Line Tension and Drop Size Dependence of Contact Angle at the Nanoscale
Abstract
:1. Introduction
2. Materials and Methods
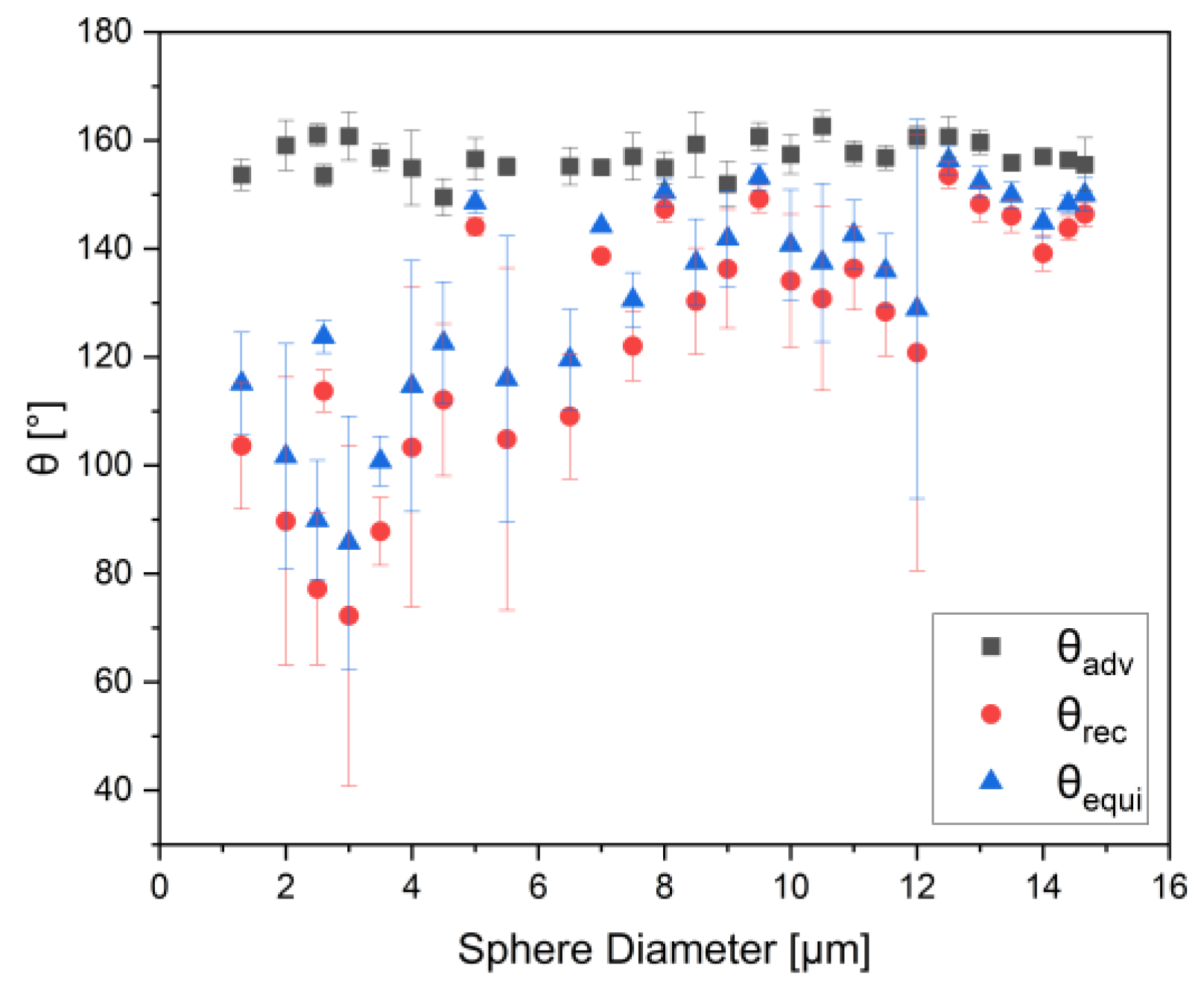
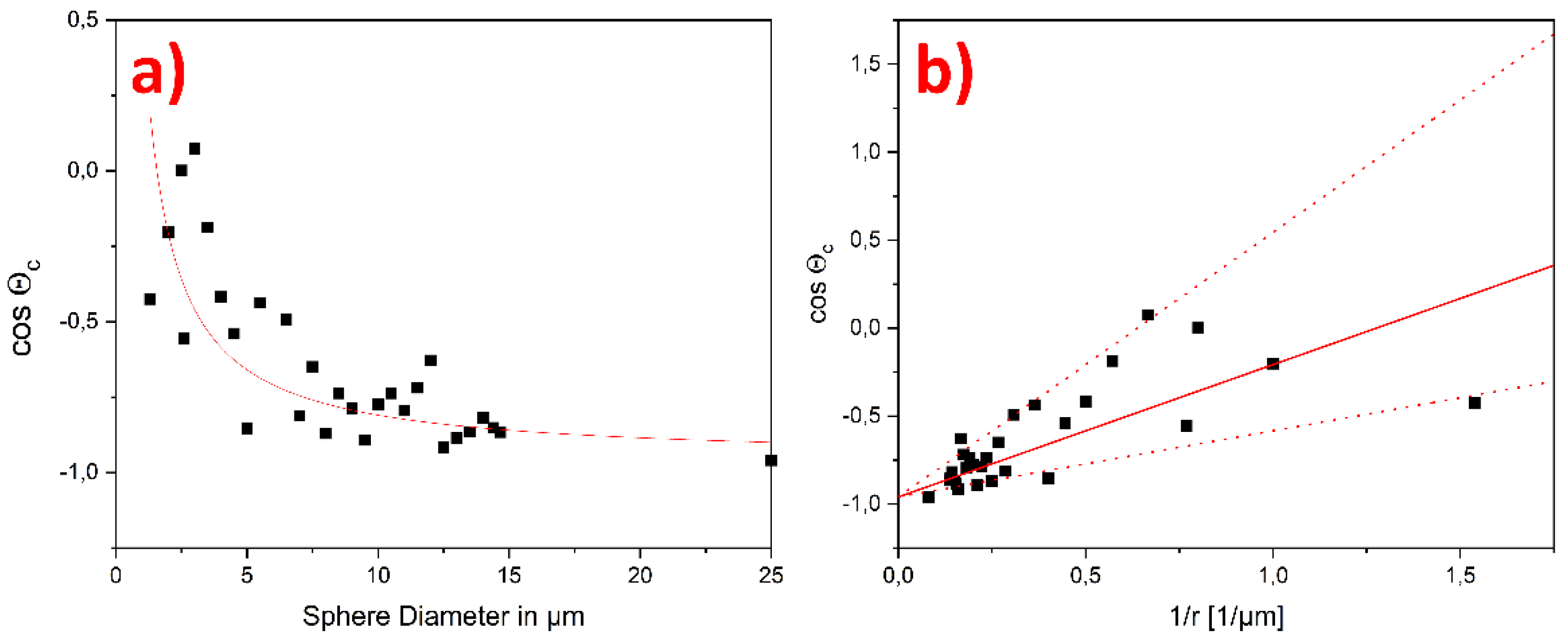
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse Double Emulsions Generated from a Microcapillary Device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Atencia, J.; Beebe, D.J. Controlled Microfluidic Interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing Soft Matter in Three Dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bieleman, J. Additives for Coatings; Wiley: Hoboken, NJ, USA, 2000; ISBN 9783527613311. [Google Scholar]
- Ulman, A. Wetting st Udies of Molecularly Engineered Surfaces. Thin Solid Film. 1996, 273, 48–53. [Google Scholar] [CrossRef]
- Amirfazli, A.; Neumann, A.W. Status of the Three-Phase Line Tension: A Review. Adv. Colloid Interface Sci. 2004, 110, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. Am. J. Sci. 1878, s3-16, 441–458. [Google Scholar] [CrossRef]
- Boruvka, L.; Neumann, A.W. Generalization of the Classical Theory of Capillarity. J. Chem. Phys. 1977, 66, 5464–5476. [Google Scholar] [CrossRef]
- An Lei, Y.; Bykov, T.; Yoo, S.; Zeng, X.C. The Tolman length: Is It Positive or Negative? J. Am. Chem. Soc. 2005, 127, 15346–15347. [Google Scholar] [CrossRef]
- Schimmele, L.; Napiórkowski, M.; Dietrich, S. Conceptual Aspects of Line Tensions. J. Chem. Phys. 2007, 127, 164715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Borg, M.K.; Reese, J.M.; Wen, D. A Critical Assessment of the Line Tension Determined by the Modified Young’s Equation. Phys. Fluids 2018, 30, 82003. [Google Scholar] [CrossRef]
- Kanduč, M.; Eixeres, L.; Liese, S.; Netz, R.R. Generalized Line Tension of Water Nanodroplets. Phys. Rev. E 2018, 98, 032804. [Google Scholar] [CrossRef]
- Law, B.M.; McBride, S.P.; Wang, J.Y.; Wi, H.S.; Paneru, G.; Betelu, S.; Ushijima, B.; Takata, Y.; Flanders, B.; Bresme, F.; et al. Line Tension and Its Influence on Droplets and Particles at Surfaces. Prog. Surf. Sci. 2017, 92, 1–39. [Google Scholar] [CrossRef]
- Zhang, J.; Leroy, F.; Müller-Plathe, F. Influence of Contact-Line Curvature on the Evaporation of Nanodroplets from Solid Substrates. Phys. Rev. Lett. 2014, 113, 46101. [Google Scholar] [CrossRef] [PubMed]
