An Endoscope-like SERS Probe Based on the Focusing Effect of Silica Nanospheres for Tyrosine and Urea Detection in Sweat
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instrumentation
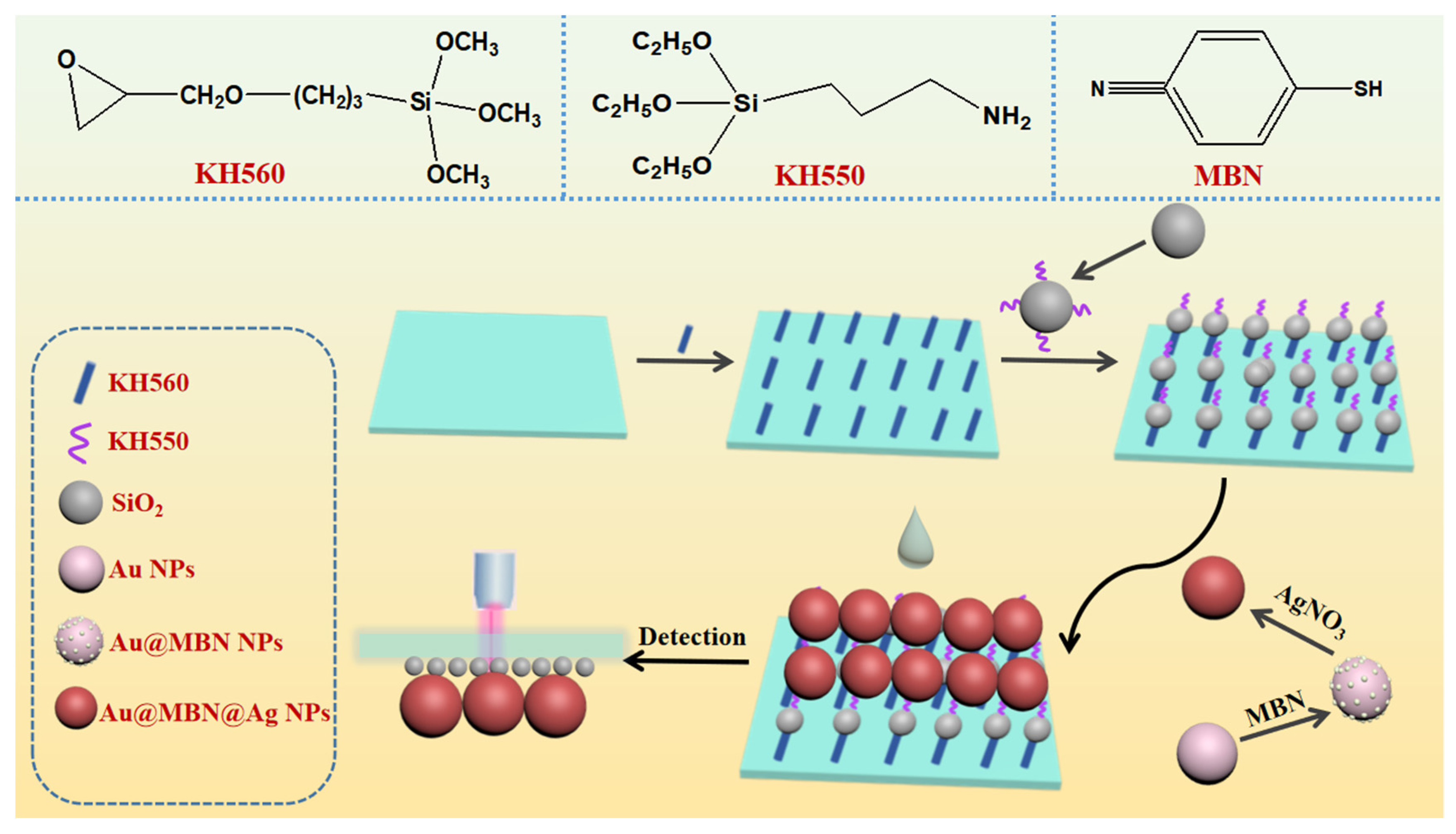
2.3. Synthesis of Au@MBN@Ag NPs
2.4. Modification of Glass Slides
2.5. Modification of SiO2 Nanospheres
2.6. Preparation of Glass-SiO2-Au@MBN@Ag NPs SERS Probe
2.7. Detection of Tyrosine and Urea
2.8. Detection of Tyrosine and Urea in Skin Sweat
3. Results and Discussion
3.1. Design Mechanism
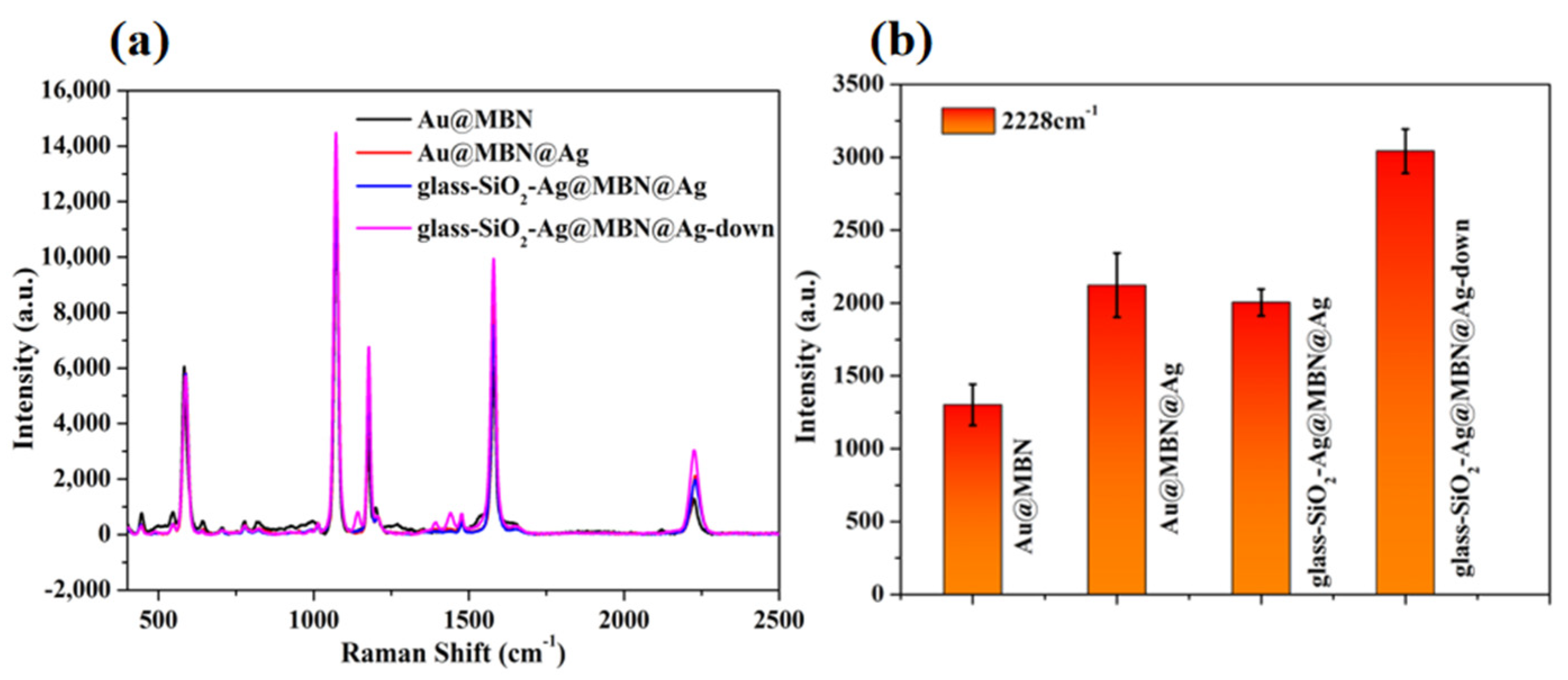
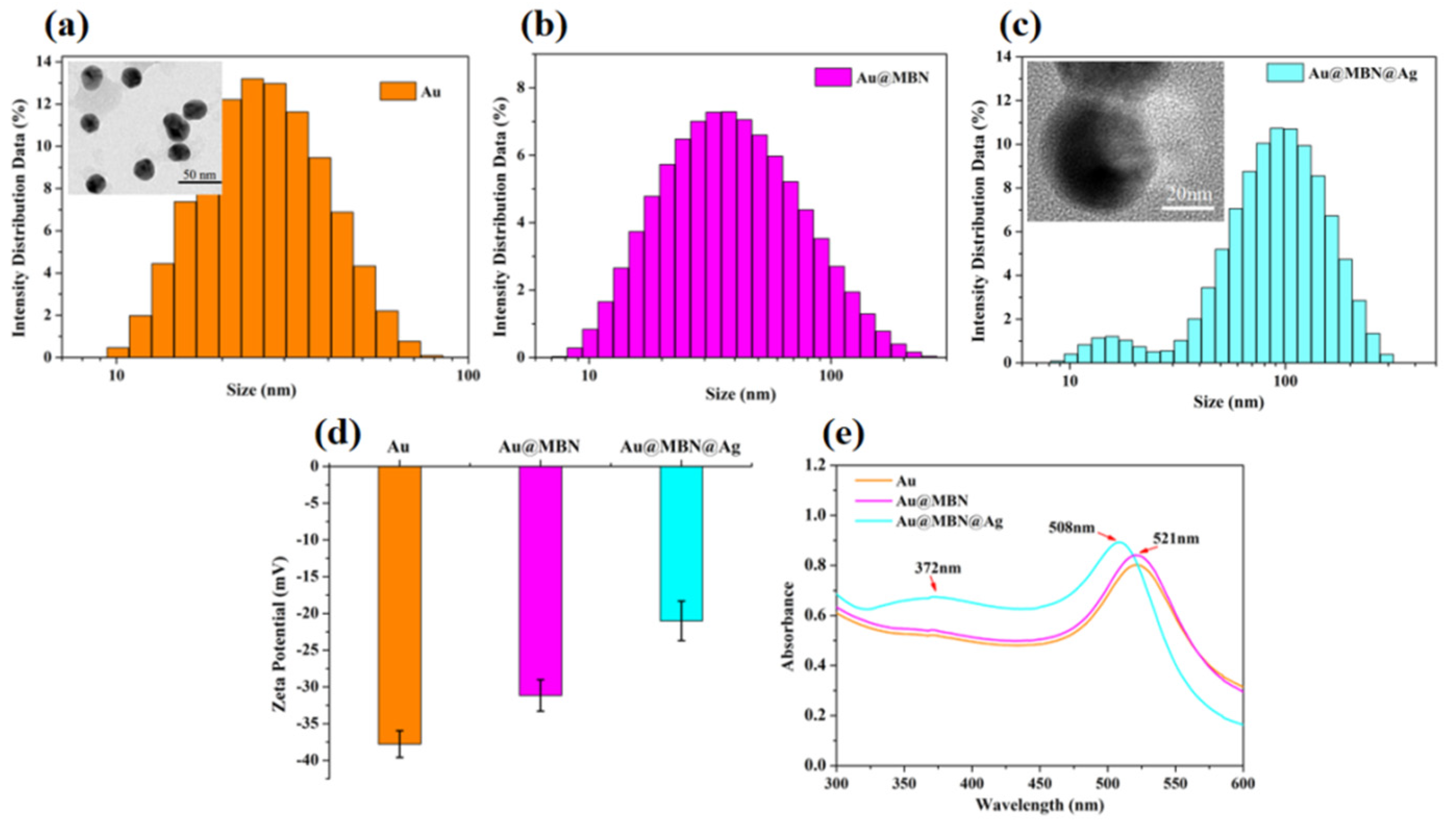
3.2. Characterization of Au@MBN@Ag NPs
3.3. Characterization of Supported SiO2
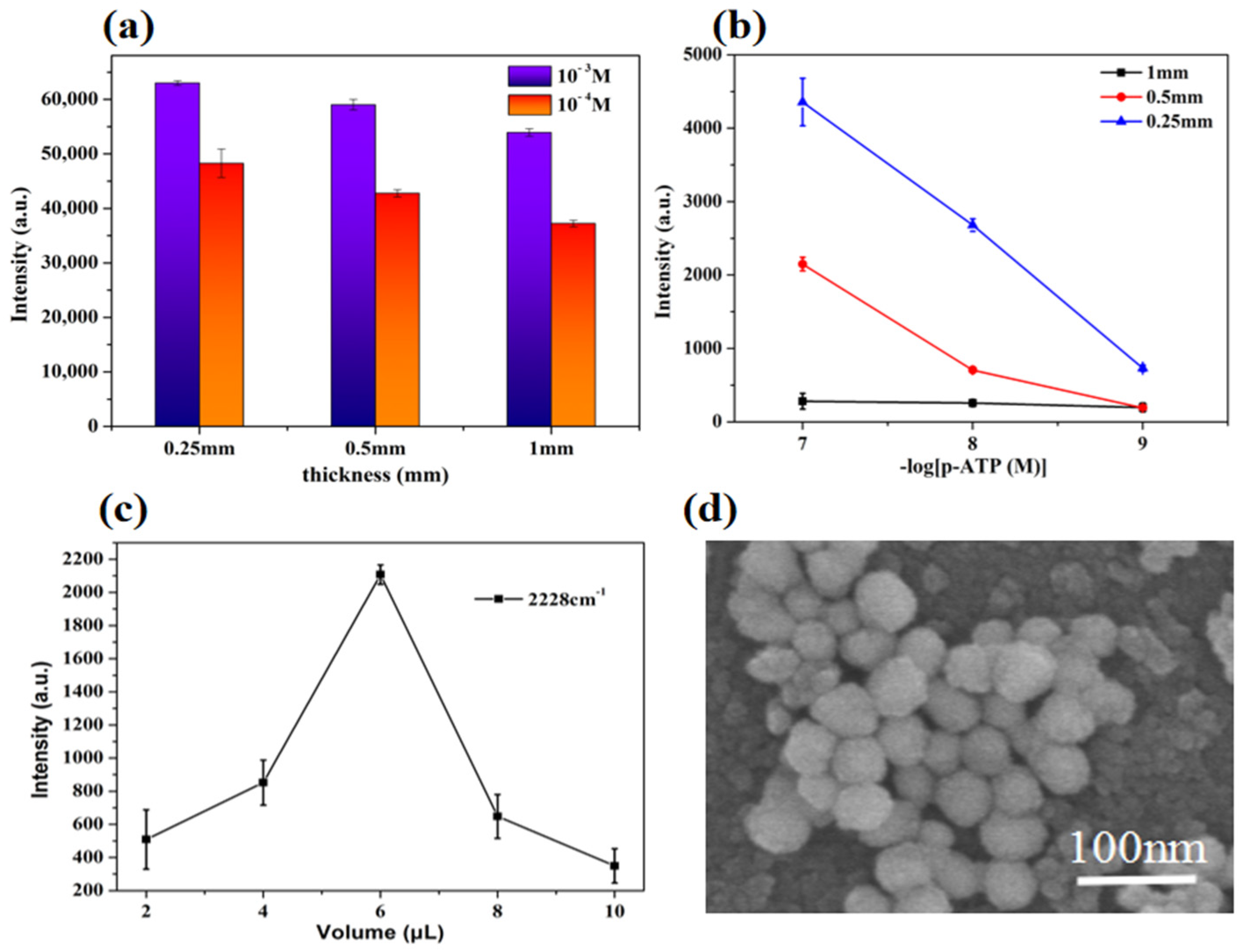
3.4. Conditions Optimization
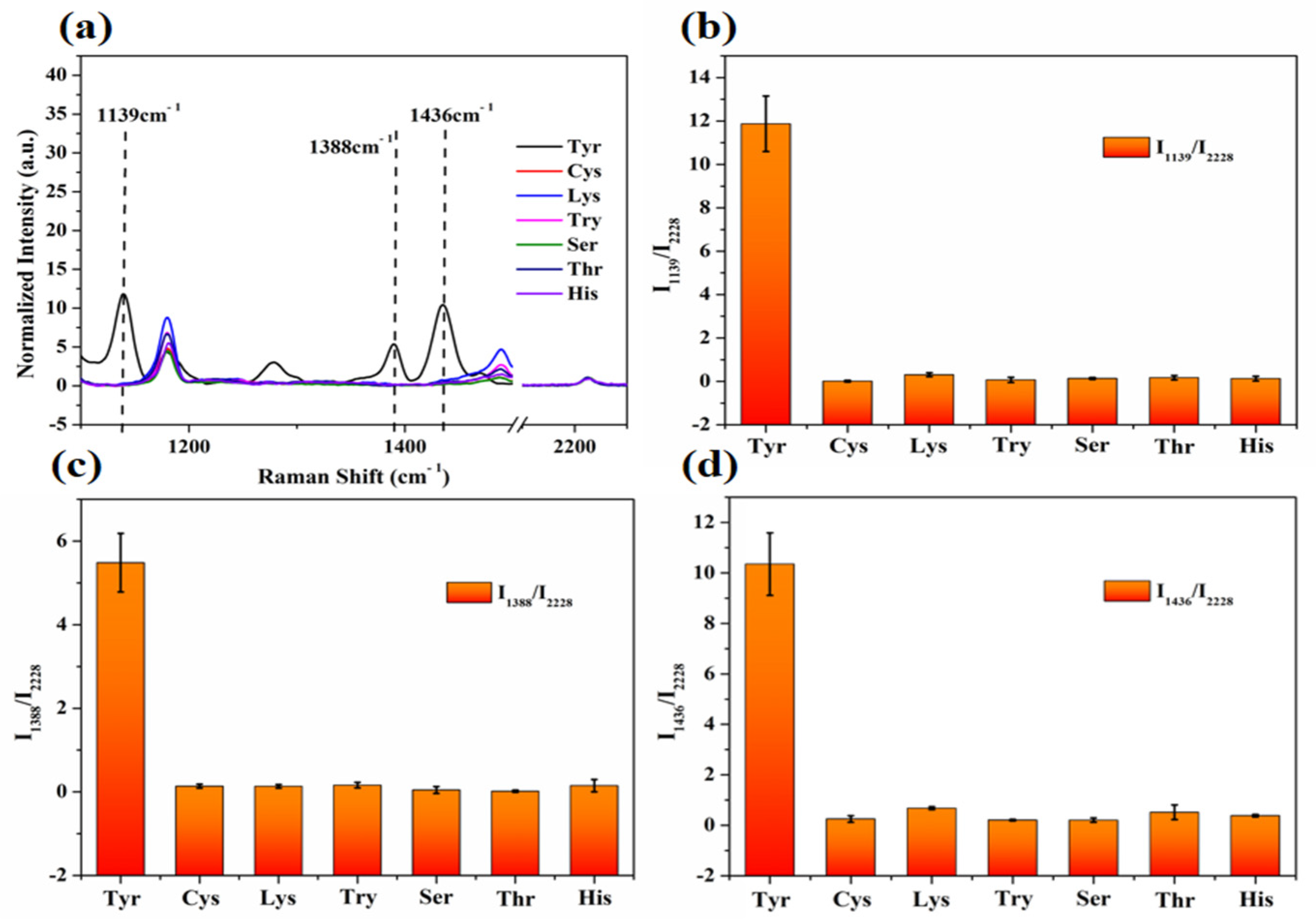
3.5. Anti-Interference Experiment
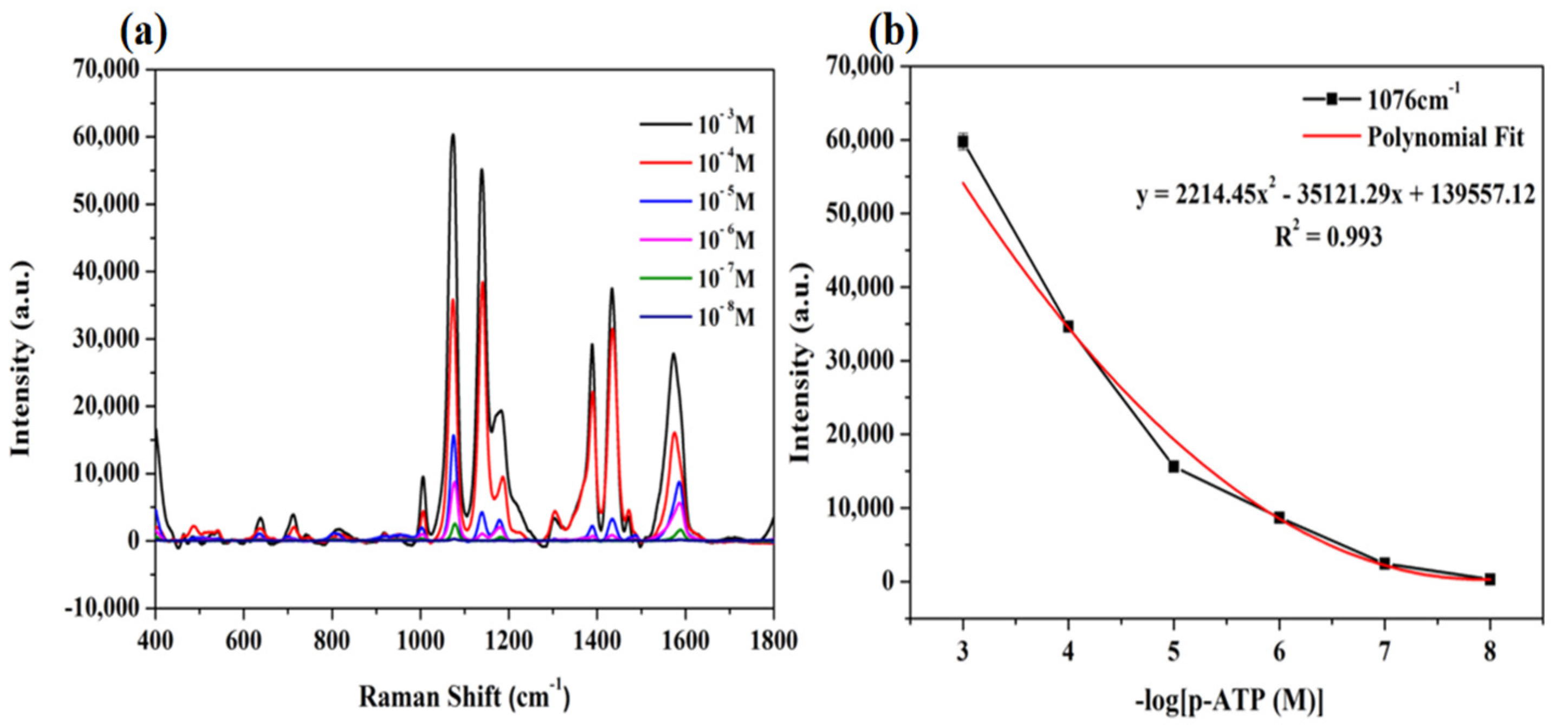
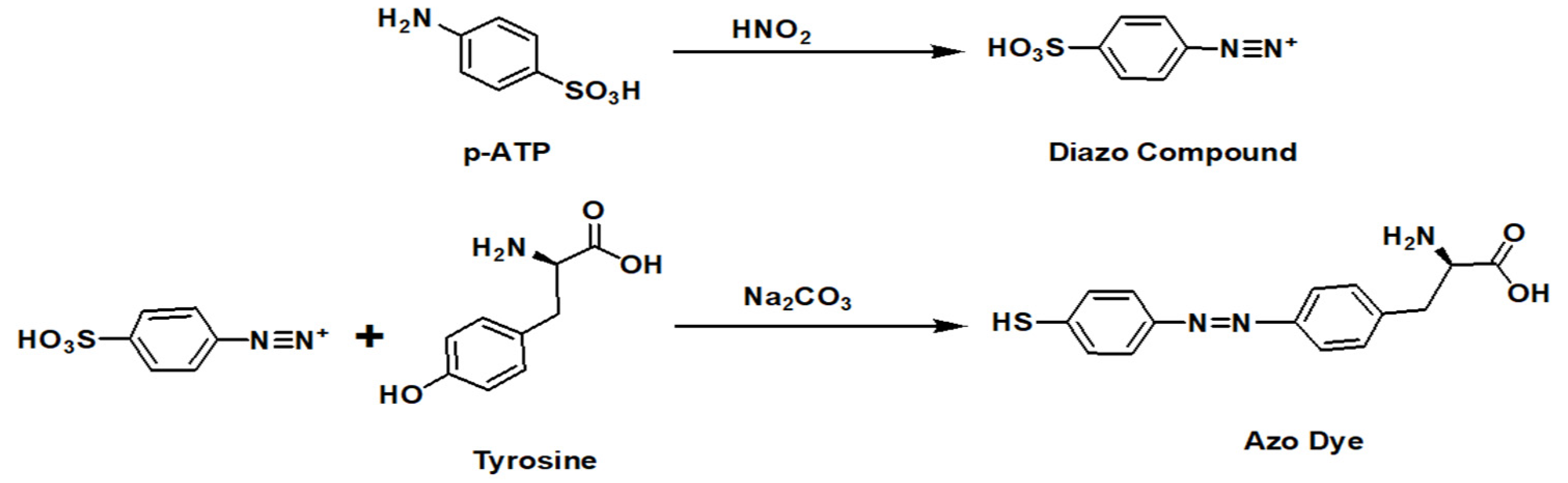
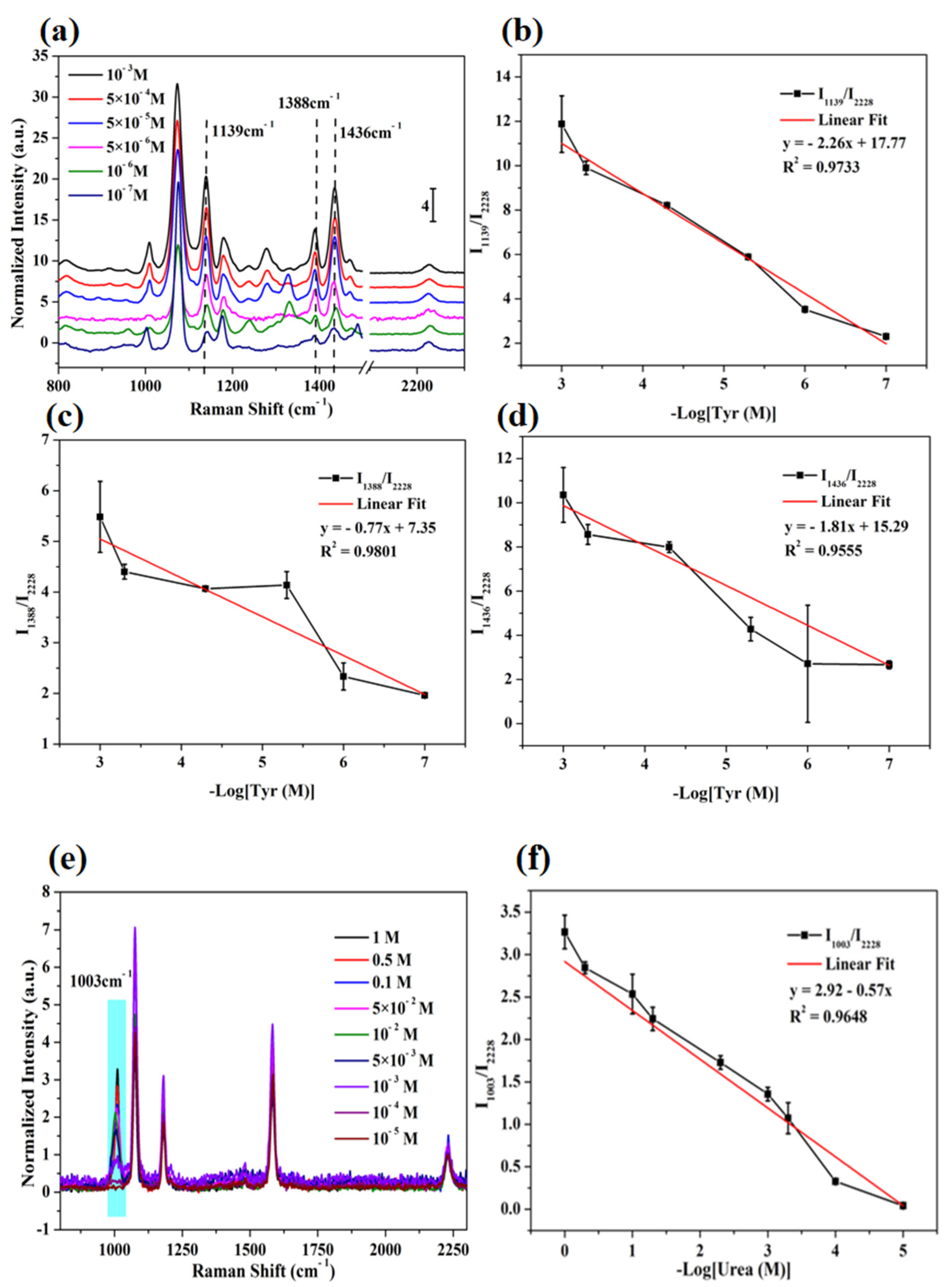
3.6. Detection of Tyrosine and Urea
3.7. Detection of Tyrosine and Urea in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celleno, L. Topical urea in skincare: A review. Dermatol. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.A.; Mitchell, G.A.; Tanguay, R.M. Tyrosinemia: A Review. Pediatric Dev. Pathol. 2001, 4, 212–221. [Google Scholar] [CrossRef]
- Levine, R.J.; Conn, H.O. Tyrosine Metabolism in Patients with Liver Disease. J. Clin. Investig. 1967, 46, 2012–2020. [Google Scholar] [CrossRef]
