The Water-Based Synthesis of Platinum Nanoparticles Using KrF Excimer Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pt-NP Synthesis
2.2. Characterization of Pt-NPs
3. Results and Discussion
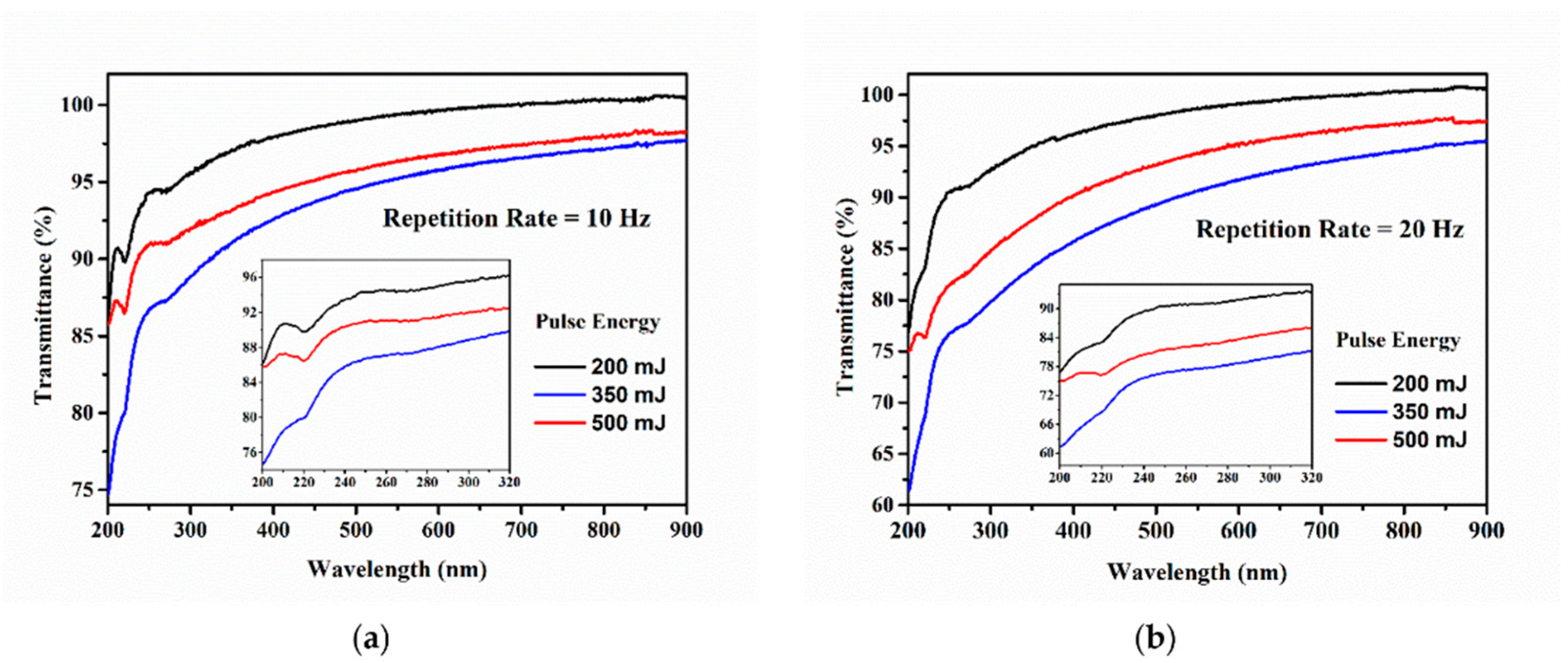
3.1. The Effect of the Repetition Rate over Pt-NP Synthesis at a Constant Laser Fluence
3.2. The Effect of Changing Laser Fluence for Pt-NP Synthesis at a Constant RR
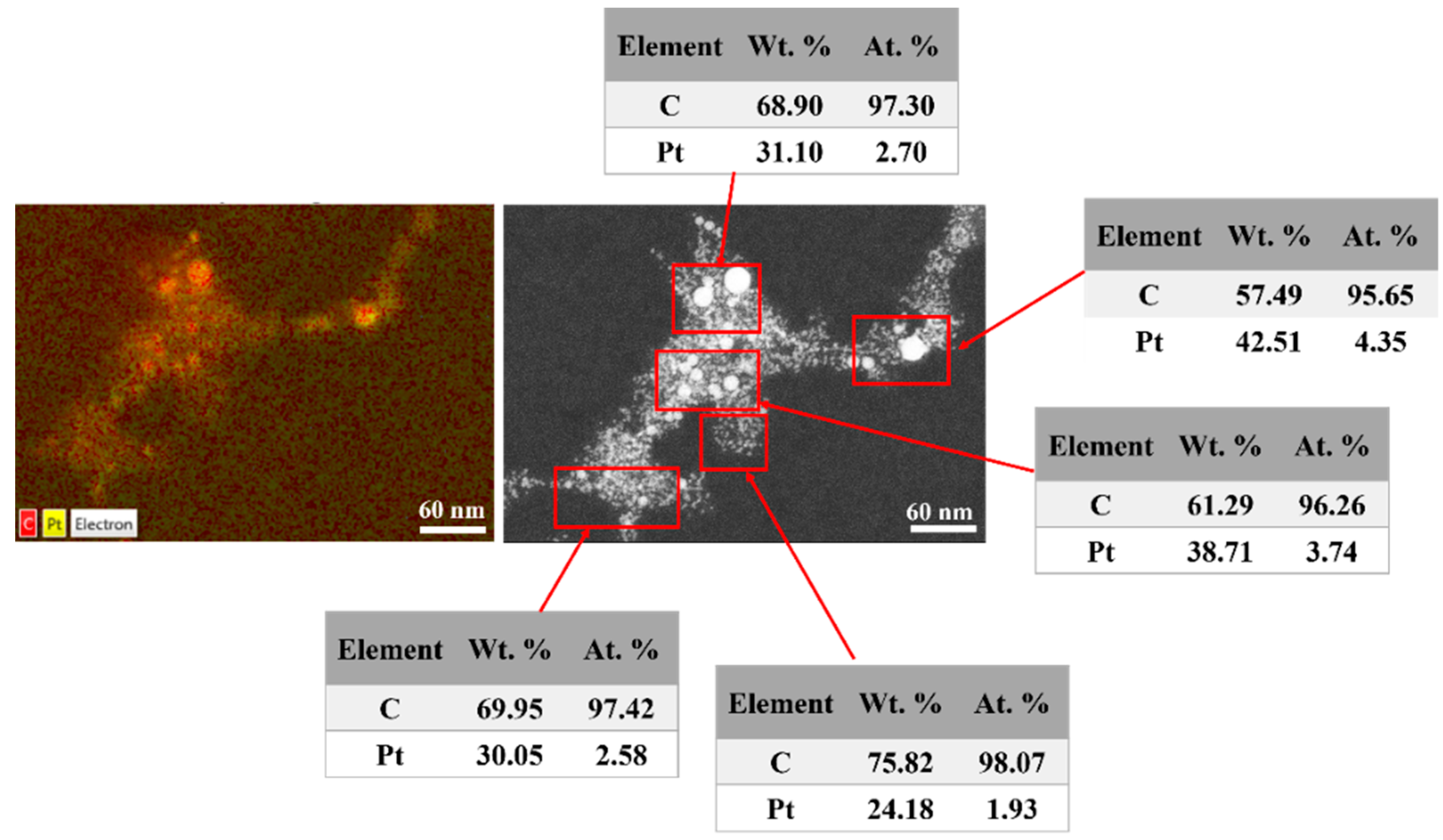
3.3. HR-STEM Investigation for Individual Pt-NPs
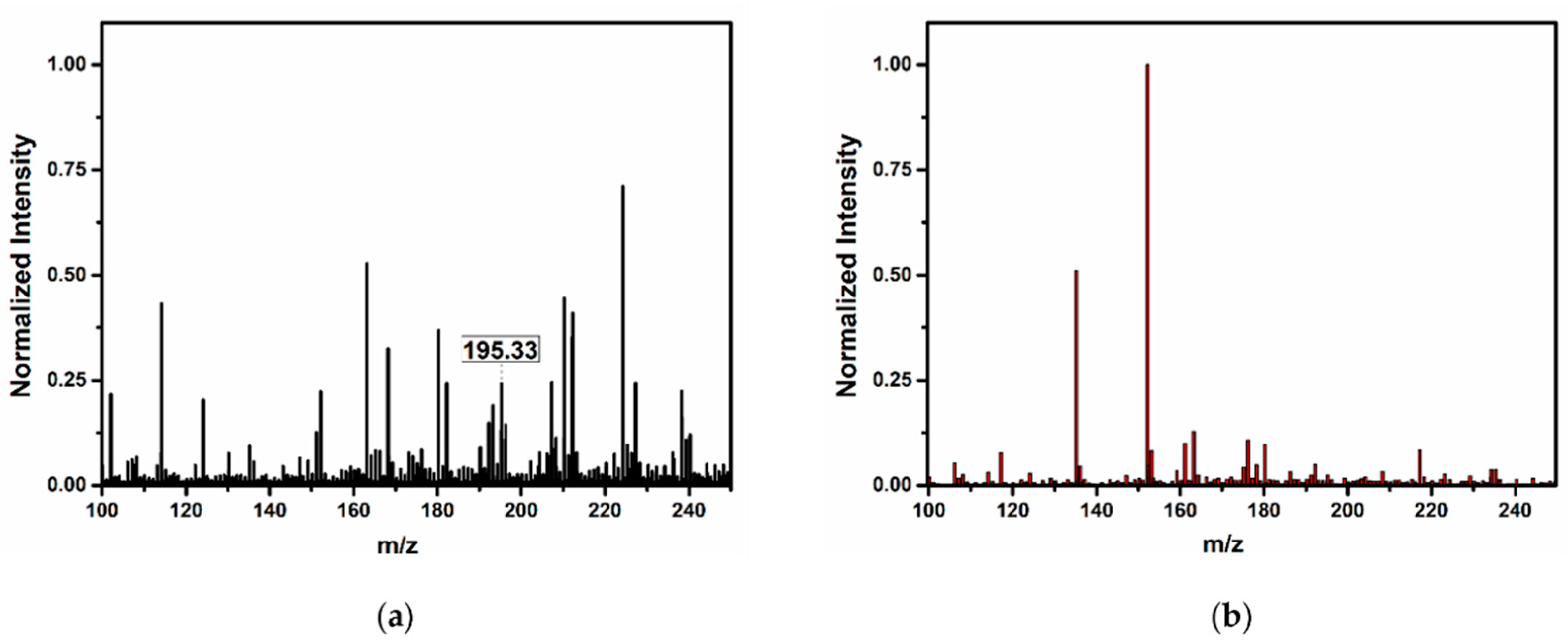
3.4. Confirmation of Platinum in the Colloids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moniri, S.; Hantehzadeh, M.R.; Ghoranneviss, M.; Asadabad, M.A. Study of the optical and structural properties of Pt nanoparticles prepared by laser ablation as a function of the applied electric field. Appl. Phys. A 2017, 123, 684. [Google Scholar] [CrossRef]
- Palma, M.I.M.; Krishnan, B.; Rodriguez, G.A.C.; Das Roy, T.K.; Avellaneda, D.; Shaji, S. Synthesis and Properties of Platinum Nanoparticles by Pulsed Laser Ablation in Liquid. J. Nanomater. 2016, 2016, 9651637. [Google Scholar] [CrossRef] [Green Version]
- Thondavada, N.; Chokkareddy, R.; Redhi, G.G. Green synthesis of platinum nanoparticles and their biomedical applications. In Green Metal Nanoparticles: Synthesis, Characterization and Their Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 603–627. [Google Scholar] [CrossRef]
- Lu, B.; Liu, Q.; Nichols, F.; Mercado, R.; Morris, D.; Li, N.; Zhang, P.; Gao, P.; Ping, Y.; Chen, S. Oxygen Reduction Reaction Catalyzed by Carbon-Supported Platinum Few-Atom Clusters: Significant Enhancement by Doping of Atomic Cobalt. Research 2020, 2020, 9167829. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhou, Y.; Gong, X.; Wang, Z.; Zhang, S.; Wang, M. Oxygen Reduction Reaction from Water Electrolysis Intensified by Pressure and O2−Oxidation Desulfurization. J. Electrochem. Soc. 2018, 165, 139–147. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zhao, K.; Shi, S.; Shao, Q.; Zhang, L.; Sun, X.; Zhao, Y.; Zhang, J. Atomically dispersed metal catalysts for the oxygen reduction reaction: Synthesis, characterization, reaction mechanisms and electrochemical energy applications. Energy Environ. Sci. 2019, 12, 2890–2923. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Nath, K.; Kumar, S.; Tiwari, R.N.; Kemp, K.; Le, N.; Youn, D.H.; Lee, J.S.; Kim, K.S. Stable platinum nanoclusters on genomic DNA–graphene oxide with a high oxygen reduction reaction activity. Nat. Commun. 2013, 4, 2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Thapa, A.; Feng, Q.; Xi, M.; Qiao, Q.; Fong, H. Electrospun TiC/C nano-felt surface-decorated with Pt nanoparticles as highly efficient and cost-effective counter electrode for dye-sensitized solar cells. Nanoscale 2013, 5, 11742–11747. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, J.; Zhang, J.; Li, J.; Tang, B.; Chen, W.; Wang, J.; Wang, X. Functionalization of Silk with In-Situ Synthesized Platinum Nanoparticles. Materials 2018, 11, 1929. [Google Scholar] [CrossRef] [Green Version]
- Formo, E.; Lee, E.; Campbell, D.; Xia, Y. Functionalization of Electrospun TiO2 Nanofibers with Pt Nanoparticles and Nanowires for Catalytic Applications. Nano Lett. 2008, 8, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Huang, Y.-T.; Miyasaka, T.; Ikegami, M.; Feng, S.-P.; Li, W.-D. Solution-Processed Transparent Nickel-Mesh Counter Electrode with in-Situ Electrodeposited Platinum Nanoparticles for Full-Plastic Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 8083–8091. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, V.; Muranszky, G.; Hashimoto, M.; Kristaly, F.; Szori, M.; Fiser, B.; Konya, Z.; Viskolcz, B.; Vanyorek, L. Combustion method combined with sonochemical step for synthesis of maghemite-supported catalysts for the hydrogenation of 2,4-dinitrotoluene. Catal. Commun. 2021, 159, 106342. [Google Scholar] [CrossRef]
