Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nitrogen-Doped Reduced Graphene Oxide (N-rGO)
2.2. Synthesis of Nitrogen-Doped Reduced Graphene Oxide-Nickel/Sliver (N-rGO-Ni/Ag) Nanocomposite
2.3. Characterization
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. X-ray Diffraction Analysis (XRD)
3.3. Scanning Electron Microscopy (SEM)
3.4. Transmission Electron Microscopy (TEM)
3.5. BET and BJH Measurements
3.6. X-ray Photoelectron Spectroscopy
3.7. ORR Activity
3.8. OER Activity
3.9. Chronoamperometric Stability
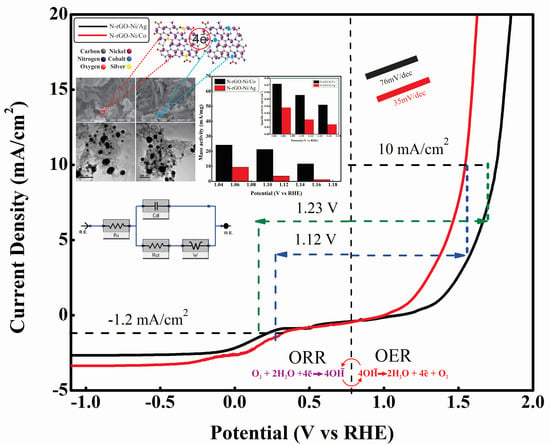
3.10. Bi-Functional Electrocatalytic Activity Measurement
3.11. Electrochemical Impedance Spectroscopic (EIS) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farooqui, U.; Ahmad, A.; Hamid, N. Challenges and potential advantages of membranes in lithium air batteries: A review. Renew. Sustain. Energy Rev. 2017, 77, 1114–1129. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Othman, R.; Dicks, A.L.; Zhu, Z. Non precious metal catalysts for the PEM fuel cell cathode. Int. J. Hydrogen Energy 2012, 37, 357–372. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wang, J.; Chen, C.; Chen, D.; Gao, Y.; Saccoccio, M.; Ciucci, F. A bi-functional catalyst for oxygen reduction and oxygen evolution reactions from used baby diapers: α-Fe2O3 wrapped in P and S dual doped graphitic carbon. RSC Adv. 2016, 6, 64258–64265. [Google Scholar] [CrossRef]
- Giorgi, L.; Leccese, F. Fuel cells: Technologies and applications. Open Fuel Cells J. 2013, 6, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Bartolomeo, A.D.; Sen, F. Enhanced electrochemical performance of MnNi2O4/rGO nanocomposite as pseudocapacitor electrode material and methanol electro-oxidation catalyst. Nanotechnology 2021, 32, 325707–325717. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-doped graphene supported transition-metals carbide electrocatalysts for oxygen reduction reaction. Sci. Rep. 2015, 5, 10389–10398. [Google Scholar] [CrossRef] [Green Version]
- Modafferi, V.; Santangelo, S.; Fiore, M.; Fazio, E.; Triolo, C.; Patane, S.; Ruffo, R.; Musolino, M.G. Transition metal oxides on reduced graphene oxide nanocomposites: Evaluation of physicochemical properties. J. Nanomater. 2019, 2019, 1703218. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhu, J.; Ma, Q.; Ma, J.; Bai, H.; Chen, L.; Mu, S. Two-dimensional MoS2: Structural properties, synthesis methods, and regulation strategies toward oxygen reduction. Micromachines 2021, 12, 240. [Google Scholar] [CrossRef]
- Lee, H.; Hong, A.J. Enhancement of catalytic activity of reduced graphene oxide via transition metal doping strategy. Nanoscale Res. Lett. 2017, 12, 426–432. [Google Scholar] [CrossRef]
- Dai, Y.; Ding, J.; Li, J.; Li, Y.; Zong, Y.; Zhang, P.; Wang, Z.; Liu, X. N, S and transition-metal co-doped graphene nanocomposites as high-performance catalyst for glucose oxidation in a direct glucose alkaline fuel cell. Nanomaterials 2021, 11, 202. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 6, 1339–1340. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Tiwary, C.S.; Ma, Z.; Huang, H.; Zhang, J.; Lu, Z.; Huang, W.; Wu, Y. Palladium nanoparticles supported on nitrogen and sulfur dual doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interfaces 2016, 8, 10858–10865. [Google Scholar] [CrossRef]
- Feng, X.M.; Li, R.M.; Ma, Y.W.; Chen, R.F.; Shi, N.E.; Fan, Q.L.; Huang, W. One-step electrochemical synthesis of graphene/polyaniline composite film and its applications. Adv. Funct. Mater. 2011, 21, 2989–2996. [Google Scholar] [CrossRef]
- Mu, X.; Yuan, B.; Feng, X.; Qiu, S.; Song, L.; Hu, Y. The effect of doped heteroatoms (nitrogen, boron, phosphorus) on inhibition thermal oxidation of reduced graphene oxide. RSC Adv. 2016, 6, 105021–105029. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mat. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Wang, S.L.; Huang, Z.C.; Yu, Y.M.; Liu, J.L. Synthesis of long chain-like nickel cobalt oxide nanoneedles-reduced graphene oxide composite material for high-performance supercapacitors. Ceram. Int. 2014, 40, 12751–12758. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.M.; Pourazadi, E.; Hassan, M.; Faisal, S.N.; Roy, A.K.; Konstantinov, K.; Harris, A.T.; Minett, A.I.; Gomes, V.G. Nitrogen doped graphene via thermal treatment of composite solid precursors as a high performance supercapacitor. RSC Adv. 2015, 5, 30679–30686. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Bao, S.-J.; Gou, Y.-T.; Cai, C.-J.; Ji, C.-C.; Xu, M.-W.; Song, J.; Wang, R. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. Rsc Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Ryu, K.-S. Synthesis of Ag/NiO honeycomb structured nanoarrays as the electrode material for high performance asymmetric supercapacitor devices. Sci. Rep. 2019, 9, 4864. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Sun, J.; Xu, C.; Xu, H.; Gao, L. Template-free approach to synthesize hierarchical porous nickel cobalt oxides for supercapacitors. Nanoscale 2012, 4, 6786–6791. [Google Scholar] [CrossRef]