- Capuron, L.; Schroecksnadel, S.; Féart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Layé, S.; Fuchs, D. Chronic Low-Grade Inflammation in Elderly Persons Is Associated with Altered Tryptophan and Tyrosine Metabolism: Role in Neuropsychiatric Symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.W.; Bailey, J.L.; Wang, Y.; Klein, J.D.; Sands, J.M. Urea transporters and sweat response to uremia. Physiol. Rep. 2016, 4, e12825. [Google Scholar] [CrossRef] [PubMed]
- Liappis, N.; Kelderbacher, S.D.; Kesseler, K.; Bantzer, P. Quantitative study of free amino acids in human eccrine sweat excreted from the forearms of healthy trained and untrained men during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 42, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; An, Y.; Wu, J. Highly sensitive turn-on detection of mercury(II) in aqueous solutions and live cells with a chemosensor based on tyrosine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118004. [Google Scholar] [CrossRef]
- Neurauter, G.; Scholl-Bürgi, S.; Haara, A.; Geisler, S.; Mayersbach, P.; Schennach, H.; Fuchs, D. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin. Biochem. 2013, 46, 1848–1851. [Google Scholar] [CrossRef]
- Deng, P.; Xiao, J.; Feng, J.; Tian, Y.; Wu, Y.; Li, J.; He, Q. Highly sensitive electrochemical sensor for tyrosine detection using a sub-millimeter electrode. Microchem. J. 2021, 165, 106106. [Google Scholar] [CrossRef]