- Yacou, C.; Ayral, A.; Giroir-Fendler, A.; Fontaine, M.-L.; Julbe, A. Hierarchical porous silica membranes with dispersed Pt nanoparticles. Microporous Mesoporous Mater. 2009, 126, 222–227. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Q.; Zhu, C.; Luo, Y.; Lu, Q.; Li, H.; Ye, R.; Du, D.; Yuehe, L. Mitochondrial-targeted multifunctional mesoporous Au@Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers. Nanoscale 2017, 9, 15813–15824. [Google Scholar] [CrossRef]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Wennemers, H. Peptide-Coated Platinum Nanoparticles with Selective Toxicity against Liver Cancer Cells. Angew. Chem. Int. Ed. 2019, 58, 4901–4905. [Google Scholar] [CrossRef] [PubMed]
- ElGuindy, M. Platinum Group Metals: Alloying, Properties, and Applications. Encycl. Mater. Sci. Technol. 2001, 7117–7121. [Google Scholar] [CrossRef]
- Davey, N.M.; Seymour, R.J. Platinum Metals in Electronics. Platin. Met. Rev. 1985, 29, 2–11. [Google Scholar]
- Cele, T. Preparation of Nanoparticles. In Engineered Nanomaterials—Health and Safety; IntechOpen: London, UK, 2020; pp. 1–14. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Rodríguez, R.A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Cabrera, C.R.; Feliu, J.M. Synthesis of Pt nanoparticles in water-in-oil microemulsion: Effect of HCl on their surface structure. J. Am. Chem. Soc. 2014, 136, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Nguyen, T.D.; Nguyen, Q.D.; Nguyen, T.T. Preparation of platinum nanoparticles in liquids by laser ablation method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Kundu, P.; Nethravathi, C.; Deshpande, P.A.; Rajamathi, M.; Madras, G.; Ravishankar, N. Ultrafast microwave-assisted route to surfactant-free ultrafine Pt nanoparticles on graphene: Synergistic co-reduction mechanism and high catalytic activity. Chem. Mater. 2011, 23, 2772–2780. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Kim, K.T.; Jin, S.-H.; Chang, S.-C.; Park, D.-S. Green synthesis of platinum nanoparticles by electroreduction of a k2ptcl6 solid-state precursor and its electrocatalytic effects on h2o 2 reduction. Bull. Korean Chem. Soc. 2013, 34, 3835–3839. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, Y.; Xie, Y.; Yang, Z.; Huang, H.; Zhou, Q. Preparation of highly dispersed CuPt nanoparticles on ionic-liquid-assisted graphene sheets for direct methanol fuel cell. Chem. Eng. J. 2012, 197, 80–87. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Oshima, R.; Maeda, Y.; Nagata, Y. Preparation of platinum nanoparticles by sonochemical reduction of the Pt(II) ion. Langmuir 1999, 15, 2733–2737. [Google Scholar] [CrossRef]
- Teranishi, T.; Kurita, R.; Miyake, M. Shape Control of Pt Nanoparticles. J. Inorg. Organomet. Polym. 2000, 10, 145–156. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Ye, Y.; Qiu, S. Radiation induced synthesis of Pt nanoparticles supported on carbon nanotubes. J. Power Sources 2006, 161, 839–842. [Google Scholar] [CrossRef]
- Mafuné, F.; Kondow, T. Selective laser fabrication of small nanoparticles and nano-networks in solution by irradiation of UV pulsed laser onto platinum nanoparticles. Chem. Phys. Lett. 2004, 383, 343–347. [Google Scholar] [CrossRef]
- Yan, Z.; Chrisey, D.B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aglio, M.; Gaudiuso, R.; DE Pascale, O.; De Giacomo, A. Mechanisms and processes of pulsed laser ablation in liquids during nanoparticle production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Marzun, G.; Bönnemann, H.; Lehmann, C.; Spliethoff, B.; Weidenthaler, C.; Barcikowski, S. Role of Dissolved and Molecular Oxygen on Cu and PtCu Alloy Particle Structure during Laser Ablation Synthesis in Liquids. ChemPhysChem 2017, 18, 1175–1184. [Google Scholar] [CrossRef]
- Hamza, L.F.; Ibrahim, D.I.M. Preparation of silver nanoparticles by pulsed laser ablation in liquid medium. Int. J. Eng. Comput. Sci. 2014, 3, 8261–8264. [Google Scholar]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- PJamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by laser ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Khan, A.R.; Al Mamun, M.S.; Ara, M.H. Review on platinum nanoparticles: Synthesis, characterization, and applications. Microchem. J. 2021, 171, 106840. [Google Scholar] [CrossRef]
- Gautam, A.; Guleria, P.; Kumar, V. Platinum Nanoparticles: Synthesis Strategies and Applications. Nanoarchitectonics 2020, 1, 70–86. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T. Formation of stable platinum nanoparticles by laser ablation in water. J. Phys. Chem. B 2003, 107, 4218–4223. [Google Scholar] [CrossRef]
- Moniri, S.; Ghoranneviss, M.; Hantehzadeh, M.R.; Asadabad, M.A. Synthesis of platinum nanoparticles by nanosecond laser irradiation of bulk Pt in different polar solvents. Res. Chem. Intermed. 2017, 43, 3015–3034. [Google Scholar] [CrossRef]
- Cueto, M.; Sanz, M.; Oujja, M.; Gámez, F.; Martínez-Haya, B.; Castillejo, M. Platinum nanoparticles prepared by laser ablation in aqueous solutions: Fabrication and application to laser desorption ionization. J. Phys. Chem. C. 2011, 115, 22217–22224. [Google Scholar] [CrossRef]
- Nichols, W.T.; Sasaki, T.; Koshizaki, N. Laser ablation of a platinum target in water. II. Ablation rate and nanoparticle size distributions. J. Appl. Phys. 2006, 100, 114912. [Google Scholar] [CrossRef]
- Censabella, M.; Torrisi, V.; Boninelli, S.; Bongiorno, C.; Grimaldi, M.; Ruffino, F. Laser ablation synthesis of mono- and bimetallic Pt and Pd nanoparticles and fabrication of Pt-Pd/Graphene nanocomposites. Appl. Surf. Sci. 2019, 475, 494–503. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Hantehzadeh, M.; Darabi, E. The effect of applied electric field on the micromorphology of Pt nanoparticles synthesized by laser ablation. Microsc. Res. Tech. 2021, 84, 3171–3181. [Google Scholar] [CrossRef]
- Condorelli, M.; D’Urso, L.; Compagnini, G.; Fazio, E.; Neri, F.; Litvinenko, V.A.; Kurmaev, E.Z.; Zhidkov, I.S. The catalytic role of platinum nanoparticles in laser generated nanocarbons. Appl. Surf. Sci. 2021, 558, 149890. [Google Scholar] [CrossRef]
- Madlum, K.N.; Khamees, E.J.; Abdulridha, S.A.; Naji, R.A. Antimicrobial and Cytotoxic Activity of Platinum Nanoparticles Synthesized by Laser Ablation Technique. J. Nanostruct. 2021, 11, 13–19. [Google Scholar] [CrossRef]
- Fathima, R.; Mujeeb, A. Enhanced nonlinear and thermo optical properties of laser synthesized surfactant-free Au-Pt bimetallic nanoparticles. J. Mol. Liq. 2021, 343, 117711. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Streubel, R.; Radev, I.; Peinecke, V.; Barcikowski, S.; Marzun, G.; Reichenberger, S. First PEM fuel cell based on ligand-free, laser-generated platinum nanoparticles. Appl. Surf. Sci. 2019, 467, 486–492. [Google Scholar] [CrossRef]
- Yan, Z.; Bao, R.; Chrisey, D.B. Excimer laser ablation of a Pt target in water: The observation of hollow particles. Nanotechnology 2010, 21, 145609. [Google Scholar] [CrossRef]
- Riabinina, D.; Irissou, E.; le Drogoff, B.; Chaker, M.; Guay, D. Influence of pressure on the Pt nanoparticle growth modes during pulsed laser ablation. J. Appl. Phys. 2010, 108, 034322. [Google Scholar] [CrossRef]
- Nikov, R.G.; Nedyalkov, N.N.; Nikolov, A.S.; Atanasov, P.A.; Alexandrov, M.T.; Karashanova, D.B. Formation of bimetallic nanoparticles by pulsed laser ablation of multicomponent thin films in water. In Proceedings of the 18th International School on Quantum Electronics: Laser Physics and Applications, Sozopol, Bulgaria, 29 September–3 October 2014; Volume 9447, p. 94470M. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadont, D.G. Ultraviolet-Visible Absorption Spectra of the Colloidal Metallic Elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Chin, C.D.; Akbarian-Tefaghi, S.; Reconco-Ramirez, J.; Wiley, J.B. Rapid microwave synthesis and optical activity of highly crystalline platinum nanocubes. MRS Commun. 2018, 8, 71–78. [Google Scholar] [CrossRef]
- Bigall, N.C.; Härtling, T.; Klose, M.; Simon, P.; Eng, L.M.; Eychmüller, A. Monodisperse platinum nanospheres with adjustable diameters from 10 to 100 nm: Synthesis and distinct optical properties. Nano Lett. 2008, 8, 4588–4592. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Hernandez, R.C.; Gleason-Villagran, R.; Torres-Torres, C.; Rodrıguez-Fernandez, L.; Crespo-Sosa, A.; Cheang-Wong, J.C.; Lopez-Suarez, A.; Rangel-Rojo, R.; Oliver, A.; Reyes-Esqueda, J.A. On the physical contributions to the third-order nonlinear optical response in plasmonic nanocomposites. J. Opt. 2012, 14, 125203. [Google Scholar] [CrossRef]
- Kovalchuk-Kogan, T.; Bulatov, V.; Schechter, I. Optical Breakdown in Liquid Suspensions and Its Analytical Applications. Adv. Chem. 2015, 2015, 463874. [Google Scholar] [CrossRef] [Green Version]
- Nichols, W.T.; Sasaki, T.; Koshizaki, N. Laser ablation of a platinum target in water. III. Laser-induced reactions. J. Appl. Phys. 2006, 100, 114913. [Google Scholar] [CrossRef]
- Torrisi, L.; Torrisi, A. Laser ablation parameters influencing gold nanoparticle synthesis in water. Radiat. Eff. Defects Solids 2018, 173, 729–739. [Google Scholar] [CrossRef]
- Zamiri, R.; Zakaria, A.; Ahangar, H.A.; Darroudi, M.; Zamiri, G.; Rizwan, Z.; Drummen, G.P.C. The effect of laser repetition rate on the LASiS synthesis of biocompatible silver nanoparticles in aqueous starch solution. Int. J. Nanomed. 2013, 8, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, M.; Hori, M. Preparation of dispersed platinum nanoparticles on a carbon nanostructured surface using supercritical fluid chemical deposition. Materials 2010, 3, 1559–1572. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.W.; Liu, Z.R.; Wang, B.L.; Zhu, L.; Yang, J.P.; Li, X.A. Preparation of graphene-supported Pt-Co nanoparticles and their use in oxygen reduction reactions. Xinxing Tan Cailiao/New Carbon Mater. 2012, 27, 250–257. [Google Scholar] [CrossRef]
- Liang, S.; Xia, Y.; Zhu, S.; Zheng, S.; He, Y.; Bi, J.; Liu, M.; Wu, L. Au and Pt co-loaded g-C 3 N 4 nanosheets for enhanced photocatalytic hydrogen production under visible light irradiation. Appl. Surf. Sci. 2015, 358, 304–312. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, P.F.; Yang, Z.J.; Jiang, T.W.; Yao, K.L.; Han, R.; Huo, X.X.; Xiong, Y.Y. Bi-functional porous carbon spheres derived from pectin as electrode material for supercapacitors and support material for Pt nanowires towards electrocatalytic methanol and ethanol oxidation. Electrochim. Acta 2015, 163, 140–148. [Google Scholar] [CrossRef]
