Synthesis, Characterization and Electrochemical Performance of a Redox-Responsive Polybenzopyrrole@Nickel Oxide Nanocomposite for Robust and Efficient Faraday Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Proceedure for the Synthesis of the Pbp@NiO Nanocomposite
2.3. Strutural and Physicochemical Characterization
2.4. Electrochemical Charaterisation in Three-Electrode Assambly
2.5. Faradaic Supercapattery Assambly and Performance Evaluation
3. Results and Discussion
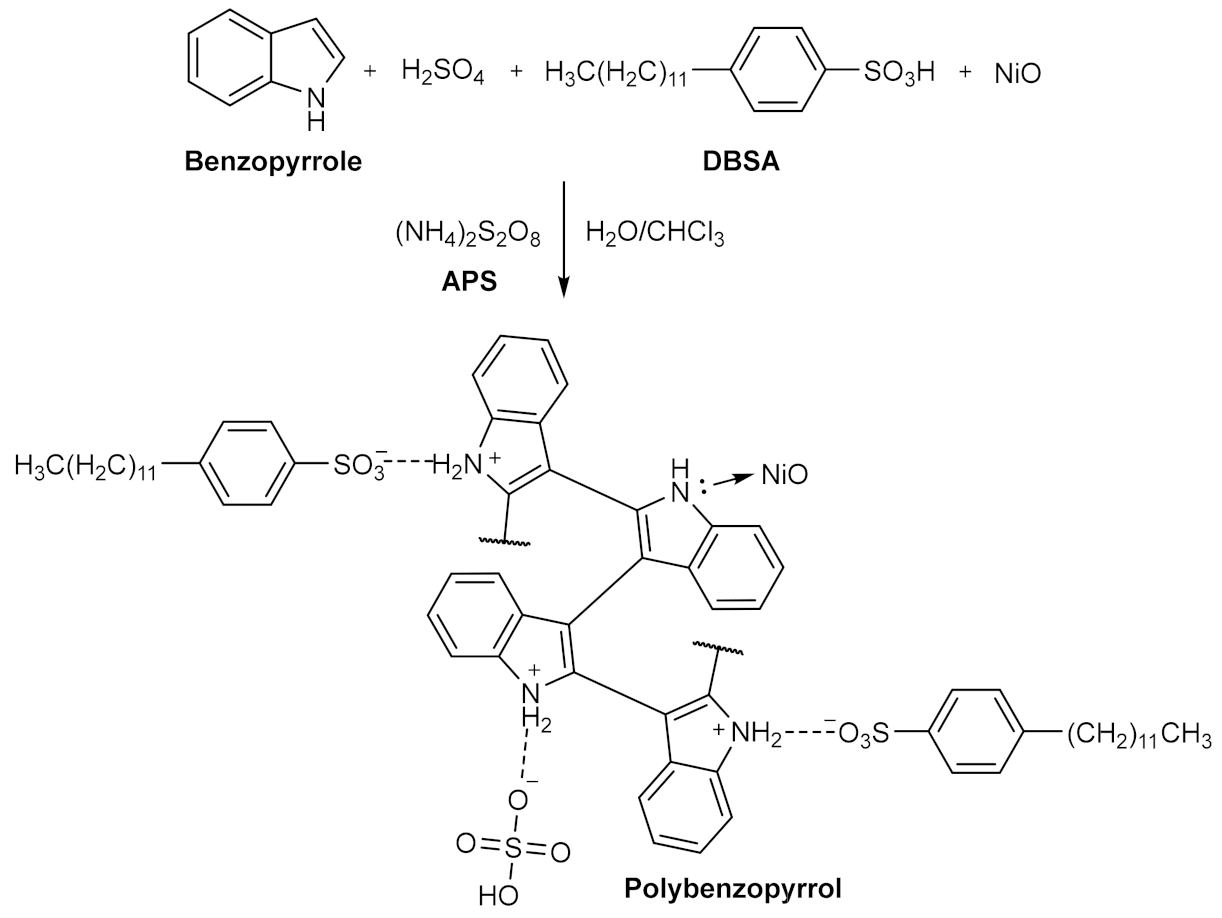
3.1. Proposed Growth Mechanism of Pbp@NiO
3.2. Analysis of the Physico-Chemical Properties of Pbp@NiO
3.3. Electrochemical Characterization and Performance Evaluation in Three-Electrode Setup
3.4. Performance Analysis of Pbp@NiO0.2 as Active Material in a Symmetric Supercapattery Device
| Electrode Material | Max. E (Wh kg−1) | Max. p (W kg−1) | Stability/Cycles | Ref. |
|---|---|---|---|---|
| Pbp/Nd2O3 | 8.9 | 1000 | 97%/5000 cycles (at 3 A g−1) | [65] |
| Pbp/Gd2O3 | <8.9 | 1000 | 85%/5000 cycles (at 3 A g−1) | [65] |
| Pbp/MoS2 | 6.0 | 2497 | 87%/5000 cycles (at 5 A g−1) | [66] |
| Cobalt oxide/Ag/PANI | 14.01 | 1650 | 121.03%/3500 cycles (at 2 A g−1) | [31] |
| Manganese dioxide-PANI | 17.9 | 261 | 84.7%/5000 cycles (at 2 A g−1) | [67] |
| NiCo2S4/PANI | 54.06 | 27.1 | 84.5%/5000 cycles (at 8 A g−1) | [68] |
| Pbp@NiO | 17.5 | 1925 | 114%/10,000 (at 1 A g−1) | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubra, K.T.; Javaid, A.; Patil, B.; Sharif, R.; Salman, A.; Shahzadi, S.; Siddique, S.; Ghani, S. Synthesis and characterization of novel Pr6O11/Mn3O4 nanocomposites for electrochemical supercapacitors. Ceram. Int. 2019, 45, 6819–6827. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Zhen, C.; Yuxuan, G.; Rui, Z.; Hui, L.; Pei, T.; Xiaohua, C.; Stefano, P.; Jilei, L. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: Synthesis, advanced characterization, and fundamentals. Adv. Energy Mater. 2020, 10, 1903780–1903804. [Google Scholar]
- Luciano, M.S.; Yang, L.; Shinjita, A.; Liana, B.; Daniel, C.; Aly, W.; Julio, M.D. Enhancing cycling stability of aqueous polyaniline electrochemical capacitors. J. Appl. Mater. Interfaces 2016, 8, 29452–29460. [Google Scholar]
- Chen, Z.; Xiaoteng, J.; Kewei, S.; Changchun, Y.; Gordon, G.; Wallace, C.W. Conducting polymer composites for unconventional solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 467–4699. [Google Scholar]
- Ishita, K.C.; Nilanjan, C.; Asim, S. CuO@NiO/Polyaniline/MWCNT nanocomposite as high-performance electrode for supercapacitor. J. Phys. Chem. C 2018, 122, 27180–27190. [Google Scholar]
- Ali, R.; Buhua, W.; Ehsan, H.; Farshad, F.K.N.; Xinyu, Z.; Tae-Sik, O. Electrophoretic deposition of nickel cobaltite/polyaniline/rGO composite electrode for high performance all-solid-state asymmetric supercapacitors. Energy Fuels 2020, 34, 6448–6461. [Google Scholar]
- Harish, M.; Parteek, P.; Mukesh, K.; Anil, K.; Zaidi, M.G.H.; Amit, K. Critical analysis of polyindole and its composites in supercapacitor application. Mater. Renew. Sustain. Energy 2019, 8, 9–27. [Google Scholar]
- Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Li, H.; Haung, Y.; Zhi, C. Polymers for supercapacitors: Boosting the development of flexible and wearable energy storage. Mater. Sci. Eng. R Rep. 2019, 139, 10052. [Google Scholar] [CrossRef]
- Torvi, A.I.; Munavalli, B.B.; Naik, S.R.; KaridurA ganavar, M.Y. Scalable fabrication of a flexible interdigital micro-supercapacitor device by in-situ polymerization of pyrrole into hybrid PVA-TEOS membrane. Electrochim. Acta 2018, 282, 469–479. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage-A review. J. Energy Storage 2019, 25, 100852–100876. [Google Scholar] [CrossRef]
- Magu, T.O.; Agobi, A.U.; HITLER, L.; Dass, P.M. A review on conducting polymers-based composites for energy storage application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar]
- Yogesh, G.; Abhik, B.; Dipit, D.; Meenal, D.; Dinesh, B.; Prakash, W.; Manjusha, S.; Satiscchandra, O. 3D polyaniline architecture by concurrent inorganic and organic acid doping for superior and robust high-rate supercapacitor performance. Sci. Rep. 2016, 6, 21002–21012. [Google Scholar]
- Yang, J.; Liu, Y.; Liu, S.; Li, L.; Zhang, C.; Liu, T. Conducting polymer composites: Material synthesis and applications in electrochemical capacitive energy storage. Mater. Chem. Front. 2017, 1, 251–268. [Google Scholar] [CrossRef]
- Jinyoung, T.; Nanjian, C.; Peining, C.; Kunkun, G.; Xuli, C. High-performance wearable supercapacitors based on PANI/N-CNT@CNT fiber with a designed hierarchical core-sheath structure. J. Mater. Chem. A 2021, 9, 20635–20644. [Google Scholar] [CrossRef]
- Josue, M.G.; Matheus, I.S.; Henrique, E.T.; Lucio, A.; Paulo, R.M.; Koiti, A. Trimetallic oxides/hydroxides as hybrid supercapacitors electrode materials: A review. J. Mater. Chem. A 2020, 8, 10534–10570. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Dong, X.; Jiang, H.; Hu, T.; Meng, C.; Huang, C. Alkali etching metal silicates derived from bamboo leaves with enhanced electrochemical properties for solid-state hybrid supercapacitors. Chem. Eng. J. 2021, 417, 127964–127976. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Xiao, J.; Jiang, H.; Li, X.; Meng, C. Novel ordered hollow spherical nickel silicate-nickel hydroxide united composite with two types of morphologies for enhanced electrochemical storage performance. Mater. Chem. Front. 2019, 3, 2090–2101. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Jiang, H.; Wang, Q.; Zheng, J.; Meng, C. Cobalt-nickel silicate hydroxide on amorphous carbon derived from bamboo leaves for hybrid supercapacitors. Chem. Eng. J. 2019, 375, 121938–121948. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Chen, X.; Dong, X.; Meng, C.; Haung, C. Bamboo leaves as sustainable sources for the preparation of amorphous carbon/iron silicate anode and nickel-cobalt silicate cathode materials for hybrid supercapacitors. ACS Appl. Energy Mater. 2021, 4, 9328–9340. [Google Scholar] [CrossRef]
