Carbon Double Coated Fe3O4@C@C Nanoparticles: Morphology Features, Magnetic Properties, Dye Adsorption
Abstract
:1. Introduction
2. Materials and Methods
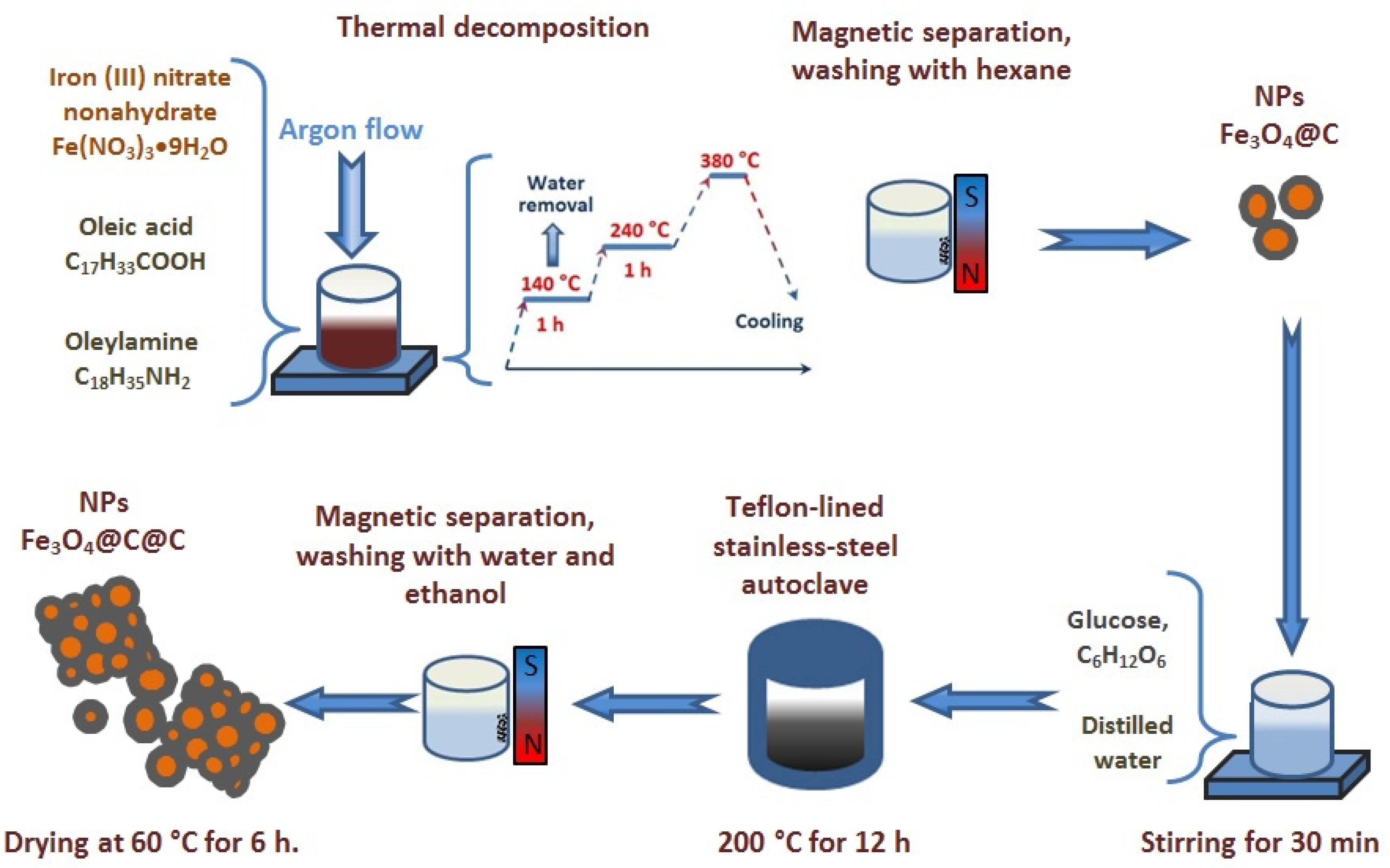
2.1. Synthesis Procedure
2.2. Characteristic Methods
3. Results
3.1. NPs Structure and Morphology
3.2. Mössbauer Spectra
3.3. NPs Magnetic Properties
3.4. Magnetic Circular Dichroism
3.5. Application of Synthesized NPs for Dye Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, L.; Pei, L.; Cao, S.; He, X.; Sang, M.; Jiang, W.; Xuan, S.; Gong, X. A high performance magnetic fluid based on carbon modified magnetite (Fe3O4) nanospheres. J. Magn. Magn. Mater. 2020, 505, 166734. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Jung, D.S.; Phiri, I.; Bae, H.-S.; Hong, J.; Kim, S.; Lee, Y.-G.; Ryou, M.-H.; Lee, K. Microalgae-templated spray drying for hierarchical and Porous Fe3O4/C Composite Microspheres as Li-ion battery anode materials. Nanomaterials 2020, 10, 2074. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Suzuki, N.; Aihara, Y.; Gabrielli, S.; Carbonari, G.; Agostini, M.; Branchi, M.; Ferrari, S.; Navarra, M.A.; et al. Highly Stable Fe3O4/C Composite: A Candidate Material for All Solid-State Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 070556. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Chen, Z.; Yang, K.; Zhang, Z.; Shi, Z.; Mei, T.; Qian, J.; Li, J.; Wang, X. Yolk-double shell Fe3O4@C@C composite as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2020, 822, 153656. [Google Scholar] [CrossRef]
- Lima, M.M.; Macuvele, D.L.P.; Muller, L.; Nones, J.; Silva, L.L.; Fiori, M.A.; Soares, C.; Riella, H.G. Synthesis and Potential Adsorption of Fe3O4@C Core-Shell Nanoparticles for to Removal of Pollutants in Aqueous Solutions: A Brief Review. J. Adv. Chem. Eng. 2017, 7, 1000172. [Google Scholar]
- Bao, X.; Qiang, Z.; Chang, J.H.; Ben, W.; Qu, J. Synthesis of carbon-coated magnetic nano composite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. J. Environ. Sci. 2014, 26, 962–969. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; El-Toni, A.M.; Labis, J.P.; Khan, A.; Al-Marghany, A.; Elafifi, H.E. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules 2017, 22, 2074. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Niu, H.; Hu, Z.; Cai, Y.; Shi, Y. Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2010, 1217, 4757–4764. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.Y.; Qiao, J.Q.; Cui, S.H.; Lian, H.Z.; Chen, H.Y. Magnetic solid phase extraction of brominated flame retardants and pentachlorophenol from environmental waters with carbon doped Fe3O4 nanoparticles. Appl. Surf. Sci. 2014, 321, 126–135. [Google Scholar] [CrossRef]
- Qu, L.; Han, T.; Luo, Z.; Liu, C.; Mei, Y.; Zhu, T. One-step fabricated Fe3O4@C core-shell composites for dye removal: Kinetics, equilibrium and thermodynamics. J. Phys. Chem. Sol. 2015, 78, 20–27. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Liu, J.; Ji, X.; Ma, J.; Tian, G. Preparation and excellent adsorption of water pollution dyes over magnetic Fe3O4/C nanoparticles with hollow grape cluster morphology. J. Nanopart. Res. 2020, 22, 196. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, H.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Hierarchical Fe3O4/C with a flower-like morphology: A highly efficient and reusable dye adsorbent. Synth. Met. 2018, 246, 45–56. [Google Scholar] [CrossRef]
- Sun, Y.; Ni, P.; Zhu, M.; Yao, Y.; Fu, S. Preparation of Fe3O4@C submicron rods for adsorption of methylene blue and fast separation from water. Micro Nano Lett. 2019, 14, 962–966. [Google Scholar] [CrossRef]
- Xiang, H.; Ren, G.; Zhong, Y.; Xu, D.; Zhang, Z.; Wang, X.; Yang, X. Fe3O4@C Nanoparticles Synthesized by In Situ Solid-Phase Method for Removal of Methylene Blue. Nanomaterials 2021, 11, 330. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Liu, J.H.; Liao, R.; Zhao, L.; Xie, J.; Wang, R.; Yang, S.T.; Wang, H.; Liu, Y. Fe3O4@C nanoparticles as high-performance Fenton-like catalyst for dye decoloration. Chin. Sci. Bull. 2014, 59, 3406–3412. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Lin, C.-R.; Tseng, Y.-T.; Spivakov, A.; Baskakov, A.O.; Starchikov, S.S.; Funtov, K.O.; Jhang, C.-J.; Tsai, Y.-J.; Hsu, H.-S. Structural and magnetic evolution of FexOy@carbon core-shell nanoparticles synthesized by a one-step thermal pyrolysis. Mater. Charact. 2019, 150, 213–219. [Google Scholar] [CrossRef]
- Velikanov, D.A. Vibration Magnetometer. Russia Patent No. 2,341,810, 20 December 2008. Bulletin No. 35. [Google Scholar]
- Velikanov, D.A. Automated vibration magnetometer with the Puzey electromagnet. Bull. Sib. State Aerosp. Univ. 2014, 1, 147–154. [Google Scholar]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Solovyov, L.A. Derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Wu, H.; Qian, X.; Chen, L.; Dan, Y.; Yu, Q. Porous Fe3O4/C nanoaggregates by the carbon polyhedrons as templates derived from metal organic framework as battery-type materials for supercapacitors. Electrochim. Acta 2020, 337, 135818. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-Controlled Synthesis of Magnetite (Fe3O4) Nanoparticles Coated with Glucose and Gluconic Acid from a Single Fe(III) Precursor by a Sucrose Bifunctional Hydrothermal. J. Phys. Chem. 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, Z. Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts. Catalysts 2020, 10, 502. [Google Scholar] [CrossRef]
- Li, Z.; Chan, C.; Berger, G.; Delaunay, S.; Graff, A.; Lefevre, G. Mechanism and kinetics of magnetite oxidation under hydrothermal conditions. RSC Adv. 2019, 9, 33633–33642. [Google Scholar] [CrossRef] [Green Version]
- Klabunde, K.J. Nanoscale Materials in Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Lee, J.-S.; Song, Y.-J.; Hsu, H.-S.; Lin, C.-R.; Huang, J.-Y.; Chen, J. Magnetic enhancement of carbon-encapsulated magnetite nanoparticles. J. Alloys Comp. 2019, 790, 716–722. [Google Scholar] [CrossRef]
- Lee, J.-S.; Wu, W.-B.; Chen, J.; Chen, C.-L.; Kuo, H.-W.; Lin, C.-R.; Lin, H.-J.; Chen, C.-T. Carbon encapsulation of magnetite nanoparticles enhances magnetism at room-temperature due to spin-polarized charge transfer. Appl. Phys. Lett. 2021, 118, 072403. [Google Scholar] [CrossRef]
- Zhang, X.; Schoenes, J.; Wachter, P. Kerr-effect and dielectric tensor elements of magnetite (Fe3O4) between 0.5 and 4.3 eV. Solid State Commun. 1981, 39, 189. [Google Scholar] [CrossRef]
- Yao, H.; Ishikawa, Y. Finite Size Effect on Magneto-Optical Responses of Chemically Modified Fe3O4 Nanoparticles Studied by MCD Spectroscopy. J. Phys. Chem. C 2015, 119, 13224–13230. [Google Scholar] [CrossRef]
- Leonov, I.; Yaresko, A.N.; Antonov, V.N.; Anisimov, V.I. Electronic structure of charge-ordered Fe3O4 from calculated optical, megneto-optical Kerr effect, and O K-edge X-ray absorption spectra. Phys. Rev. B 2006, 74, 165117. [Google Scholar] [CrossRef] [Green Version]
- Neal, J.R.; Behan, A.J.; Mokhtari, A.; Ahmed, M.R.; Blythe, H.J.; Fox, A.M.; Gehring, G.A. Magneto-optical study of the Verwey transition in magnetite. J. Magn. Magn. Mater. 2007, 310, 246–248. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Starchikov, S.S.; Lin, C.-R.; Lu, S.-Z.; Shaikh, M.O.; Funtov, K.O.; Dmitrieva, T.V.; Ovchinnikov, S.G.; Edelman, I.S.; Ivantsov, R. Magnetic, structural, and electronic properties of iron sulfide Fe3S4 nanoparticles synthesized by the polyol mediated process. J. Nanopart. Res. 2013, 15, 1397. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, J. Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J. Hazard. Mater. 2011, 193, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Freundlich, H. Kapillarchemie, eine Darstellung der Chemie der Kolloide und Verwandter Gebiete; Akademische Verlagsgesellschaft: Leipzig, Germany, 1909. [Google Scholar]
- Langmuir, I. Vapor pressures, evaporation, condensation and adsorption. J. Am. Chem. Soc. 1932, 542, 798–2832. [Google Scholar] [CrossRef]
- Calace, N.E.; Nardi, B.; Petronio, M.; Pietroletti, M. Adsorption of phenols by papermill sludges. Environ. Pollut. 2002, 118, 315–318. [Google Scholar] [CrossRef]
- Slyusareva, E.; Gerasimova, M.; Plotnikov, A.; Sizykh, A. Spectral study of fluorone dyes adsorption on chitosan-based polyelectrolyte complexes. J. Colloid Interface Sci. 2014, 417, 80–87. [Google Scholar] [CrossRef]
- Slyusarenko, N.; Gerasimova, M.; Atamanova, M.; Plotnikov, A.; Slyusareva, E. Adsorption of eosin Y on polyelectrolyte complexes based on chitosan and arabinogalactan sulfate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125731. [Google Scholar] [CrossRef]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Namvari, M.; Namazi, H. Preparation of efficient magnetic biosorbents by clicking carbohydrates onto graphene oxide. J. Mater. Sci. 2015, 50, 5348–5361. [Google Scholar] [CrossRef]