- Huang, W.; Wang, H.; Zhou, J.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef]

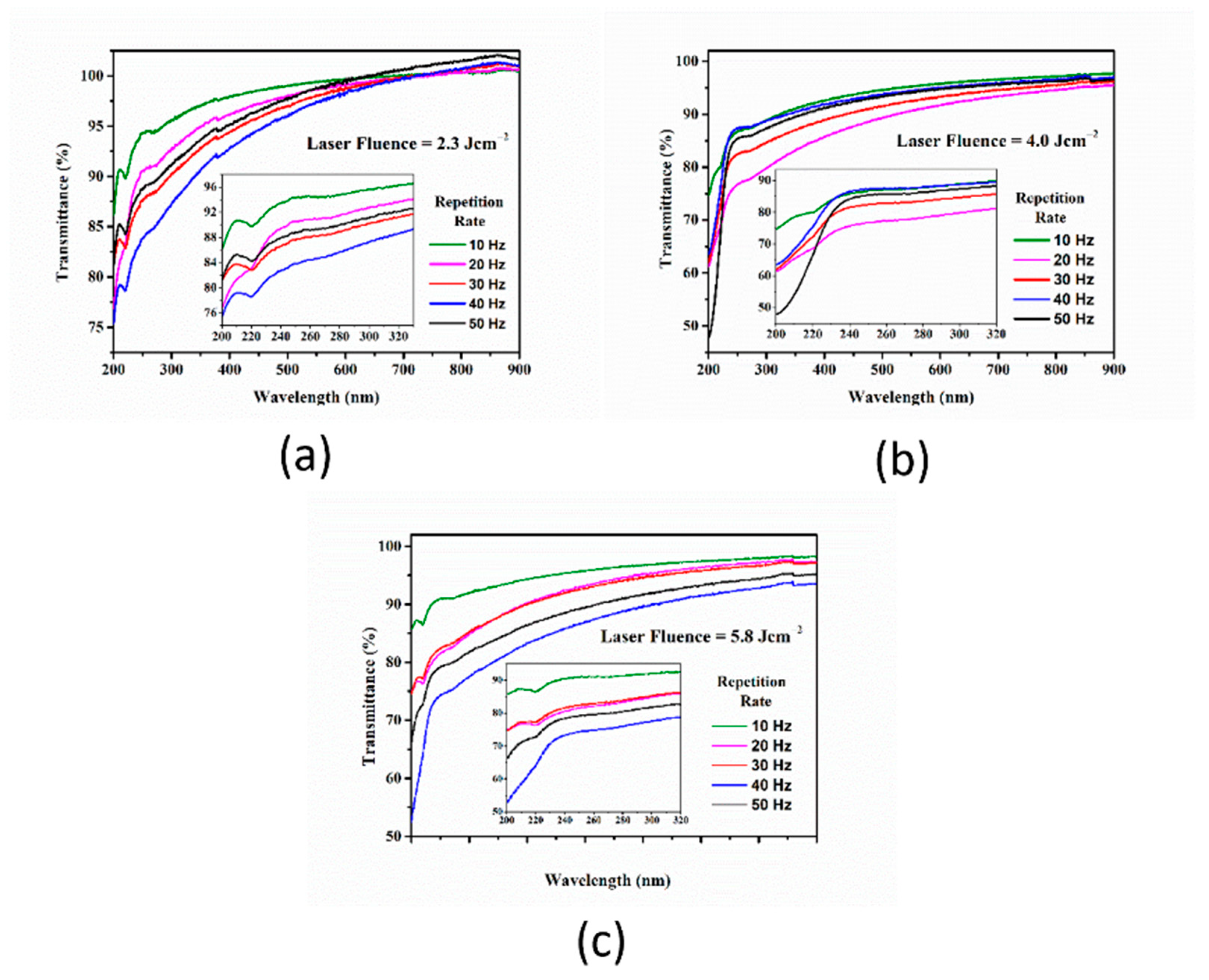
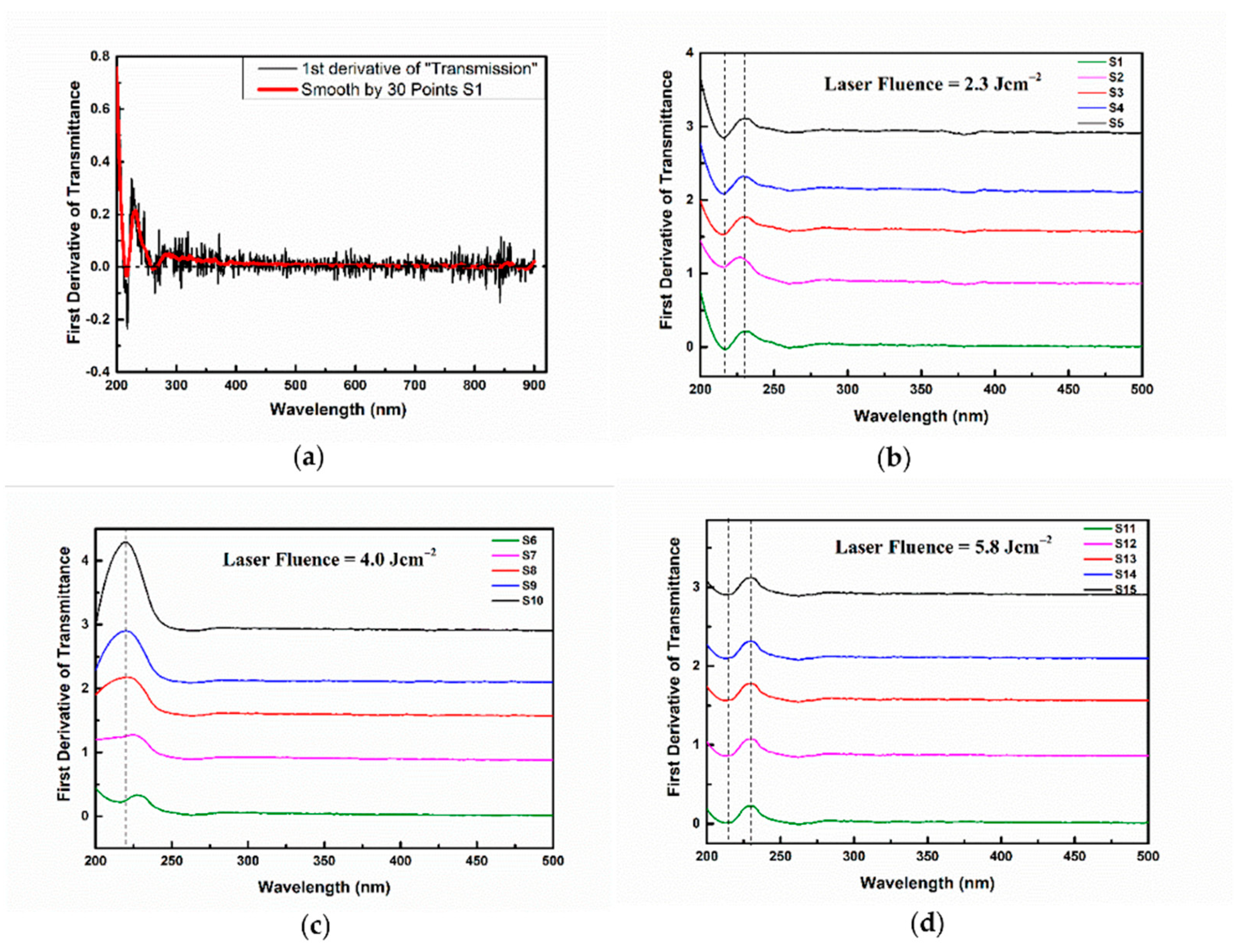

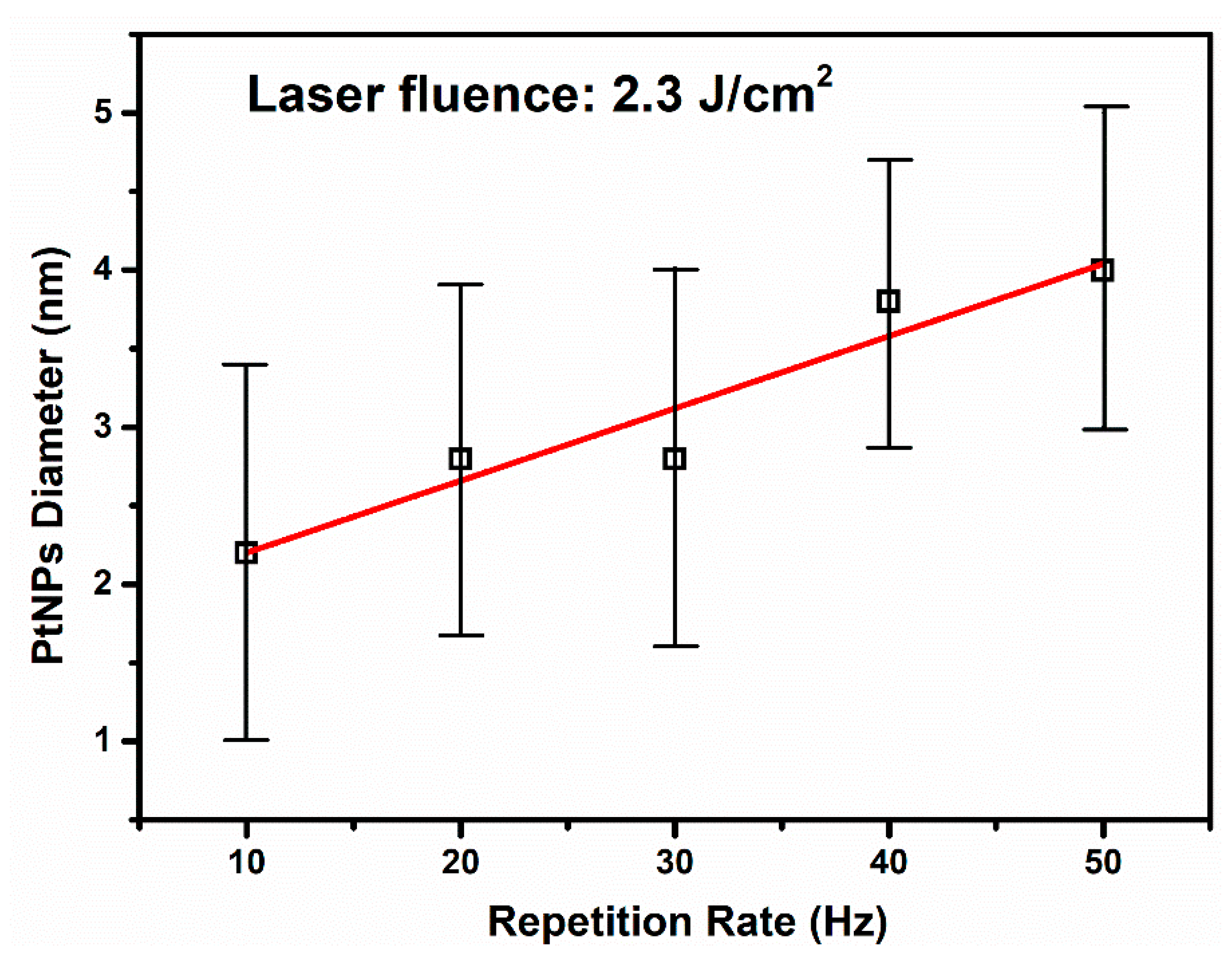
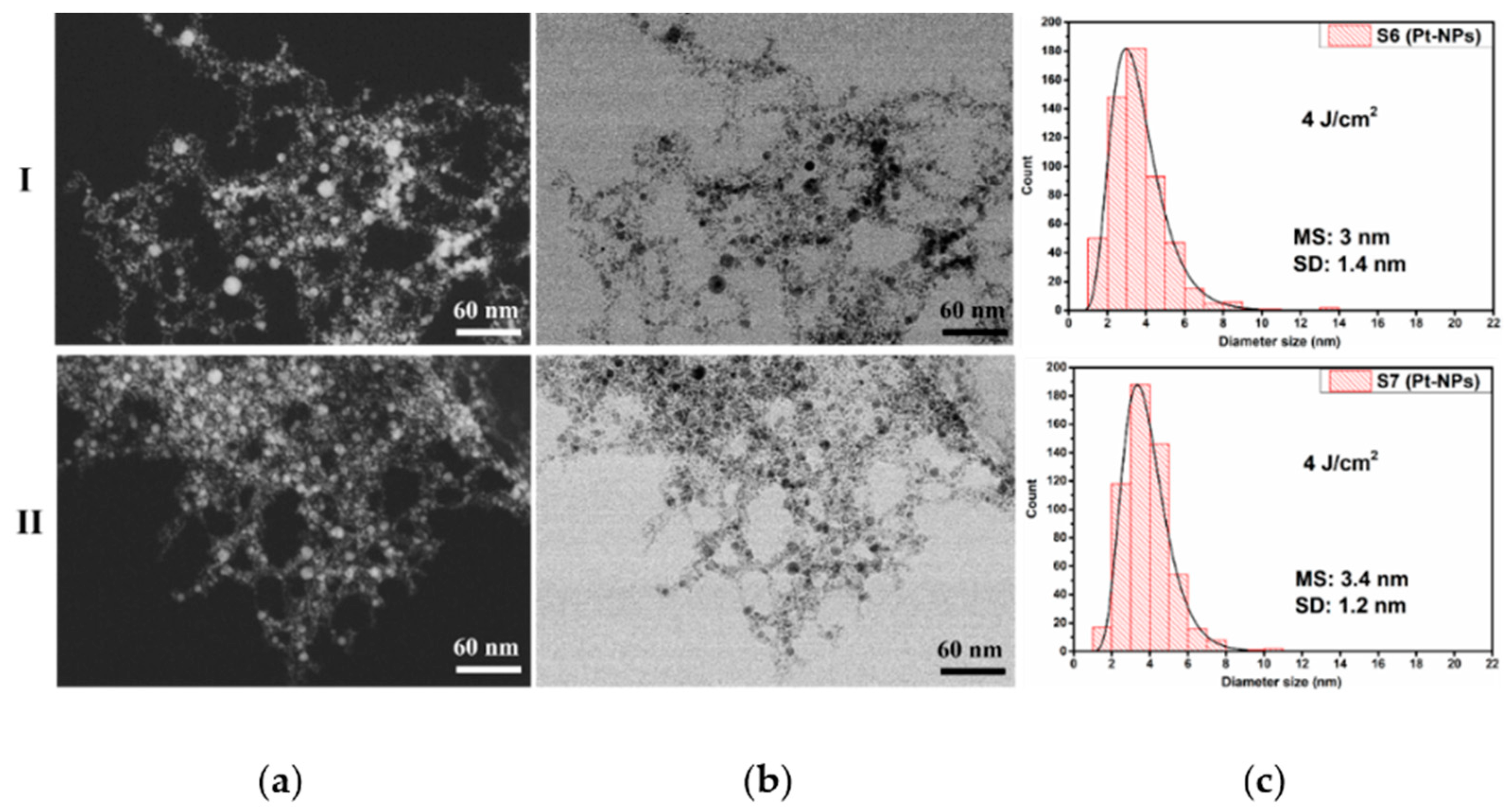


| Sample Index | E (mJ) | RR (Hz) | No. of Laser Shots | Fluence (J cm−2) | Final Volume (mL) | Lost Volume (%) |
|---|---|---|---|---|---|---|
| S1 | 200 | 10 | 9000 | 2.3 | 6.5 | 3 |
| S2 | 200 | 20 | 18,000 | 2.3 | 6.5 | 3 |
| S3 | 200 | 30 | 27,000 | 2.3 | 5.9 | 12 |
| S4 | 200 | 40 | 36,000 | 2.3 | 5.9 | 12 |
| S5 | 200 | 50 | 45,000 | 2.3 | 5.7 | 15 |
| S6 | 350 | 10 | 9000 | 4.0 | 6.5 | 3 |
| S7 | 350 | 20 | 18,000 | 4.0 | 6.2 | 7.5 |
| S8 | 350 | 30 | 27,000 | 4.0 | 5.9 | 12 |
| S9 | 350 | 40 | 36,000 | 4.0 | 5.1 | 24 |
| S10 | 350 | 50 | 45,000 | 4.0 | 4.7 | 30 |
| S11 | 500 | 10 | 9000 | 5.8 | 6.2 | 7.5 |
| S12 | 500 | 20 | 18,000 | 5.8 | 5.4 | 19 |
| S13 | 500 | 30 | 27,000 | 5.8 | 4 | 40 |
| S14 | 500 | 40 | 36,000 | 5.8 | 3.5 | 48 |
| S15 | 500 | 50 | 45,000 | 5.8 | 3 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, O.A.; Moise, C.C.; Nikolov, A.S.; Enache, L.-B.; Mihai, G.V.; Enachescu, M. The Water-Based Synthesis of Platinum Nanoparticles Using KrF Excimer Laser Ablation. Nanomaterials 2022, 12, 348. https://doi.org/10.3390/nano12030348
Lazar OA, Moise CC, Nikolov AS, Enache L-B, Mihai GV, Enachescu M. The Water-Based Synthesis of Platinum Nanoparticles Using KrF Excimer Laser Ablation. Nanomaterials. 2022; 12(3):348. https://doi.org/10.3390/nano12030348
Chicago/Turabian StyleLazar, Oana Andreea, Călin Constantin Moise, Anastas Savov Nikolov, Laura-Bianca Enache, Geanina Valentina Mihai, and Marius Enachescu. 2022. "The Water-Based Synthesis of Platinum Nanoparticles Using KrF Excimer Laser Ablation" Nanomaterials 12, no. 3: 348. https://doi.org/10.3390/nano12030348