- Jing, X.; Zhang, Y.; Dong, X.; Mu, Y.; Liu, X.; Meng, C. Layered silicate magadiite-derived three-dimensional honeycomb-like cobalt-nickel silicates as excellent cathode for hybrid supercapacitors. Mater. Today Chem. 2021, 22, 100550–100560. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Xu, Z.; Li, W.; Wang, X.; Marriam, I.; Peng, S.; Ramkrishna, S.; Yang, S.; Zhu, M. Surface self-assembly of functional electroactive nanofibers on textile yarns as a facile approach toward super flexible energy storage. ACS Appl. Energy Mater. 2018, 1, 377–386. [Google Scholar] [CrossRef]
- Shao, Y.; El-kady, M.F.; Sun, J.; Li, J.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Designs and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Bangning, S.; Xinping, H.; Xijin, L.; Yang, J.; Yudong, Z.; Hui, S.; Chun, Z. Flower-like polyaniline-NiO structures: A high specific capacity supercapacitor electrode material with remarkable cycling stability. RSC Adv. 2016, 6, 43959–43963. [Google Scholar]
- Wenjing, J.; Junyi, J.; Xinghong, C.; Jianjun, C.; Daijun, L.; Hua, D.; Qiang, F. Polypyrrole encapsulation on flower-like porous NiO for advanced high-performance supercapacitors. Chem. Commun. 2015, 51, 7669–7672. [Google Scholar]
- Huiling, Y.; Xu, H.; Ming, L.; Zhang, L.; Yunhui, H.; Xianluo, H. Assembly of NiO/Ni(OH)2/PEDOT nanocomposites on contra wires for fiber-shaped flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 1774–1779. [Google Scholar]
- Purty, B.; Choudhary, R.B.; Biswas, A.; Udayabhanu, G. Chemically grown mesoporous f-CNT/-MnO2/PIn nanocomposites as electrode materials for supercapacitors. Polym. Bull. 2019, 76, 1619–1640. [Google Scholar] [CrossRef]
- Shubhra, G.; Nasreen, A.M.; Alka, G. Growth of one-dimensional polyindole nanostructures. J. Nanosci. Nanotechnol. 2011, 11, 10164–10172. [Google Scholar]
- Javed, I.; Mohammad, O.A.; Arshid, N.; Sayed, A.; Mohd, G.A.; Pramod, K.; Rashida, J.; Shahid, B.; Rajpa, A.H. Hydrothermally assisted synthesis of porous polyaniline@carbon nanotubes-manganese dioxide ternary composite for potential application in supercapattery. Polymers 2020, 12, 2918. [Google Scholar]
- Javed, I.; Arshid, N.; Mohammad, O.A.; Rashida, J.; Priyanka, R.J.; Shahid, B.; Hasan, P.M.Z.; Anwar, L.B.; Sharifah, M.; Ramesh, K.; et al. Cobalt oxide nanograins and silver nanoparticles decorated fibrous polyaniline nanocomposite as battery-type electrode for high performance supercapattery. Polymers 2020, 12, 2816. [Google Scholar]
- Arshid, N.; Perumal, R.K.; Mohammad, K.; Ramesh, S.; Ramesh, K.; Shamsudin, E.M.; Yiqiang, Z.; Priyanka, J. Facile sonochemical snthesis of 2D porous Co3O4 nanoflakes for supercapattery. J. Alloys Compd. 2020, 819, 153019. [Google Scholar]
- Xi, Z.; Anqi, W.; Yumei, P.; Chenfei, Y.; Yun, Z.; Yang, Z.; Qiang, C.; Shishan, W. Facile synthesis of a Co3O4@carbon nanotubes/polyindole composite and its application in all-solid-state flexible supercapacitors. J. Mater. Chem. A 2015, 3, 13011–13015. [Google Scholar]
- Chunhai, Y.; Hao, C.; Cao, G. Hybrid CoO nanowires coated with uniform polypyrrole nanolayers for high-performance energy storage devices. Nanomaterials 2019, 9, 586. [Google Scholar]
- Amar, M.P.; Nutthaphak, K.; Xiaowei, A.; Xiaoqiong, H.; Shasha, L.; Xiaogang, H.; Abuliti, A.; Guoqing, G. Fabrication of a high-energy flexible all-solid-state supercapacitor using pseudocapacitive 2D-Ti3C2Tx-MXene and battery-type reduced graphene oxide/nickel-cobalt bimetal oxide electrode materials. ACS App. Mater. Interfaces 2020, 12, 52749–52762. [Google Scholar]
- Hajera, G.; Anwar-ul-Haq, S.; Ulrike, K.; Salma, B. Study on direct synthesis of energy efficient multifunctional polyaniline-graphene oxide nanocomposite and its application in aqueous symmetric supercapacitor devices. Nanomaterials 2020, 10, 118–141. [Google Scholar]
- Katesara, P.; Anuvat, S. Synthesis of nano-sized polyindole via emulsion polymerization and doping. Synth. Met. 2016, 219, 142–153. [Google Scholar]
- Inamuddin, N.S.; Mohd, I.A.; Suvardhan, K.; Heba, A.K. Green synthesis of ZnO nanoparticles decorated on polyindole functionalized-MCNTs and used as anode material for enzymatic biofuel cell applications. Sci. Rep. 2020, 10, 5052. [Google Scholar] [CrossRef] [Green Version]
- Shambharkar, B.H.; Umare, S.S. Synthesis and characterization of polyaniline/NiO nanocomposite. J. Appl. Polym. Sci. 2011, 122, 1905–1912. [Google Scholar] [CrossRef]
- Rajasudha, G.; Nancy, A.P.; Paramasivam, T.; Boulos, N.; Narayanan, V.; Stephen, A. Synthesis and characterization of polyindole-NiO-based composite polymer electrolyte with LiClO4. Int. J. Polym. Mater. 2011, 60, 877–892. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Nushhat, K.; Parvathi, K.; Anilkumar, T. Nickel oxide@polyindole/phenothiazine blend nanocomposites: Preparation, characterization, thermal, electrical properties and gas sensing applications. J. Mater. Sci. Mater. Electron. 2019, 30, 13719–13728. [Google Scholar] [CrossRef]
- Chandra, J.V.; Achal, S.K.; Prashant, D.; Rajiv, P. Polyindole modified g-C3N4 nanohybrids via in-situ chemical polymerization for its improved electrochemical performance. Vacuum 2020, 177, 109363. [Google Scholar]
- Misoon, O.; Seok, K. Effect of dodecyl benzene sulfonic acid on the preparation of polyaniline/activated carbon composites by in situ emulsion polymerization. Electrochim. Acta 2012, 59, 196–201. [Google Scholar] [CrossRef]
- Shah AH, A.; Yasmeen, N.; Rahman, G.; Bilal, S. High electrocatalytic behavior of Ni impregnated conducting polymer coated platinum and graphite electrodes for electrooxidation of methanol. Electrochim. Acta 2017, 224, 468–474. [Google Scholar] [CrossRef]
- Begum, B.; Bilal, S.; Rose, P. Physical, chemical, and electrochemical properties of redox-responsive polybenzopyrrole as electrode material for faradaic energy storage. Polymers 2021, 13, 2883. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.M.A.; Mohammad, F. Graphene/nickel oxide-based nanocomposite of polyaniline with special reference to ammonia sensing. ACS Omega 2018, 3, 9378–9387. [Google Scholar] [CrossRef] [Green Version]
- Xiaomin, C.; Xiuguo, C.; Lei, Z.; You, Z.; Xing, G.; Huiqin, L.; Yang, L.; Xiaodong, W. Ultra high electrical performance of nano nickel oxide and polyaniline composite materials. Polymers 2017, 9, 288–314. [Google Scholar]
- Mini, V.; Devendrappa, H. Electrical conductivity and supercapacitor properties of polyaniline/chitosan/nickel oxide honeycomb nanocomposite. J. Appl. Polym. Sci. 2016, 134, 44536–44547. [Google Scholar]
- Bela, P.; Ram, B.C.; Malati, M.; Ruby, K. Augmented optical, dielectric, and electrochemical performance for morphologically crushed nanorods decorated Fe:MnO2/PIn nanocomposite. Optik 2018, 158, 767–778. [Google Scholar]
- Jayakrishnan, P.; Ramesan, M.T. Synthesis, characterization, electrical conductivity and material properties of magnetite/polyindole/poly(vinyl alcohol) blend nanocomposite. J. Inorg. Organomet. Polym. 2017, 27, 323–333. [Google Scholar] [CrossRef]
- Dongxue, H.; Ying, C.; Likun, Y.; Yang, L.; Zhongxian, L. Reversed micelle polymerization: A new route for the synthesis of DBSA-polyaniline nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2005, 259, 175–187. [Google Scholar]
- Prakash, C.; Kalpana, H.; Amit, H.; Kakasaheb, M.; Atul, C.; Sabrina, D.; Vasant, C. Synthesis and characterization of polyindole and its catalytic performance study as a heterogeneous catalyst. J. Chem. Sci. 2016, 128, 467–475. [Google Scholar]
- Salma, B.; Bushra, B.; Salma, G.; Anwar-ul-Haq, A.S. PANI/DBSA/H2SO4: A promising and highly efficient electrode material for supercapacitors. Synth. Met. 2018, 235, 1–15. [Google Scholar]
- Mini, V.; Devendrappa, H. Polyaniline/nickel oxide-a core/shell structure nanocomposite as electrode material in supercapacitor application. Mat. Today Proceed. 2018, 5, 23148–23155. [Google Scholar] [CrossRef]
- Chengxiang, H.; Chen, H.; Wanhao, Z.; Saisai, Z.; Lignze, Y.; Xiaohong, W.; Chenglong, J.; Linli, Z. Synthesis of polyaniline/nickel oxide/sulfonated graphene ternary composite for all-solid-state asymmetric supercapacitor. Appl. Surf. Sci. 2020, 5, 144589. [Google Scholar]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrode: Mini review. Nanoscale Adv. 2019, 1, 2817. [Google Scholar] [CrossRef] [Green Version]
- Vimal, K.M.; Karthikeyan, K.; Parthiban, P.; Subramanian, N.; Surjit, S.; Swapnil, S.N.; Sang-Jae, K. Antimonene dendritic nanostructures: Dual-functional material for high-performance energy storage and harvesting devices. Nano Energy 2020, 77, 105248. [Google Scholar]
- John, W.; Julien, P.; James, L.; Bruce, D. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticels. J. Phy. Chem. C 2007, 111, 14925–14931. [Google Scholar]
- Fathi, M.; Saghafi, M.; Mahboubi, F.; Mohajerzadeh, S. Synthesis and electrochemical investigation of polyaniline/unzipped carbon nanotube composites as electrode material in supercapacitors. Synth. Met. 2014, 198, 345–356. [Google Scholar] [CrossRef]
- Danhua, Z.; Qianjie, Z.; Aiqin, L.; Weiqiang, Z.; Yanan, C.; Danqin, L.; Jing, W.; Guo, Y.; Jingkun, X.; Yong, R. Two-step preparation of carbon nanotubes/RuO2/polyindole ternary nanocomposites and their application as high-performance supercapacitors. Front. Mater. Sci. 2020, 14, 109–119. [Google Scholar]
- Raj, R.P.; Ragupathy, P.; Mohan, S. Remarkable capacitive behavior of Co3O4-polyindole composite s electrode material for supercapacitor applications. J. Mater. Chem. A 2015, 3, 24338–24348. [Google Scholar] [CrossRef]
- Maqsood, R.W.; Rasal, A.S.; Shinde, N.S.; Dhas, S.D.; Moholkar, A.V.; Shirsat, M.D.; Chakarvarti, S.K.; Sonkawade, R.G. Electrochemical performance of polyaniline based symmetrical energy storage device. Mater. Sci. Semicond. Process. 2020, 120, 105291. [Google Scholar]
- Qianjie, Z.; Danhua, Z.; Xiumei, M.; Daize, M.; Fengxing, J.; Jingkun, X.; Weiqiang, Z. PEDOT:PSS-assisted polyindole hollow nanospheres modified carbon cloth as high performance electrochemical capacitor electrodes. Electrochim. Acta 2016, 212, 662–670. [Google Scholar]
- Mandira, M.; Ram, B.C.; Anukul, K.T.; Chandra, S.P.; Govind, G. Rare earth metal oxide (RE2O3; RE = Nd, Gd, and Yb) incorporated polyindole composites: Gravimetric and volumetric capacitive performance for supercapacitor applications. New J. Chem. 2018, 42, 5295–5308. [Google Scholar]
- Ram, B.C.; Sarfaraz, A.; Bela, P. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. J. Energy Storage 2020, 29, 101302. [Google Scholar]
- Choudahary, R.B.; Majumder, M.; Thakur, A.K. Two-dimensional exfoliated MoS2 flakes integrated with polyindole for supercapacitor application. Chem. Select 2019, 4, 6906–6912. [Google Scholar]
- Kalyan, G.; Chee, Y.Y.; Md, M.S.; Rajeeb, K.J. Development of 3-D urchin shaped coaxial MnO2/PANI composite and self-assembled 3-D pollared graphene foam for asymmetric all-solid-state flexible supercapacitor application. ACS Appl. Mater. Interfaces 2017, 9, 15350–15363. [Google Scholar]
- Xinyi, H.; Qi, L.; Jingyuan, L.; Rumin, L.; Hongsen, Z.; Rongrong, C.; Jun, W. High-performance all-solid-state asymmetrical supercapacitors based on petal-like NiCo2S4/polyaniline nanosheets. Chem. Eng. J. 2017, 325, 134–143. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, B.; Bilal, S.; Shah, A.u.H.A.; Röse, P. Synthesis, Characterization and Electrochemical Performance of a Redox-Responsive Polybenzopyrrole@Nickel Oxide Nanocomposite for Robust and Efficient Faraday Energy Storage. Nanomaterials 2022, 12, 513. https://doi.org/10.3390/nano12030513
Begum B, Bilal S, Shah AuHA, Röse P. Synthesis, Characterization and Electrochemical Performance of a Redox-Responsive Polybenzopyrrole@Nickel Oxide Nanocomposite for Robust and Efficient Faraday Energy Storage. Nanomaterials. 2022; 12(3):513. https://doi.org/10.3390/nano12030513
Chicago/Turabian StyleBegum, Bushra, Salma Bilal, Anwar ul Haq Ali Shah, and Philipp Röse. 2022. "Synthesis, Characterization and Electrochemical Performance of a Redox-Responsive Polybenzopyrrole@Nickel Oxide Nanocomposite for Robust and Efficient Faraday Energy Storage" Nanomaterials 12, no. 3: 513. https://doi.org/10.3390/nano12030513






