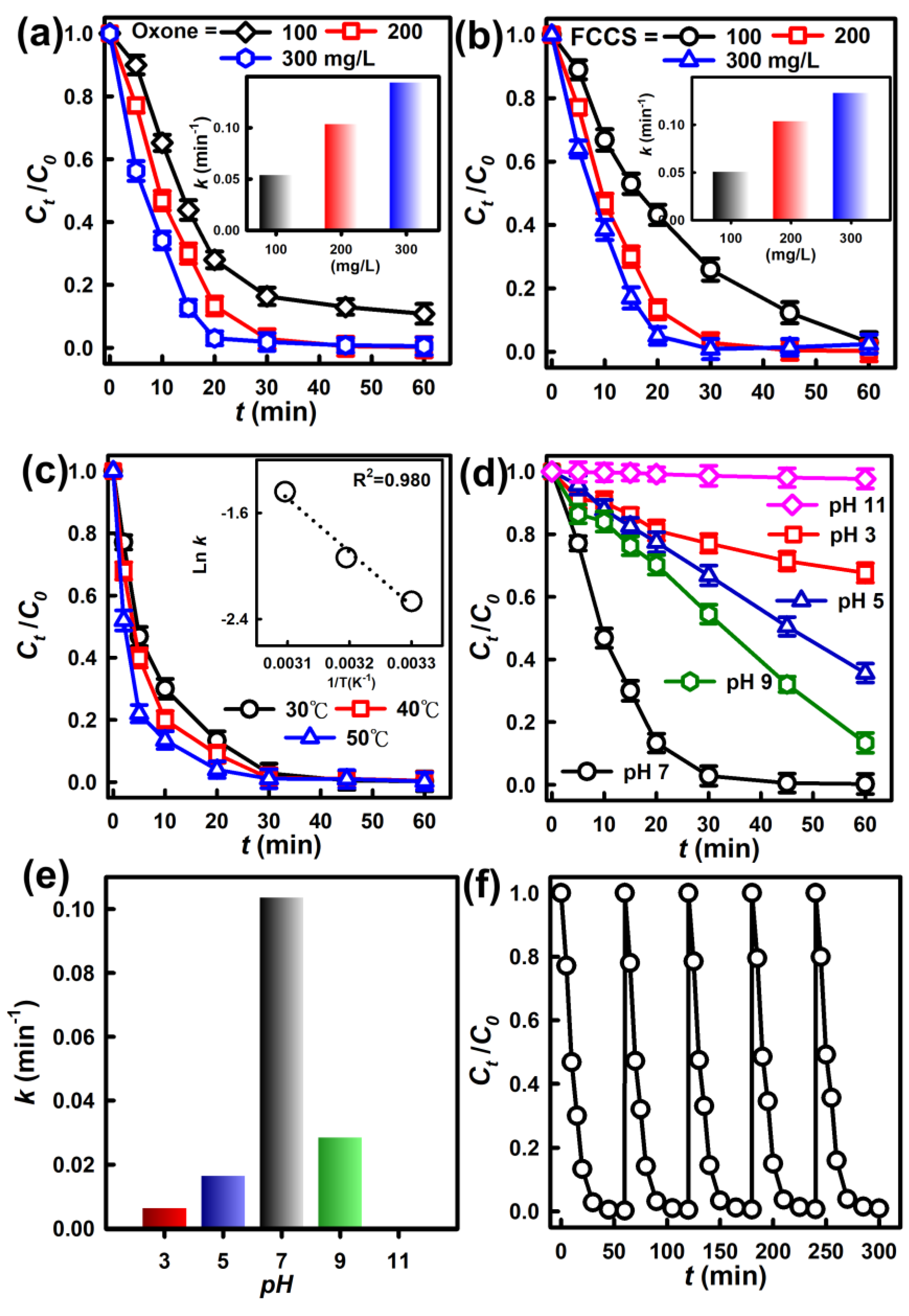
3.2. Degradation of AR by Oxone Activation Using Different Catalysts
3.3. Effects of Oxone and Catalyst Dosages
3.4. Other Effects and Reusability
3.4.1. Variation in Temperature
3.4.2. Effect of pH
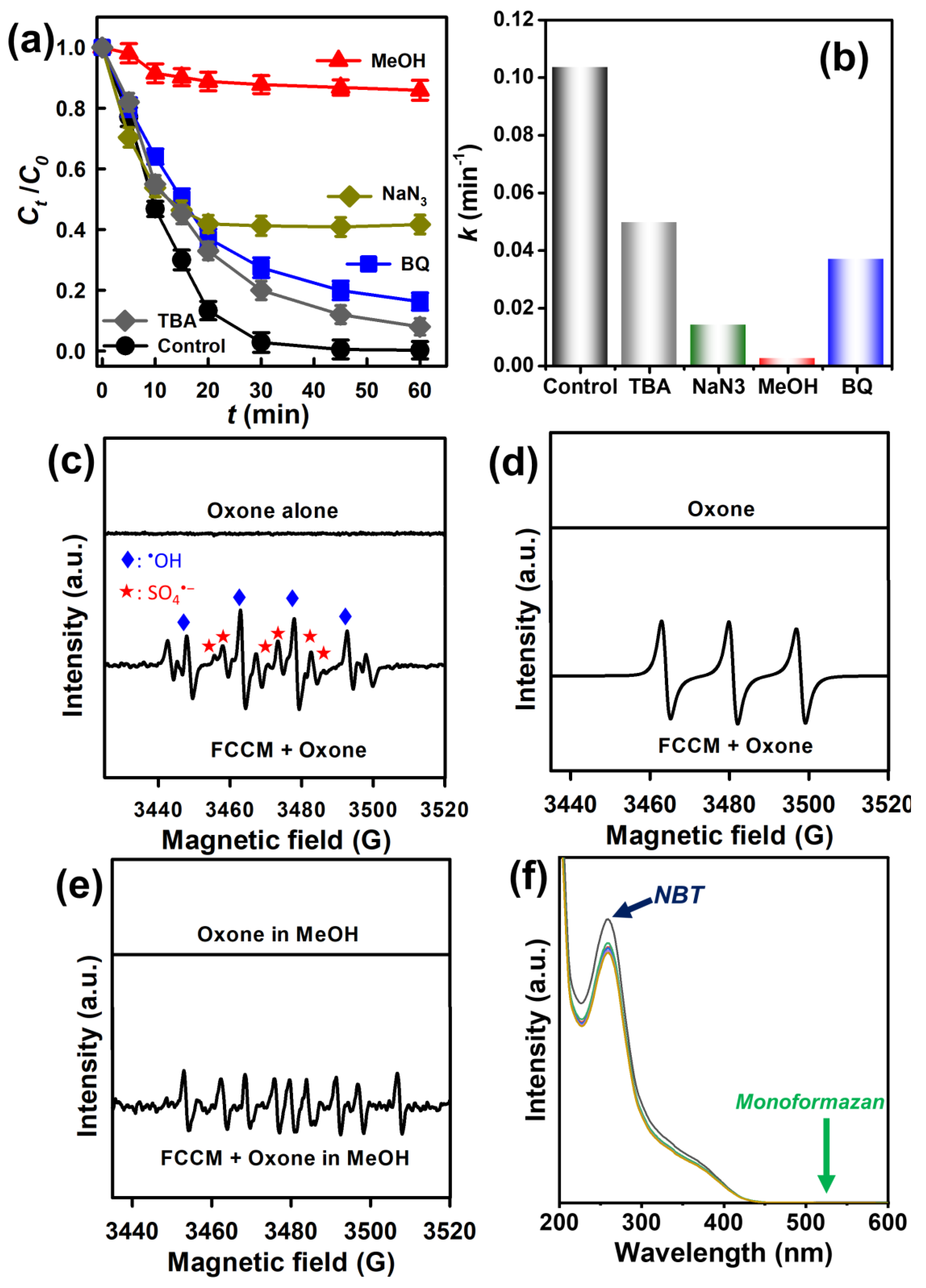
3.4.3. Reusability of the FCCM and the Activation Mechanism of the FCCM for Oxone
3.5. Mechanistic Insights into AR Elimination
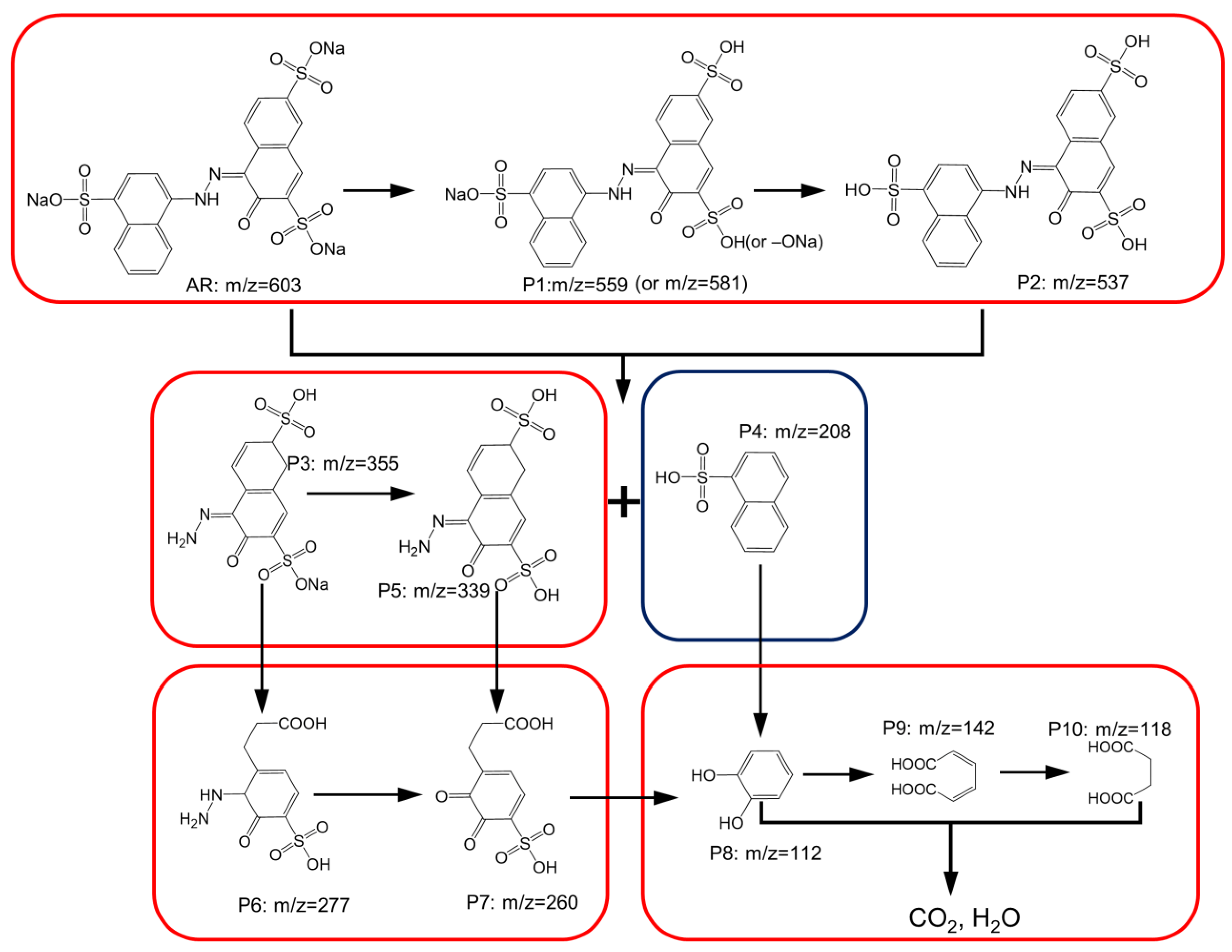
3.6. Computational Calculation and Possible AR Degradation Pathways and
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, S.-A.; Min, O.-M.; Ho, L.-N.; Wong, Y.-S. Comparative Study on Photocatalytic Degradation of Mono Azo Dye Acid Orange 7 and Methyl Orange under Solar Light Irradiation. Water Air Soil Pollut. 2012, 223, 5483–5493. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry; VCH: Weinheim, Germany, 1998. [Google Scholar]
- Mohan, S.V.; Prasad, K.K.; Rao, N.C.; Sarma, P.N. Acid azo dye degradation by free and immobilized horseradish peroxidase (HRP) catalyzed process. Chemosphere 2005, 58, 1097–1105. [Google Scholar] [CrossRef]
- Sarıkaya, R.; Selvi, M.; Erkoç, F. Evaluation of potential genotoxicity of five food dyes using the somatic mutation and recombination test. Chemosphere 2012, 88, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Kiron, M.I. Acid Dyes|Properties of Acid Dyes|Mechanism of Dyeing with Acid Dyes. 2012. Available online: https://textilelearner.net/acid-dyes-properties/ (accessed on 5 November 2022).
- Mittal, A.; Kurup, L.; Gupta, V.K. Use of waste materials—Bottom Ash and De-Oiled Soya, as potential adsorbents for the removal of Amaranth from aqueous solutions. J. Hazard. Mater. 2005, 117, 171–178. [Google Scholar] [CrossRef]
- Chung, K.T.; Fulk, G.E.; Egan, M. Reduction of azo dyes by intestinal anaerobes. Appl. Environ. Microbiol. 1978, 35, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Ng, W.-Y.; Choong, Z.-Y.; Gasim, M.F.; Khoerunnisa, F.; Lin, K.-Y.A.; Oh, W.-D. Insights into the performance and kinetics of face mask-derived nitrogen-doped porous carbon as peroxymonosulfate activator for gatifloxacin removal. J. Water Process Eng. 2022, 50, 103239. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.-Y.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Naik, A.P.; Salkar, A.V.; Majik, M.S.; Morajkar, P.P. Enhanced photocatalytic degradation of Amaranth dye on mesoporous anatase TiO2: Evidence of C–N, N=N bond cleavage and identification of new intermediates. Photochem. Photobiol. Sci. 2017, 16, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, S.; Zhao, S.; Fu, L.; Chen, G.; Yang, F. Oxidative degradation of azo dye by hydrogen peroxide electrogenerated in situ on anthraquinonemonosulphonate/polypyrrole composite cathode with heterogeneous CuO/γ-Al2O3 catalyst. Appl. Catal. B 2011, 106, 370–378. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl. Catal. B 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Cong Khiem, T.; Dinh Tuan, D.; Kwon, E.; Nhat Huy, N.; Oh, W.-D.; Chen, W.-H.; Lin, K.-Y.A. Degradation of dihydroxybenzophenone through monopersulfate activation over nanostructured cobalt ferrites with various morphologies: A comparative study. Chem. Eng. J. 2022, 450, 137798. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D. Iron–cobalt mixed oxide nanocatalysts: Heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications. Appl. Catal. B Environ. 2009, 88, 462–469. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef]
- Guo, W.; Su, S.; Yi, C.; Ma, Z. Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system. Environ. Prog. Sustain. Energy 2013, 32, 193–197. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lee, J.; Oh, W.D.; Kwon, E.; Tsai, Y.-C.; Lisak, G.; Phattarapattamawong, S.; Hu, C.; Lin, K.-Y.A. Hierarchical ZIF-decorated nanoflower-covered 3-dimensional foam for enhanced catalytic reduction of nitrogen-containing contaminants. J. Colloid Interface Sci. 2021, 602, 95–104. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Chen, P.-Y.; Kwon, E.; Oh, W.D.; You, S.; Huang, C.-W.; Ghanbari, F.; Wi-Afedzi, T.; Lin, K.-Y.A. One-step synthesized 3D-structured MOF foam for efficient and convenient catalytic reduction of nitrogen-containing aromatic compounds. J. Water Process Eng. 2021, 40, 101933. [Google Scholar] [CrossRef]
- Liu, F.; Gao, X.; Shi, R.; Tse, E.C.M.; Chen, Y. A general electrochemical strategy for upcycling polyester plastics into added-value chemicals by a CuCo2O4 catalyst. Green Chem. 2022, 24, 6571–6577. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Poudel, T.; Neupane, D.; Gupta, R.K.; Mishra, S.R. Influence of Urea on the Synthesis of NiCo2O4 Nanostructure: Morphological and Electrochemical Studies. J. Nanosci. Nanotechnol. 2020, 20, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Park, Y.-K.; Chen, W.-H.; Bui, H.M.; Munagapati, V.S.; Tuan, D.D.; Wen, J.-C.; You, S.; Da Oh, W.; Lin, K.-Y.A. Highly-efficient degradation of ensulizole using monopersulfate activated by nanostructured cobalt oxide: A comparative study on effects of different nanostructures. J. Environ. Chem. Eng. 2022, 10, 107137. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Lyu, C.; Niu, S.; Dong, Z.; Lyu, C. Enhanced catalytic oxidation of benzotriazole via peroxymonosulfate activated by CoFe2O4 supported onto nitrogen-doped three-dimensional graphene aerogels. Chem. Eng. J. 2020, 400, 125897. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Wi-Afedzi, T.; Liu, Y.-T.; Ghanbari, F.; Lin, K.-Y.A. Cobalt Oxides with Various 3D Nanostructured Morphologies for Catalytic Reduction of 4-Nitrophenol: A Comparative Study. J. Water Process Eng. 2020, 37, 101379. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Nhat Huy, N.; Lee, J.; Lin, Y.-F.; Lin, K.-Y.A. Catalytic soot oxidation using hierarchical cobalt oxide microspheres with various nanostructures: Insights into relationships of morphology, property and reactivity. Chem. Eng. J. 2020, 395, 124939. [Google Scholar] [CrossRef]
- Liu, W.-J.; Kwon, E.; Huy, N.N.; Khiem, T.C.; Lisak, G.; Wi-Afedzi, T.; Wu, C.-C.; Ghanbari, F.; Lin, K.-Y.A. Facilely-prepared sulfide-doped Co3O4 nanocomposite as a boosted catalyst for activating Oxone to degrade a sunscreen agent. J. Taiwan Inst. Chem. Eng. 2022, 133, 104253. [Google Scholar] [CrossRef]
- Lv, X.; Leng, Y.; Wang, R.; Wei, Y.; Ren, X.; Guo, W. Persulfate activation by ferrocene-based metal–organic framework microspheres for efficient oxidation of orange acid 7. Environ. Sci. Pollut. Res. 2022, 29, 34464–34474. [Google Scholar] [CrossRef]
- Cong Khiem, T.; Duan, X.; Liu, W.-J.; Park, Y.-K.; Manh Bui, H.; Oh, W.-D.; Ghotekar, S.; Fai Tsang, Y.; Andrew Lin, K.-Y. MOF-templated Hollow Cobalt Sulfide as an Enhanced Oxone Activator for Degradation of UV Absorber: Key Role of Sulfur Vacancy-Induced Highly Active CoII Sites. Chem. Eng. J. 2022, 453, 139699. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, W.; Li, Z.; Ho, K.H.; Li, X.; Jiao, X.; Chen, D. Synthesis of amorphous cobalt sulfide polyhedral nanocages for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 8603–8606. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.-M.; Meng, X.-Y.; Li, S.-N.; Zeng, J.-H.; Chen, Y. Ultrathin Co3O4 Nanomeshes for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Chen, M.; Ahmad, F.; Fida, H.; Zhang, H. Heterogeneous Activation of Peroxymonosulfate by a Spinel CoAl2O4 Catalyst for the Degradation of Organic Pollutants. Catalysts 2022, 12, 847. [Google Scholar] [CrossRef]
- Tuan, D.D.; Oh, W.D.; Ghanbari, F.; Lisak, G.; Tong, S.; Andrew Lin, K.-Y. Coordination polymer-derived cobalt-embedded and N/S-doped carbon nanosheet with a hexagonal core-shell nanostructure as an efficient catalyst for activation of oxone in water. J. Colloid Interface Sci. 2020, 579, 109–118. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, Y.-C.; Huang, C.-F. Magnetic carbon-supported cobalt prepared from one-step carbonization of hexacyanocobaltate as an efficient and recyclable catalyst for activating Oxone. Sep. Purif. Technol. 2016, 170, 173–182. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Lin, T.-Y. Degradation of Acid Azo Dyes Using Oxone Activated by Cobalt Titanate Perovskite. Water Air Soil Pollut. 2018, 229, 10. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Lin, J.-T.; Lu, X.-Y.; Hung, C.; Lin, Y.-F. Electrospun magnetic cobalt-embedded carbon nanofiber as a heterogeneous catalyst for activation of oxone for degradation of Amaranth dye. J. Colloid Interface Sci. 2017, 505, 728–735. [Google Scholar] [CrossRef]
- Lin, K.A.; Yang, M.T.; Lin, J.T.; Du, Y. Cobalt ferrite nanoparticles supported on electrospun carbon fiber as a magnetic heterogeneous catalyst for activating peroxymonosulfate. Chemosphere 2018, 208, 502–511. [Google Scholar] [CrossRef]
- Yang, M.-T.; Zhang, Z.-Y.; Lin, K.-Y.A. One-step fabrication of cobalt-embedded carbon nitride as a magnetic and efficient heterogeneous catalyst for activating oxone to degrade pollutants in water. Sep. Purif. Technol. 2019, 210, 1–9. [Google Scholar] [CrossRef]
- Yang, M.T.; Tong, W.C.; Lee, J.; Kwon, E.; Lin, K.A. CO2 as a reaction medium for pyrolysis of lignin leading to magnetic cobalt-embedded biochar as an enhanced catalyst for Oxone activation. J. Colloid Interface Sci. 2019, 545, 16–24. [Google Scholar] [CrossRef]
- Li, M.-C.; Tong, S.; Lin, J.-T.; Lin, K.-Y.A.; Lin, Y.-F. Electrospun Co3O4 nanofiber as an efficient heterogeneous catalyst for activating peroxymonosulfate in water. J. Taiwan Inst. Chem. Eng. 2020, 106, 110–117. [Google Scholar] [CrossRef]
- Li, M.H.; Lin, K.A.; Yang, M.T.; Thanh, B.X.; Tsang, D.C.W. Prussian Blue Analogue-derived co/fe bimetallic nanoparticles immobilized on S/N-doped carbon sheet as a magnetic heterogeneous catalyst for activating peroxymonosulfate in water. Chemosphere 2020, 244, 125444. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lee, J.; Kwon, E.; Lisak, G.; Thanh, B.X.; Oh, W.D.; Lin, K.-Y.A. Metal-complexed covalent organic frameworks derived N-doped carbon nanobubble–embedded cobalt nanoparticle as a magnetic and efficient catalyst for oxone activation. J. Colloid Interface Sci. 2021, 591, 161–172. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Lin, J.-T.; Jochems, A.P. Oxidation of amaranth dye by persulfate and peroxymonosulfate activated by ferrocene. J. Chem. Technol. Biotechnol. 2017, 92, 163–172. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Deng, J.; Zhou, S.; Li, J.; Xin, X. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef]
- Lai, L.; Yan, J.; Li, J.; Lai, B. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism. Chem. Eng. J. 2018, 343, 676–688. [Google Scholar] [CrossRef]
- Khiem, T.C.; Tuan, D.D.; Kwon, E.; Thanh, B.X.; Tsang, Y.F.; Munagapati, V.S.; Wen, J.-C.; Hu, C.; Lin, K.-Y.A. Hollow and oval-configured ultrafine Co3O4 as a highly-efficient activator of monopersulfate for catalytic elimination of Azorubin S. Sustain. Environ. Res. 2022, 32, 48. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Zhang, Y.; Lai, Y.; Fang, Q.; Duan, Y. Activation of peroxymonosulfate by CuFe2O4-CoFe2O4 composite catalyst for efficient bisphenol a degradation: Synthesis, catalytic mechanism and products toxicity assessment. Chem. Eng. J. 2021, 423, 130093. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem. Eng. J. 2021, 406, 127158. [Google Scholar] [CrossRef]
- Cui, K.-P.; He, Y.-Y.; Xu, K.-J.; Zhang, Y.; Chen, C.-B.; Xu, Z.-J.; Chen, X. Degradation of Tetracycline Hydrochloride by Cu-Doped MIL-101(Fe) Loaded Diatomite Heterogeneous Fenton Catalyst. Nanomaterials 2022, 12, 811. [Google Scholar] [CrossRef]
- Tuan, D.D.; Kwon, E.; Phattarapattamawong, S.; Thanh, B.X.; Khiem, T.C.; Lisak, G.; Wang, H.; Lin, K.-Y.A. Nitrogen-containing carbon hollow nanocube-confined cobalt nanoparticle as a magnetic and efficient catalyst for activating monopersulfate to degrade a UV filter in water. J. Environ. Chem. Eng. 2022, 10, 106989. [Google Scholar] [CrossRef]
- Chen, H.-H.; Park, Y.-K.; Kwon, E.; Fai Tsang, Y.; Xuan Thanh, B.; Cong Khiem, T.; You, S.; Hu, C.; Lin, K.-Y.A. Nanoneedle-Assembled Copper/Cobalt sulfides on nickel foam as an enhanced 3D hierarchical catalyst to activate monopersulfate for Rhodamine b degradation. J. Colloid Interface Sci. 2022, 613, 168–181. [Google Scholar] [CrossRef]
- Li, M.-H.; Zhao, L.-X.; Xie, M.; Li, N.; Wang, X.-L.; Zhao, R.-S.; Lin, J.-M. Singlet oxygen-oriented degradation of sulfamethoxazole by Li–Al LDH activated peroxymonosulfate. Sep. Purif. Technol. 2022, 290, 120898. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Liu, W.-J.; Kwon, E.; Thanh, B.X.; Lee, J.; Ta, C.K.; Sirivithayapakorn, S.; Lin, K.-Y.A. Nanoscale CoNi alloy@carbon derived from Hofmann-MOF as a magnetic/effective activator for monopersulfate to eliminate an ultraviolet filter. J. Nanostructure Chem. 2022. [Google Scholar] [CrossRef]
- Liu, W.-J.; Kwon, E.; Xuan Thanh, B.; Cong Khiem, T.; Dinh Tuan, D.; Lin, J.-Y.; Wi-Afedzi, T.; Hu, C.; Sirivithayapakorn, S.; Lin, K.-Y.A. Hofmann-MOF derived nanoball assembled by FeNi alloy confined in carbon nanotubes as a magnetic catalyst for activating peroxydisulfate to degrade an ionic liquid. Sep. Purif. Technol. 2022, 295, 120945. [Google Scholar] [CrossRef]
- Tuan, D.D.; Khiem, C.; Kwon, E.; Tsang, Y.F.; Sirivithayapakorn, S.; Thanh, B.X.; Lisak, G.; Yang, H.; Lin, K.-Y.A. Hollow porous cobalt oxide nanobox as an enhanced for activating monopersulfate to degrade 2-hydroxybenzoic acid in water. Chemosphere 2022, 294, 133441. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, P.-H.; Kwon, E.; Chang, H.-C.; Bui, H.M.; Phattarapattamawong, S.; Tsai, Y.-C.; Lin, K.-Y.A.; Ebrahimi, A.; Yee, Y.F.; Yuan, M.-H. Modulating Direct Growth of Copper Cobaltite Nanostructure on Copper Mesh as a Hierarchical Catalyst of Oxone Activation for Efficient Elimination of Azo Toxicant. Nanomaterials 2022, 12, 4396. https://doi.org/10.3390/nano12244396
Mao P-H, Kwon E, Chang H-C, Bui HM, Phattarapattamawong S, Tsai Y-C, Lin K-YA, Ebrahimi A, Yee YF, Yuan M-H. Modulating Direct Growth of Copper Cobaltite Nanostructure on Copper Mesh as a Hierarchical Catalyst of Oxone Activation for Efficient Elimination of Azo Toxicant. Nanomaterials. 2022; 12(24):4396. https://doi.org/10.3390/nano12244396
Chicago/Turabian StyleMao, Po-Hsin, Eilhann Kwon, Hou-Chien Chang, Ha Manh Bui, Songkeart Phattarapattamawong, Yu-Chih Tsai, Kun-Yi Andrew Lin, Afshin Ebrahimi, Yeoh Fei Yee, and Min-Hao Yuan. 2022. "Modulating Direct Growth of Copper Cobaltite Nanostructure on Copper Mesh as a Hierarchical Catalyst of Oxone Activation for Efficient Elimination of Azo Toxicant" Nanomaterials 12, no. 24: 4396. https://doi.org/10.3390/nano12244396