- Kharkwal, H.; Joshi, H.; Singh, K. Fabrication and Characterization of Layered Graphene Oxide Biocompatible Nano-Film by Various Methods. J. Biochem. Biophys. 2018, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kartick, B.; Srivastava, S. Simple facile route for the preparation of graphite oxide and graphene. J. Nanosci. Nanotechnol. 2011, 11, 8586–8592. [Google Scholar] [CrossRef]
- Liu, X.; Shao, X.Y.; Fang, G.B.; He, H.F.; Wan, Z.G. Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites. e-Polymers 2017, 17, 3–14. [Google Scholar] [CrossRef]
- Malinga, N.N.; Jarvis, A.L. Synthesis, characterization and magnetic properties of Ni, Co and FeCo nanoparticles on reduced graphene oxide for removal of Cr (VI). J. Nanostruct. Chem. 2020, 10, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Matthias, T.; Katsumi, K.; Alexander, V.; Neimark, J.P.; Olivier, F.R.; Jean, R.; Kenneth, S.W.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Wang, Y.; Yu, F.; Zhu, M.; Ma, C.; Zhao, D.; Wang, C.; Zhou, A.; Dai, B.; Ji, J.; Guo, X. N-Doping of plasma exfoliated graphene oxide via dielectric barrier discharge plasma treatment for the oxygen reduction reaction. J. Mater. Chem. 2018, 6, 2011–2017. [Google Scholar] [CrossRef]
- Paranthaman, V.; Sundaramoorthy, K.; Chandra, B.; Muthu, S.P.; Alagarsamy, P.; Perumalsamy, R. Investigation on the performance of reduced graphene oxide as counter electrode in dye sensitized solar cell applications. Phys. Status Solidi A 2018, 215, 1800298. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Method 2018, 2, 1800173. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.; Entezari, M.H. Facile and fast synthesis of graphene oxide nanosheets via bath ultrasonic irradiation. J. Colloid Interface Sci. 2014, 432, 19–25. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Yu, C.; Zhu, T.; Li, Y.; Cui1, J.; Wu, J.; Shu, X.; Qin, Y.; Sun, J.; et al. Hierarchical NiCo2O4/MnO2 core-shell nanosheets arrays for flexible asymmetric supercapacitor. J. Mater. Sci. 2019, 55, 688–700. [Google Scholar] [CrossRef]
- Gorlin, Y.; Jaramillo, T.F. A bifunctional nonprecious metal catalyst for oxygen reduction and water oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar] [CrossRef]
- Zhao, B.; Zheng, Y.; Ye, F.; Deng, X.; Xu, X.; Liu, M.; Shao, Z. Multifunctional iron-oxide-nanoflake/graphene composites derived from mechanochemical synthesis for enhanced lithium storage and electrocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 14446–14455. [Google Scholar] [CrossRef]
- Zhan, Y.; Xu, C.; Lu, M.; Liu, Z.; Lee, J.Y. Mn and Co co-substituted Fe3O4 nanoparticles on nitrogen-doped reduced graphene oxide for oxygen electrocatalysis in alkaline solution. J. Mater. Chem. 2014, 2, 16217–16223. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Mukherjee, A.; Su, W.N.; Basu, S. Improved bi-functional ORR and OER catalytic activity of reduced graphene oxide supported ZnCo2O4 microsphere. Int. J. Hydrogen Energy 2019, 44, 1565–1578. [Google Scholar] [CrossRef]
- Su, C.; Yang, T.; Zhou, W.; Wang, W.; Xu, X.; Shao, Z. Pt/C-LiCoO2 composites with ultralow Pt loadings as synergistic bifunctional electrocatalysts for oxygen reduction and evolution reactions. J. Mater. Chem. 2016, 4, 4516–4524. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S. Determination of the electron transfer number for the oxygen reduction reaction: From theory to experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Sapner, V.S.; Mulik, B.B.; Digraskar, R.V.; Narwade, S.S.; Sathe, B.R. Enhanced oxygen evolution reaction on amine functionalized graphene oxide in alkaline medium. RSC Adv. 2019, 9, 6444–6451. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, S.; Sun, B.; Su, D.; Huang, X.; Liu, H.; Yan, Y.; Sun, K.; Wang, G. Graphene-Co3O4 nanocomposite as electrocatalyst with high performance for oxygen evolution reaction. Sci. Rep. 2015, 5, 7629. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Zheng, M.; Cai, M.; He, L.; Xu, C. Improved electrocatalytic performance of core-shell NiCo/NiCoOx with amorphous FeOOH for oxygen-evolution reaction. Electrochim. Acta 2017, 257, 1–8. [Google Scholar] [CrossRef]
- Basnayaka, P.A.; Ram, M.K.; Stefanakos, E.K.; Kumar, A. Supercapacitors based on graphene-polyaniline derivative nanocomposite electrode materials. Electrochim. Acta 2013, 92, 376–382. [Google Scholar] [CrossRef]
- Palaniappan, S.; Srinivas, P. Nano fibre polyaniline containing long chain and small molecule dopants and carbon composites for supercapacitor. Electrochim. