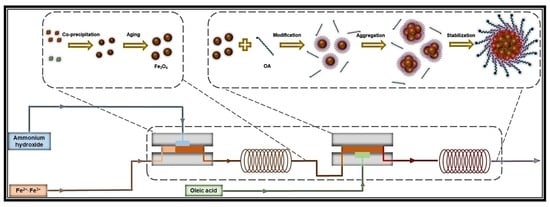
Flexible and Effective Preparation of Magnetic Nanoclusters via One-Step Flow Synthesis
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
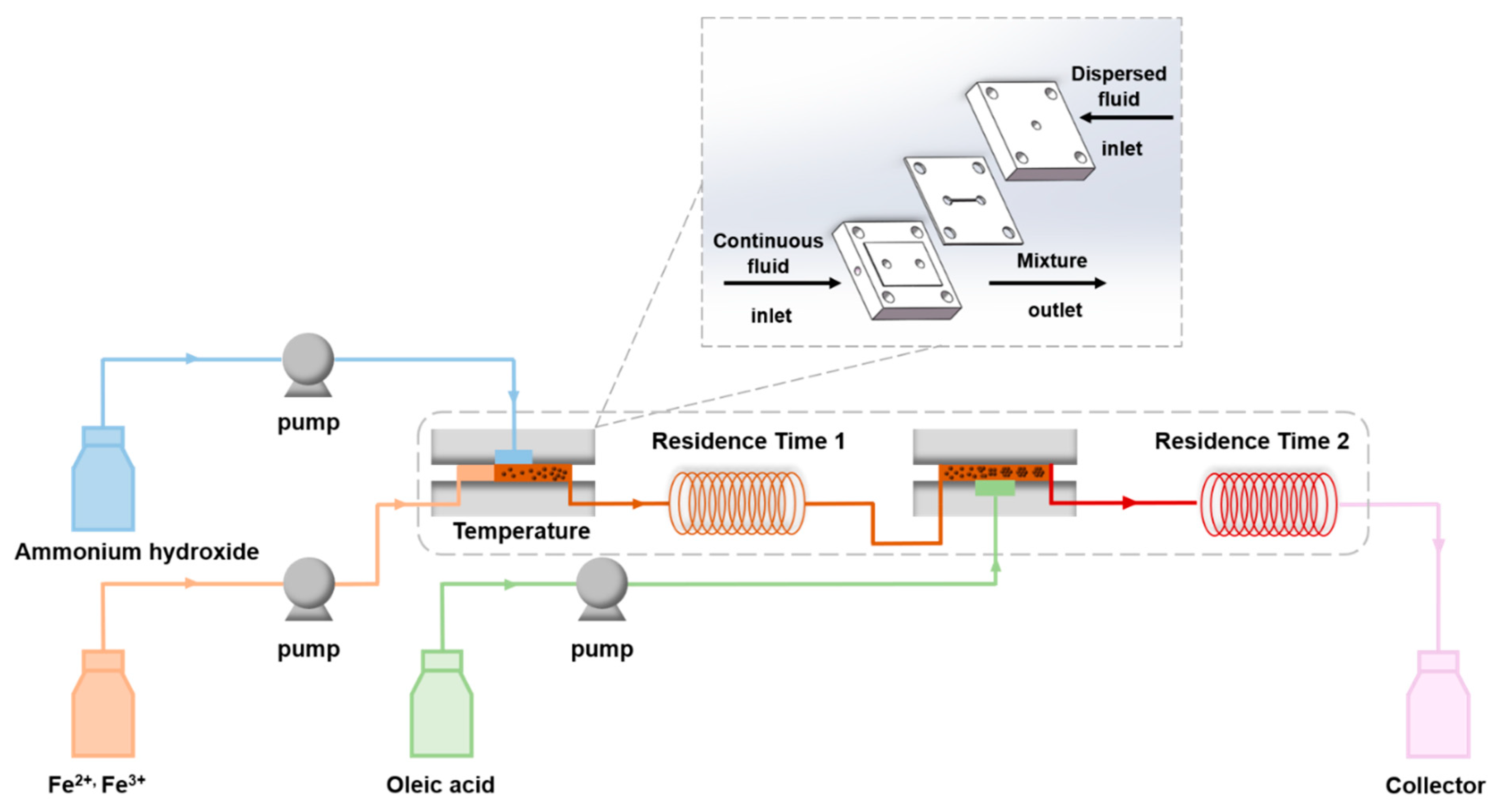
2.3. Continuous Synthesis of Bilayer OA-Coated Fe3O4 Nanoclusters
3. Results and Discussion
3.1. Feasibility of Continuous Preparation of Bilayer OA-coated Fe3O4 Nanoclusters
3.2. Influence Factors on the Formation of Bilayer OA-Coated Fe3O4 Nanoclusters
3.2.1. Effect of the Residence Time
3.2.2. Effect of the Amount of OA
3.2.3. Effect of the pH
3.2.4. Effect of the Temperature
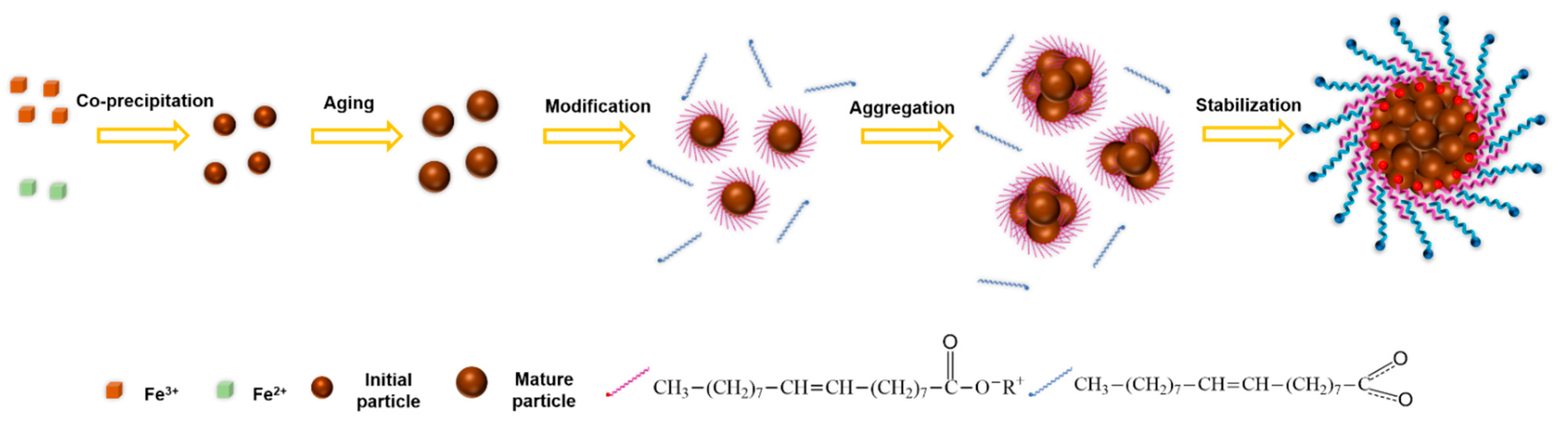
3.3. Proposed Mechanism of Bilayer OA-Coated Fe3O4 Nanocluster Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drbohlavova, J.; Hrdy, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and properties of various magnetic nanoparticles. Sensors 2009, 9, 2352–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Formation and corrosion behavior of Nickel/Alumina nanocomposites. Solid State Phenom. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Trukhanov, A.V. Thermal Stability of Nano-Crystalline Nickel Electrodeposited into Porous Alumina. Solid State Phenom. 2020, 299, 281–286. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Zhang, L.; Wang, T.; Yu, H.; Wang, C.; Su, Z. Multifunctional magnetic–fluorescent eccentric-(concentric-Fe3O4@SiO2)@Polyacrylic acid Core–Shell nanocomposites for cell imaging and pH-responsive drug delivery. Nanoscale 2013, 5, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 nanoparticles for complex targeted delivery and boron neutron capture therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Zhou, X.; Nie, W.; Chen, L.; Qiu, K.; Zhang, Y.; He, C. Au/Polypyrrole@Fe3O4 nanocomposites for MR/CT Dual-Modal imaging guided-photothermal therapy: An in vitro study. ACS Appl. Mater. Interfaces 2015, 7, 4354–4367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, W.; Wang, D.; Ran, H.; Wang, R.; Yao, Y.; Wang, Z.; Zheng, Y.; Li, P. Phase-shifted PFH@PLGA/Fe3O4 nanocapsules for MRI/US imaging and photothermal therapy with near-infrared irradiation. ACS Appl. Mater. Interfaces 2015, 7, 14231–14242. [Google Scholar] [CrossRef]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron oxide mediated photothermal therapy in the second biological window: A comparative study between magnetite/maghemite nanospheres and nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Jafarzadeh, H.; Ramezani, M. Preparation and characterization of uniform-sized PLGA nanospheres encapsulated with oleic acid-coated magnetic-Fe3O4 nanoparticles for simultaneous diagnostic and therapeutic applications. Colloids Surf. A 2017, 514, 146–154. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, V.; Magro, M.; Gedanken, A.; Baratella, D.; Vianello, F.; Zboril, R. Nanocrystalline iron oxides, composites, and related materials as a platform for electrochemical, magnetic, and chemical biosensors. Chem. Mater. 2014, 26, 6653–6673. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Yu, S.; Zou, Y.; Wang, J.; Chen, Z.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Chen, Y.; et al. Silica coated Fe3O4 magnetic nanospheres for high removal of organic pollutants from wastewater. Chem. Eng. J. 2016, 306, 280–288. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, B.; Zhang, J.; Wang, A. Magnetic, durable, and superhydrophobic polyurethane @Fe3O4@SiO2@ fluoropolymer sponges for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 4936–4946. [Google Scholar] [CrossRef]
- Kandathil, V.; Fahlman, B.D.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. A Convenient, efficient and reusable N-Heterocyclic Carbene-Palladium(II) Based Catalyst Supported on Magnetite for Suzuki–Miyaura and Mizoroki–Heck Cross-Coupling Reactions. New J. Chem. 2017, 41, 9531–9545. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Misztalewska, I. Direct synthesis of imidazolinium salt on magnetic nanoparticles and its palladium complex application in the heck reaction. Organometallics 2014, 33, 5203–5208. [Google Scholar] [CrossRef]
- Laska, U.; Frost, C.G.; Price, G.J.; Plucinski, P.K. Easy-separable magnetic nanoparticle-supported Pd catalysts: Kinetics, stability and catalyst Re-use. J. Catal. 2009, 268, 318–328. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D. Magnetic Assembly of Superparamagnetic Iron Oxide Nanoparticle Clusters into Nano chains and Nanobundles. Acs Nano 2015, 9, 9700–9707. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Park, S.-E.; Lee, S.-W.; Yang, D.; Lee, J.K. Encapsulated Fe3O4 nanoparticles with silica thin layer as an anode material for lithium secondary batteries. Phys. Scr. 2010, 139, 014027. [Google Scholar] [CrossRef]
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Bustamante, D.A.; De Los Santos Valladares, L.; Albino Aguiar, J. Synthesis and Characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, C.; Guo, J.; Xu, K.; Wang, C. Biopolymer-directed synthesis of high-surface-area magnetite colloidal nanocrystal clusters for dual drug delivery in prostate cancer. J. Mater. Chem. 2012, 22, 19067. [Google Scholar] [CrossRef]
- Nikitin, A.A.; Shchetinin, I.V.; Tabachkova, N.Y.; Soldatov, M.A.; Soldatov, A.V.; Sviridenkova, N.V.; Beloglazkina, E.K.; Savchenko, A.G.; Fedorova, N.D.; Abakumov, M.A.; et al. Synthesis of iron oxide nanoclusters by thermal decomposition. Langmuir 2018, 34, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Li, W.; Xie, Y.; Li, Y.; Wu, J.; Wang, S.; Tian, Y.; Tian, W.; Teng, Z.; et al. Synthesis of sub-100 nm biocompatible superparamagnetic Fe3O4 colloidal nanocrystal clusters as contrast agents for magnetic resonance imaging. RSC Adv. 2016, 6, 62550–62555. [Google Scholar] [CrossRef]
- Kim, J.; Tran, V.T.; Oh, S.; Kim, C.S.; Hong, J.C.; Kim, S.; Joo, Y.S.; Mun, S.; Kim, M.H.; Jung, J.W.; et al. Scalable solvothermal synthesis of superparamagnetic Fe3O4 nanoclusters for bioseparation and theragnostic probes. ACS Appl. Mater. Interfaces 2018, 10, 41935–41946. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, J.K.; Deak, A.; Brougham, D.F. Nanoparticle clusters: Assembly and control over internal order, current capabilities, and future potential. Adv. Mater. 2016, 28, 5400–5424. [Google Scholar] [CrossRef]
- Kim, B.-S.; Qiu, J.-M.; Wang, J.-P.; Taton, T.A. Magnetomicelles: Composite nanostructures from magnetic nanoparticles and cross-linked amphiphilic block copolymers. Nano Lett. 2005, 5, 1987–1991. [Google Scholar] [CrossRef]
- Euliss, L.E.; Grancharov, S.G.; O’Brien, S.; Deming, T.J.; Stucky, G.D.; Murray, C.B.; Held, G.A. Cooperative assembly of magnetic nanoparticles and block copolypeptides in aqueous media. Nano Lett. 2003, 3, 1489–1493. [Google Scholar] [CrossRef]
- Zhuang, J.; Wu, H.; Yang, Y.; Cao, Y.C. Supercrystalline colloidal particles from artificial atoms. J. Am. Chem. Soc. 2007, 129, 14166–14167. [Google Scholar] [CrossRef]
- Fu, J.; He, L.; Xu, W.; Zhuang, J.; Yang, X.; Zhang, X.; Wu, M.; Yin, Y. Formation of colloidal nanocrystal clusters of iron oxide by controlled ligand stripping. Chem. Commun. 2016, 52, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.P.; Hatton, T.A. Membrane emulsification and solvent pervaporation processes for the continuous synthesis of functional magnetic and janus nanobeads. Langmuir 2012, 28, 9748–9758. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Y. Highly efficient and flexible preparation of water-dispersed Fe3O4 nanoclusters using a micromixer. Particuology 2019, 45, 42–48. [Google Scholar] [CrossRef]
- Zou, H.; Weder, C.; Simon, Y.C. Shape-memory polyurethane nanocomposites with single layer or bilayer oleic acid-coated Fe3O4 nanoparticles. Macromol. Mater. Eng. 2015, 300, 885–892. [Google Scholar] [CrossRef]
- Wu, H.; Hao, L.; Chen, C.; Zhou, J. Superhydrophobic Fe3O4/OA magnetorheological fluid for removing oil slick from water surfaces effectively and quickly. ACS Omega 2020, 5, 27425–27432. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yang, D.; Xie, L.; Liu, Q.; Tang, T.; Zhang, H.; Zeng, H. Probing the interactions between pickering emulsion droplets stabilized with pH-responsive nanoparticles. J. Phys. Chem. B 2021, 125, 7320–7331. [Google Scholar] [CrossRef]
- Li, H.; Qin, L.; Feng, Y.; Hu, L.; Zhou, C. Preparation and characterization of highly water-soluble magnetic Fe3O4 Nanoparticles via surface double-layered self-assembly method of sodium alpha-olefin sulfonate. J. Magn. Magn. Mater. 2015, 384, 213–218. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Liu, X.; Kaminski, M.D.; Guan, Y.; Chen, H.; Liu, H.; Rosengart, A.J. Preparation and characterization of hydrophobic superparamagnetic magnetite gel. J. Magn. Magn. Mater. 2006, 306, 248–253. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Laia, C.A.T.; Carvalho, A.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. iron oxide nanoparticles stabilized with a bilayer of oleic acid for magnetic hyperthermia and MRI applications. Appl. Surf. Sci. 2016, 383, 240–247. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Chen, M.J.; Shen, H.; Li, X.; Liu, H.F. Facile synthesis of oil-soluble Fe3O4 nanoparticles based on a phase transfer mechanism. Appl. Surf. Sci. 2014, 307, 306–310. [Google Scholar] [CrossRef]
- Wang, Y.M.; Cao, X.; Liu, G.H.; Hong, R.Y.; Chen, Y.M.; Chen, X.F.; Li, H.Z.; Xu, B.; Wei, D.G. Synthesis of Fe3O4 magnetic fluid used for magnetic resonance imaging and hyperthermia. J. Magn. Magn. Mater. 2011, 323, 2953–2959. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, Y.; Luo, G. Synthesis of Micro–Nano-assembled manganese carbonate via aqueous precipitation assisted by ethanol. Ind. Eng. Chem. Res. 2017, 56, 10036–10043. [Google Scholar] [CrossRef]
- Shouheng Sun, H.Z.; David, B.R.; Simone, R.; Philip, M.R.; Shan, X.W.; Guanxiong, L. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Ho, C.-H.; Tsai, C.-P.; Chung, C.-C.; Tsai, C.-Y.; Chen, F.-R.; Lin, H.-J.; Lai, C.-H. Shape-controlled growth and shape-dependent cation site occupancy of monodisperse Fe3O4 nanoparticles. Chem. Mater. 2011, 23, 1753–1760. [Google Scholar] [CrossRef]
- Fan, T.; Pan, D.; Zhang, H. Study on Formation mechanism by monitoring the morphology and structure evolution of nearly monodispersed Fe3O4 submicroparticles with controlled particle sizes. Ind. Eng. Chem. Res. 2011, 50, 9009–9018. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, C.; Yang, F.; Liu, S.; Xu, J.; Sun, D. Synthesis of bilayer oleic acid-coated Fe3O4 nanoparticles and their application in pH-responsive pickering emulsions. J. Colloid Interface Sci. 2007, 310, 260–269. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.-C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Hitesh, G.; Bagaria, E.T.A.; Mohammed, S.; David, E.N.; Duane, T.J. Understanding mercapto ligand exchange on the surface of FePt nanoparticles. Langmuir 2006, 22, 7732–7737. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ Ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Poulin, S.F.R.; Moreau-Bélanger, L.; Sacher, E. Confirmation of X-ray photoelectron spectroscopy peak attributions of nanoparticulate Iron oxides, using symmetric peak component line shapes. J. Phys. Chem. C 2010, 114, 10711–10718. [Google Scholar] [CrossRef]
- Lifen, S.; Labinis, P.E.; Hatton, T.A. Bilayer surfactant stabilized magnetic fluids: Synthesis and interactions at interfaces. Langmuir 1999, 15, 447–453. [Google Scholar] [CrossRef]
- Gu, S.; Shiratori, T.; Konno, M. Synthesis of monodisperse, magnetic latex particles with polystyrene core. Colloid. Polym. Sci. 2003, 281, 1076–1081. [Google Scholar] [CrossRef]

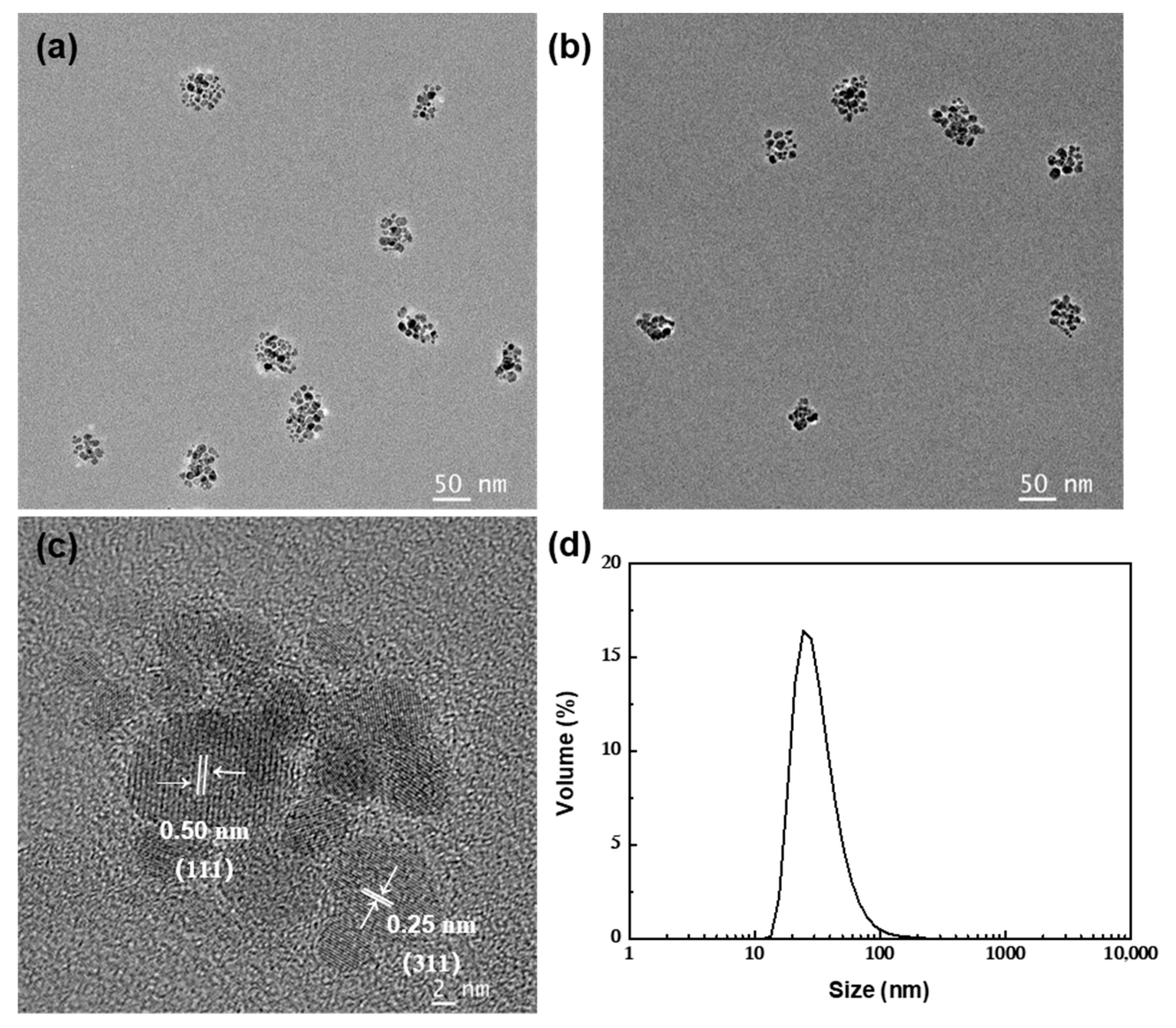
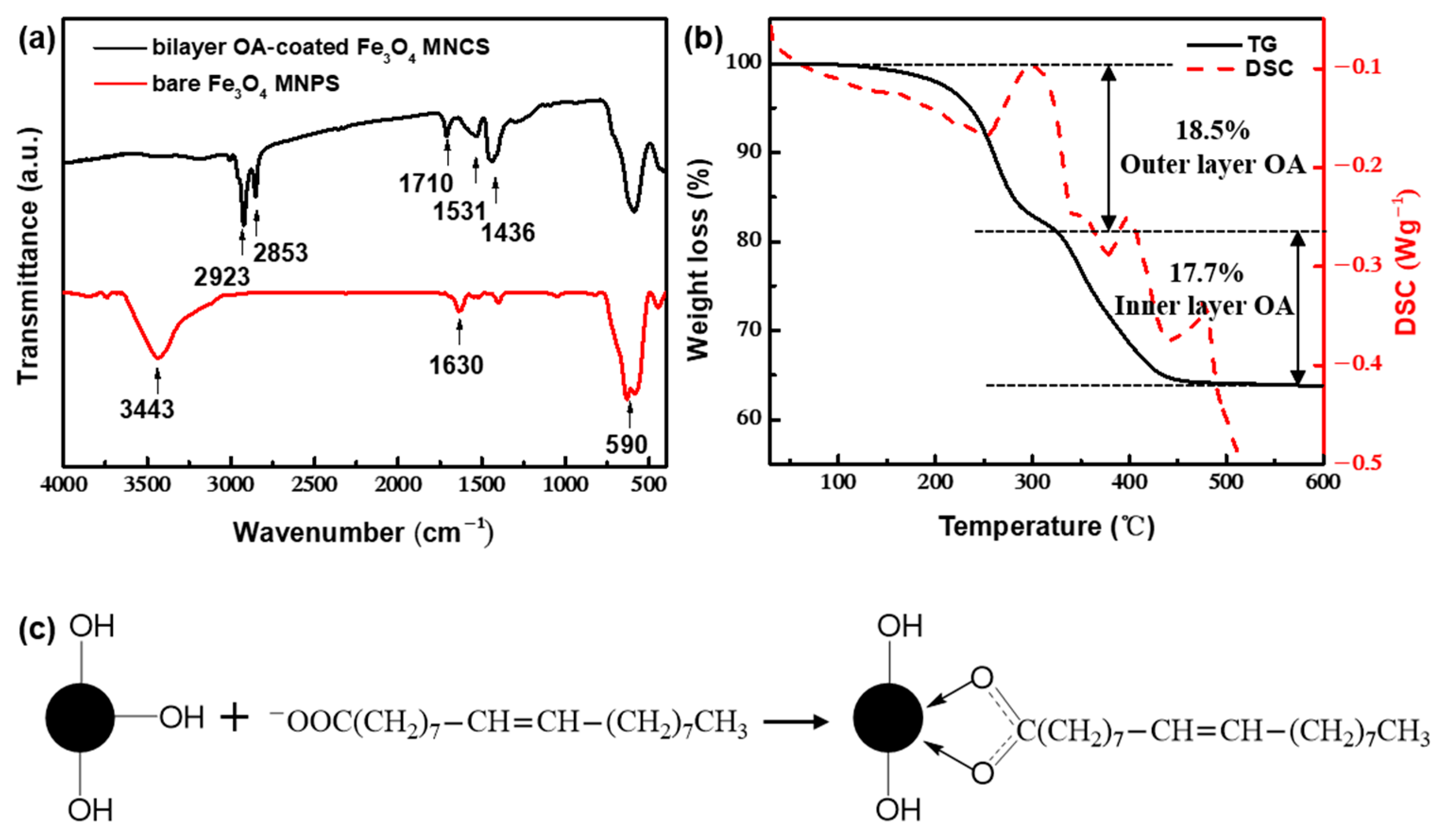

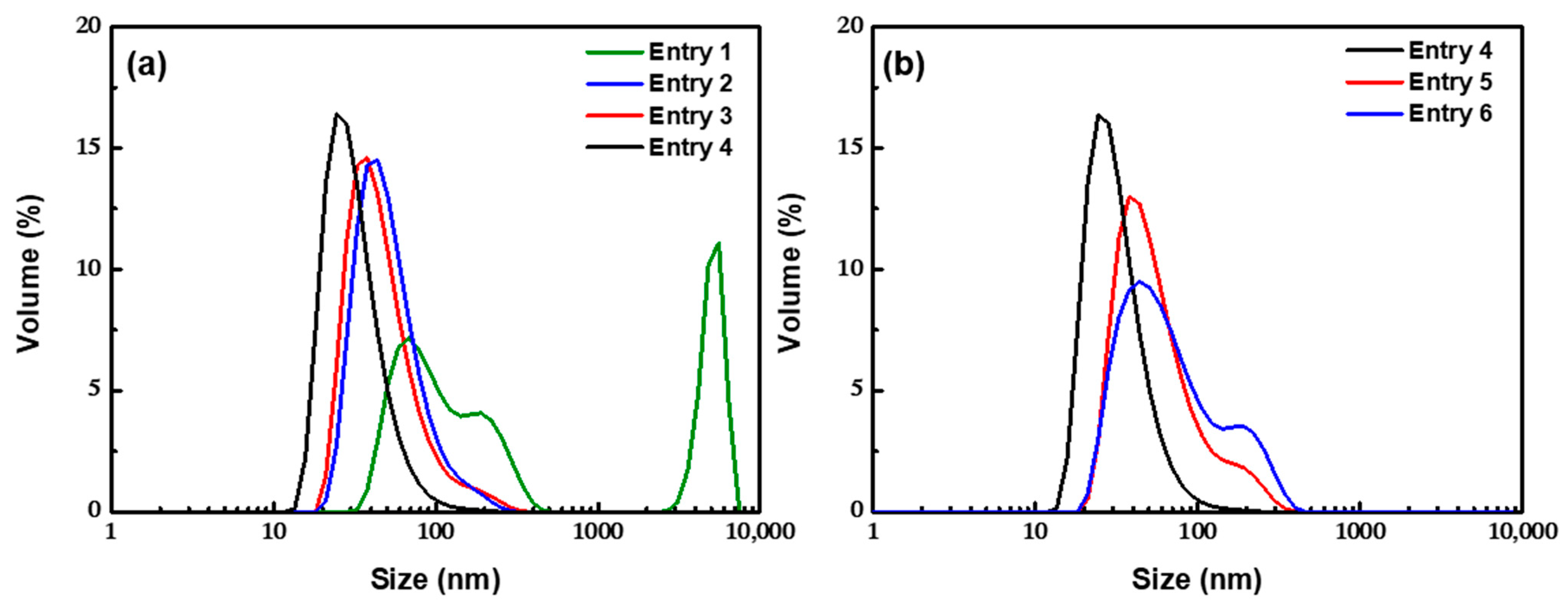

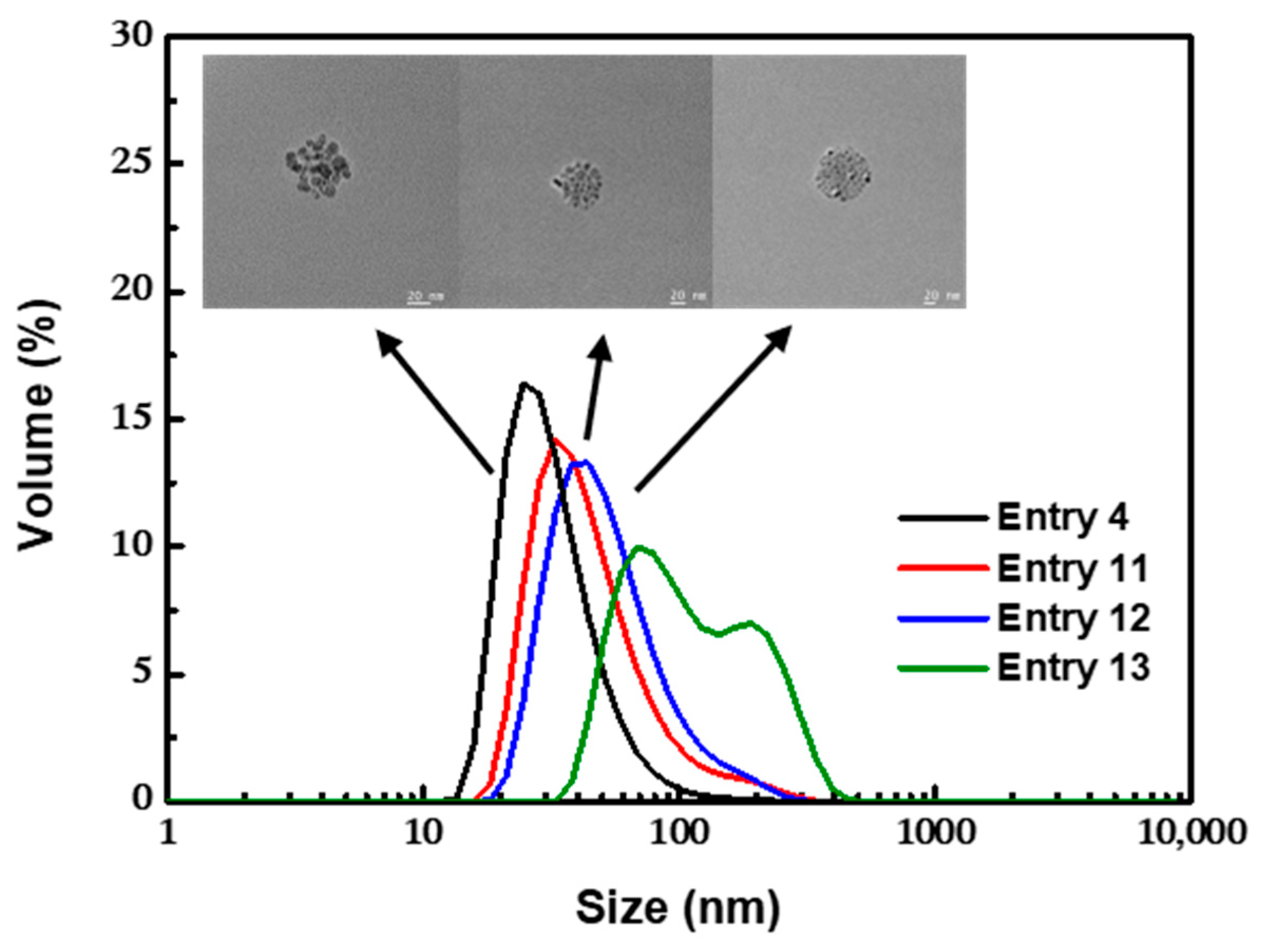

| Entry | NH3·H2O (M) | Oleic Acid (M) | Residence Time 1 (s) | Residence Time 2 (s) | pH | Temperature (°C) | Cluster Size (nm) | PDI |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.3 | 0.1 | 0 | 12.56 | 10.3 | 60 | 110.9 | 0.443 |
| 2 | 7.3 | 0.1 | 6.28 | 12.56 | 10.3 | 60 | 71.1 | 0.201 |
| 3 | 7.3 | 0.1 | 12.56 | 12.56 | 10.3 | 60 | 70.2 | 0.197 |
| 4 | 7.3 | 0.1 | 18.84 | 12.56 | 10.3 | 60 | 47.9 | 0.165 |
| 5 | 7.3 | 0.1 | 18.84 | 6.28 | 10.3 | 60 | 88.6 | 0.189 |
| 6 | 7.3 | 0.1 | 18.84 | 0 | 10.3 | 60 | 97.55 | 0.203 |
| 7 | 7.3 | 0.07 | 18.84 | 12.56 | 10.3 | 60 | 57.5 | 0.172 |
| 8 | 7.3 | 0.060 | 18.84 | 12.56 | 10.3 | 60 | 97.6 | 0.183 |
| 9 | 7.3 | 0.055 | 18.84 | 12.56 | 10.3 | 60 | 116.2 | 0.216 |
| 10 | 7.3 | 0.035 | 18.84 | 12.56 | 10.3 | 60 | - | - |
| 11 | 5.5 | 0.1 | 18.84 | 12.56 | 10.1 | 60 | 62.2 | 0.174 |
| 12 | 3.7 | 0.1 | 18.84 | 12.56 | 9.9 | 60 | 75.2 | 0.175 |
| 13 | 2.0 | 0.1 | 18.84 | 12.56 | 9.7 | 60 | 122.6 | 0.215 |
| 14 | 7.3 | 0.1 | 18.84 | 12.56 | 10.3 | 70 | 49.7 | 0.165 |
| 15 | 7.3 | 0.1 | 18.84 | 12.56 | 10.3 | 50 | 62.9 | 0.184 |
| 16 | 7.3 | 0.1 | 18.84 | 12.56 | 10.3 | 35 | 103.2 | 0.210 |
| 17 | 7.3 | 0.1 | 18.84 | 12.56 | 10.3 | RT | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ye, L.; Lu, Y. Flexible and Effective Preparation of Magnetic Nanoclusters via One-Step Flow Synthesis. Nanomaterials 2022, 12, 350. https://doi.org/10.3390/nano12030350
Zhou L, Ye L, Lu Y. Flexible and Effective Preparation of Magnetic Nanoclusters via One-Step Flow Synthesis. Nanomaterials. 2022; 12(3):350. https://doi.org/10.3390/nano12030350
Chicago/Turabian StyleZhou, Lin, Lu Ye, and Yangcheng Lu. 2022. "Flexible and Effective Preparation of Magnetic Nanoclusters via One-Step Flow Synthesis" Nanomaterials 12, no. 3: 350. https://doi.org/10.3390/nano12030350
APA StyleZhou, L., Ye, L., & Lu, Y. (2022). Flexible and Effective Preparation of Magnetic Nanoclusters via One-Step Flow Synthesis. Nanomaterials, 12(3), 350. https://doi.org/10.3390/nano12030350