- Vyas, G.; Bhatt, S.; Si, M.K.; Jindani, S.; Suresh, E.; Ganguly, B.; Paul, P. Colorimetric dual sensor for Cu(II) and tyrosine and its application as paper strips for detection in water and human saliva as real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118052. [Google Scholar] [CrossRef]
- Pang, C.; Han, S.; Li, Y.; Zhang, J. Graphene quantum dot-enhanced chemiluminescence through energy and electron transfer for the sensitive detection of tyrosine. J. Chin. Chem. Soc. 2018, 65, 1504–1509. [Google Scholar] [CrossRef]
- Kyunghee, K.; Jeongeun, L.; Mi, M.B.; Been, S.Y.; Hum, P.C.; Min, P.; Yong, S.G. Fabrication of a Urea Biosensor for Real-Time Dynamic Fluid Measurement. Sensors 2018, 18, 2607. [Google Scholar]
- Hu, X.; Takenaka, N.; Kitano, M.; Bandow, H.; Maeda, Y.; Hattori, M. Determination of trace amounts of urea by using flow injection with chemiluminescence detection. Analyst 1994, 119, 1829–1833. [Google Scholar] [CrossRef]
- Miyauchi, T.; Miyachi, Y.; Takahashi, M.; Ishikawa, N.; Mori, H. Determination of Urea in Serum Based on the Combination of an Enzymatic Reaction with Immobilized Urease and Ion Chromatographic Analysis. Anal. Sci. 2010, 26, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krämer, M.; Fry, H.; Kappenstein, O. Development and validation of two analytical methods for urea determination in compound feed, including pet food, and yeast using high-performance liquid chromatography coupled with fluorescence detection and tandem mass spectrometry. Food Addit. Contam. Part A 2021, 38, 931–942. [Google Scholar] [CrossRef]
- Soundharraj, P.; Dhinasekaran, D.; Rajendran, A.R.; Prakasarao, A.; Ganesan, S. N-Doped zinc oxide as an effective fluorescence sensor for urea detection. New J. Chem. 2021, 45, 6080–6090. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, G.; Qiu, S.; Feng, S.; Lin, D. A target-triggered and self-calibration aptasensor based on SERS for precise detection of a prostate cancer biomarker in human blood. Nanoscale 2021, 13, 7574. [Google Scholar] [CrossRef]
- Zhang, W.S.; Wang, Y.N.; Xu, Z.R. High sensitivity and non-background SERS detection of endogenous hydrogen sulfide in living cells using core-shell nanoparticles. Anal. Chim. Acta 2020, 1094, 106–112. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Tsai, B.-C.; Yang, J. Silver nanoparticle-treated filter paper as a highly sensitive surface-enhanced Raman scattering (SERS) substrate for detection of tyrosine in aqueous solution. Anal. Chim. Acta 2011, 708, 89–96. [Google Scholar] [CrossRef]
- Mukanova, Z.; Gudun, K.; Elemessova, Z.; Khamkhash, L.; Ralchenko, E.; Bukasov, R. Detection of Paracetamol in Water and Urea in Artificial Urine with Gold Nanoparticle@Al Foil Cost-efficient SERS Substrate. Anal. Sci. 2018, 34, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Wang, Y.; Wang, L.; Lu, Y.; Li, J.; Lu, D.; Zhou, T.; Huang, Z.; Huang, J.; Huang, H.; et al. Interference-free and high precision biosensor based on surface enhanced Raman spectroscopy integrated with surface molecularly imprinted polymer technology for tumor biomarker detection in human blood. Biosens. Bioelectron. 2019, 143, 111599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Lu, S.; Ren, H.; Li, Z.; Zhang, Y.; Pan, F.; Liu, W.; Zhang, J.; Liu, Z. Gold nanoparticle probe-based immunoassay as a new tool for tetrodotoxin detection in puffer fish tissues. Sens. Actuators B Chem. 2010, 146, 368–372. [Google Scholar] [CrossRef]
- Cai, R.; Lu, D.; She, Q.; You, R.; Feng, S.; Lin, X.; Lu, Y. Reusable 3D silver superposed silica SERS substrate based on the Griess reaction for the ratiometric detection of nitrite. Anal. Bioanal. Chem. 2021, 413, 4751–4761. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; She, Q.; You, R.; Huang, Y.; Chen, J.; Su, H.; Lu, Y. “On-off” SERS sensor triggered by IDO for non-interference and ultrasensitive quantitative detection of IDO. Sens. Actuators B Chem. 2021, 344, 130166. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, D.; You, R.; Liu, J.; Huang, L.; Su, J.; Feng, S. Diazotization-Coupling Reaction-Based Determination of Tyrosine in Urine Using Ag Nanocubes by Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 400. [Google Scholar] [CrossRef] [Green Version]
- Luca, F.; De, L.B.; Vincenzo, M.; Elena, S.; Renato, M.; Maria, G.B.; Danila, M.; Fabiana, A. Smartphone-assisted electrochemical sensor for reliable detection of tyrosine in serum. Talanta 2021, 237, 122869. [Google Scholar]
- Chen, H.Y.; Yeh, Y.C. Detection of tyrosine and monitoring tyrosinase activity using an enzyme cascade-triggered colorimetric reaction. RSC Adv. 2020, 10, 29745–29750. [Google Scholar] [CrossRef]
- Lin, C.; Jair, Y.C.; Chou, Y.C.; Chen, P.S.; Yeh, Y.C. Transcription Factor-Based Biosensor for Detection of Phenylalanine and Tyrosine in Urine for Diagnosis of Phenylketonuria. Anal. Chim. Acta 2018, 1041, 108–113. [Google Scholar] [CrossRef]
- Feng, D.Q.; Liu, G.; Ma, G.; Nan, Z.; Wang, W. Phosphodiesters quaternary ammonium nanoparticles as label-free light scattering probe for turn-off detection of tyrosine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 208, 1–6. [Google Scholar] [CrossRef]
- Huang, G.G.; Yang, J. Development of infrared optical sensor for selective detection of tyrosine in biological fluids. Biosens. Bioelectron. 2005, 21, 408–418. [Google Scholar] [CrossRef]
- Huan, W.; Hui, Z.; Dagan, Z.; Jie, W.; Hui, T.; Tiantian, K. Enzyme-functionalized structural color hydrogel particles for urea detection and elimination. J. Clean. Prod. 2021, 315, 128149. [Google Scholar]
- Rinky, S.; Kikuo, K.; Sushmee, B. Graphene–Polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim. Acta 2017, 233, 44–51. [Google Scholar]
- Lu, S.; Gu, Z.; Hummel, M.; Zhou, Y.; Wang, K.; Xu, B.B.; Wang, Y.; Li, Y.; Qi, X.; Liu, X. Nickel Oxide Immobilized on the Carbonized Eggshell Membrane for Electrochemical Detection of Urea. J. Electrochem. Soc. 2020, 167, 10. [Google Scholar] [CrossRef]
- Liu, J.; Moakhar, R.S.; Perumal, A.S.; Roman, H.N.; Mahshid, S.; Wachsmann-Hogiu, S. An AgNP-deposited commercial electrochemistry test strip as a platform for urea detection. Sci. Rep. 2020, 10, 9527. [Google Scholar] [CrossRef]
- Liu, Q.; Stenbæk Schmidt, M.; Thienpont, H.; Ottevaere, H. A Tunable Freeform-Segmented Reflector in a Microfluidic System for Conventional and Surface-Enhanced Raman Spectroscopy. Sensors 2020, 20, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Biomarkers | Methods | Application | Analytical Range | LOD | Reference |
|---|---|---|---|---|---|
| Tyr | Electrochemistry | Serum | 30–500 µM | 4.4 µM | [26] |
| Colorimetry | Urine | 5–100 µM | 2.74 µM | [27] | |
| Fluorescence | Urine | 5–100 µM | 4.9 µM | [28] | |
| Light scattering probe | Biological samples | 0.055–4.68 µM | 0.0138 µM | [29] | |
| IR spectroscopic | Biological samples | 10–200 µM | 5.88 µM | [30] | |
| Our method | Sweat | 0.1–103 µM | 0.00357 µM | This work | |
| Urea | Electrochemistry | Aqueous solution and milk | 10–200 µM | 5.88 µM | [31] |
| Enzymatic catalysis | Aqueous solution | 1–30 mM | 0.48 mM | [32] | |
| Electrochemistry | Alkaline buffer and tap water | 0.05–2.5 mM | 20 µM | [33] | |
| Electrochemistry | Human blood | 1–8 mM | 0.14 mM | [34] | |
| SERS | Aqueous solution | 15–300 mM | 0.1 mM | [35] | |
| Our method | Sweat | 10–106 µM | 7.54 µM | This work |
| Spiked (μM) | Characteristic Peak (cm−1) | SERS (μM) | SERS Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 200 | 1139 | 198.8 | 99.39 | 3.82 |
| 1388 | 183.1 | 91.55 | 2.32 | |
| 1436 | 201.6 | 100.79 | 10.18 | |
| 100 | 1139 | 96.6 | 96.58 | 1.11 |
| 1388 | 99.9 | 99.92 | 1.54 | |
| 1436 | 96.7 | 96.72 | 2.17 | |
| 50 | 1139 | 46.0 | 91.98 | 2.48 |
| 1388 | 52.1 | 104.26 | 1.72 | |
| 1436 | 47.5 | 95.03 | 2.65 |
| Spiked (μM) | Characteristic Peak (cm−1) | SERS (μM) | SERS Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 500 | 1003 | 545.1 | 109.03 | 7.75 |
| 100 | 1003 | 98.6 | 98.58 | 2.65 |
| 50 | 1003 | 52.2 | 104.45 | 3.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, R.; Yin, L.; Huang, Q.; You, R.; Feng, S.; Lu, Y. An Endoscope-like SERS Probe Based on the Focusing Effect of Silica Nanospheres for Tyrosine and Urea Detection in Sweat. Nanomaterials 2022, 12, 421. https://doi.org/10.3390/nano12030421
Cai R, Yin L, Huang Q, You R, Feng S, Lu Y. An Endoscope-like SERS Probe Based on the Focusing Effect of Silica Nanospheres for Tyrosine and Urea Detection in Sweat. Nanomaterials. 2022; 12(3):421. https://doi.org/10.3390/nano12030421
Chicago/Turabian StyleCai, Rongyuan, Lijun Yin, Qian Huang, Ruiyun You, Shangyuan Feng, and Yudong Lu. 2022. "An Endoscope-like SERS Probe Based on the Focusing Effect of Silica Nanospheres for Tyrosine and Urea Detection in Sweat" Nanomaterials 12, no. 3: 421. https://doi.org/10.3390/nano12030421
APA StyleCai, R., Yin, L., Huang, Q., You, R., Feng, S., & Lu, Y. (2022). An Endoscope-like SERS Probe Based on the Focusing Effect of Silica Nanospheres for Tyrosine and Urea Detection in Sweat. Nanomaterials, 12(3), 421. https://doi.org/10.3390/nano12030421