- McBride, S.P.; Law, B.M. Influence of Line Tension on Spherical Colloidal Particles at Liquid-Vapor Interfaces. Phys. Rev. Lett. 2012, 109, 196101. [Google Scholar] [CrossRef]
- Heim, L.-O.; Bonaccurso, E. Measurement of Line Tension on Droplets in the Submicrometer Range. Langmuir 2013, 29, 14147–14153. [Google Scholar] [CrossRef]
- Toshev, B.V.; Platikanov, D.; Scheludko, A. Line Tension in Three-Phase Equilibrium Systems. Langmuir 1988, 4, 489–499. [Google Scholar] [CrossRef]
- Berg, J.K.; Weber, C.M.; Riegler, H. Impact of Negative Line Tension on the Shape of Nanometer-Size Sessile Droplets. Phys. Rev. Lett. 2010, 105, 76103. [Google Scholar] [CrossRef]
- Wang, J.Y.; Betelu, S.; Law, B.M. Line Tension Approaching a First-Order Wetting Transition: Experimental Results from Contact Angle Measurements. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2001, 63, 31601. [Google Scholar] [CrossRef]
- Wang, J.Y.; Betelu, S.; Law, B.M. Line Tension Effects near First-Order Wetting Transitions. Phys. Rev. Lett. 1999, 83, 3677–3680. [Google Scholar] [CrossRef]
- Pompe, T. Line Tension Behavior of a First-Order Wetting System. Phys. Rev. Lett. 2002, 89, 76102. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, S.; Bonaccurso, E.; Auernhammer, G.K.; Deng, X.; Li, Z.; Chen, L. Resolving the Apparent Line Tension of Sessile Droplets and Understanding its Sign Change at a Critical Wetting Angle. Phys. Rev. Lett. 2009, 123, 94501. [Google Scholar] [CrossRef]
- von Kleist-Retzow, F.; Klauser, W.; Zimmermann, S.; Fatikow, S. Assessing Micro- and Nanoscale Adhesion via Liquid Metal-Based Contact Angle Measurements in Vacuum. J. Mater. Sci. 2020, 55, 4073–4080. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Persson, K.A.; Truong, V.K.; Oh, J.-H.; Kong, M.; Vong, M.H.; Ni, C.; Alsafatwi, M.; Parekh, D.P.; Zhao, H.; et al. Are Contact Angle Measurements Useful for Oxide-Coated Liquid Metals? Langmuir 2021, 37, 10914–10923. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Tiemerding, T.; Fatikow, S. Automated Robotic Manipulation of Individual Colloidal Particles Using Vision-Based Control. IEEE/ASME Trans. Mechatron. 2015, 20, 2031–2038. [Google Scholar] [CrossRef]
- Von Kleist-Retzow, F.T.; Haenssler, O.C.; Fatikow, S. Manipulation of Liquid Metal Inside an SEM by Taking Advantage of Electromigration. J. Microelectromech. Syst. 2019, 28, 88–94. [Google Scholar] [CrossRef]