| IS, ±0.005 mm/s | Hhf, ±5 kOe | QS, ±0.02 mm/s | W, ±0.03 mm/s | A, ±0.03 o.e. | Position/Phase | |
|---|---|---|---|---|---|---|
| Fe3O4@C | ||||||
| S1 | 0.668 | 460 | 0 | 0.24 | 0.64 | [Fe]—Fe3O4 |
| S2 | 0.283 | 490 | 0 | 0.24 | 0.36 | (Fe)—Fe3O4 |
| Fe3O4@C@C | ||||||
| S1 | 0.670 | 456 | 0.02 | 0.27 | 0.50 | [Fe]—Fe3O4 |
| S2 | 0.254 | 488 | 0 | 0.31 | 0.22 | (Fe)—Fe3O4 |
| S3 | 0.356 | 490 | 0.0 | 0.24 | 0.28 | γ-Fe2O3 |
| Kinetic Model | Parameters | CR | MB |
|---|---|---|---|
| Pseudo-first order | k1 (1/min) | 0.00638 ± 0.0004 | 0.0305 ± 0.004 |
| qe (mg/g) | 5.19 ± 0.10 | 9.39 ± 0.28 | |
| R2 | 0.9576 | 0.8366 | |
| Pseudo-second order | k2 (g/(mg min)) | 0.0011 ± 0.0001 | 0.004 ± 0.0001 |
| qe (mg/g) | 6.33 ± 0.13 | 10.49 ± 0.25 | |
| R2 | 0.9798 | 0.9482 | |
| Intraparticle diffusion | ki1 (mg/(g min0.5)) | 0.1842 ± 0.0077 | 0.4617 ± 0.012 |
| C1 (mg/g) | 0.71 ± 0.15 | 3.41 ± 0.14 | |
| R2 | 0.9521 | 0.9786 | |
| ki2 (mg/g min0.5) | −1.6018 ± 0.001 | ||
| C2 (mg/g) | 9.82 ± 0.003 | ||
| R2 | 0.9999 |
| Dye | qm (mg/g) | KLF (L/mg) | nLF | R2 |
|---|---|---|---|---|
| MB | 20.04 | 3.48 | 0.53 | 0.995 |
| CR | 21.07 | 21.82 | 1.66 | 0.992 |
| Cationic Dye | Adsorbent | qmax (mg/g) | Anionic Dye | Adsorbent | qmax (mg/g) |
|---|---|---|---|---|---|
| MB | Fe3O4@C@C a | 16 [This work] | CR | Fe3O4@C@C b | 19 [This work] |
| Fe3O4/C core–shell NPs (~250 nm) c | 44.4 [34] | Cresol red | Fe3O4/C core–shell nanoparticles (~ 250 nm)c | 11.2 [34] | |
| Fe3O4@C (~3000 nm) d B-Fe3O4@C (~2000 nm) e | 15 [10] 40 [10] | Cresol red | Fe3O4@C (~ 3000 nm) d | 11 [10] | |
| Fe3O4 NPs (~200 nm)on the graphene layers f | 35 [12] | ||||
| Fe3O4@C submicron rods g | 24 [14] | ||||
| Fe3O4 NPs on glucose-functionalized hydrophilic graphene nanolayers h | 18–20 [44] | ||||
| Fe3O4@C NPs (~30 nm) i | 17 [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-R.; Ivanova, O.S.; Edelman, I.S.; Knyazev, Y.V.; Zharkov, S.M.; Petrov, D.A.; Sokolov, A.E.; Svetlitsky, E.S.; Velikanov, D.A.; Solovyov, L.A.; et al. Carbon Double Coated Fe3O4@C@C Nanoparticles: Morphology Features, Magnetic Properties, Dye Adsorption. Nanomaterials 2022, 12, 376. https://doi.org/10.3390/nano12030376
Lin C-R, Ivanova OS, Edelman IS, Knyazev YV, Zharkov SM, Petrov DA, Sokolov AE, Svetlitsky ES, Velikanov DA, Solovyov LA, et al. Carbon Double Coated Fe3O4@C@C Nanoparticles: Morphology Features, Magnetic Properties, Dye Adsorption. Nanomaterials. 2022; 12(3):376. https://doi.org/10.3390/nano12030376
Chicago/Turabian StyleLin, Chun-Rong, Oxana S. Ivanova, Irina S. Edelman, Yuriy V. Knyazev, Sergey M. Zharkov, Dmitry A. Petrov, Alexey E. Sokolov, Eugeniy S. Svetlitsky, Dmitry A. Velikanov, Leonid A. Solovyov, and et al. 2022. "Carbon Double Coated Fe3O4@C@C Nanoparticles: Morphology Features, Magnetic Properties, Dye Adsorption" Nanomaterials 12, no. 3: 376. https://doi.org/10.3390/nano12030376