- Von Kleist-Retzow, F. Robotic Liquid Metal Manipulation and Electrical Contact Probing at Small Scales; Verlag Dr. Hut: Munich, Germany, 2021; ISBN 978-3-8439-4828-9. [Google Scholar]
- Doudrick, K.; Liu, S.; Mutunga, E.M.; Klein, K.L.; Damle, V.; Varanasi, K.K.; Rykaczewski, K. Different Shades of Oxide: From Nanoscale Wetting Mechanisms to Contact Printing of Gallium-Based Liquid Metals. Langmuir 2014, 30, 6867–6877. [Google Scholar] [CrossRef]
- Zimmermann, S.; Huang, H. Investigating the Effects of Electron Beam Irradiation on Nanoscale Adhesion. In Proceedings of the 2019 IEEE 14th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Bangkok, Thailand, 11–14 April 2019; pp. 33–38. [Google Scholar] [CrossRef]
- Tadmor, R. Line Energy and the Relation between Advancing, Receding, and Young Contact Angles. Langmuir 2004, 20, 7659–7664. [Google Scholar] [CrossRef]
- Lin, F.; Li, D.; Neumann, A.W. Effect of Surface Roughness on the Dependence of Contact Angles on Drop Size. J. Colloid Interface Sci. 1993, 159, 86–95. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Liquid Metal Inks for Flexible Electronics and 3D Printing: A Review. Proc. ASME Int. Mech. Eng. Congr. Expo. 2014, 46445, V02BT02A044. [Google Scholar] [CrossRef]
- Gennes, P.-G.d.; Brochard-Wyart, F.; Quere, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves: Drops, Bubbles, Pearls, Waves, 1st ed.; Springer: New York, NY, USA, 2004; ISBN 9780387216560. [Google Scholar]
- Tadmor, R. Line Energy, Line Tension and Drop Size. Surf. Sci. 2008, 602, L108–L111. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klauser, W.; von Kleist-Retzow, F.T.; Fatikow, S. Line Tension and Drop Size Dependence of Contact Angle at the Nanoscale. Nanomaterials 2022, 12, 369. https://doi.org/10.3390/nano12030369
Klauser W, von Kleist-Retzow FT, Fatikow S. Line Tension and Drop Size Dependence of Contact Angle at the Nanoscale. Nanomaterials. 2022; 12(3):369. https://doi.org/10.3390/nano12030369
Chicago/Turabian StyleKlauser, Waldemar, Fabian T. von Kleist-Retzow, and Sergej Fatikow. 2022. "Line Tension and Drop Size Dependence of Contact Angle at the Nanoscale" Nanomaterials 12, no. 3: 369. https://doi.org/10.3390/nano12030369





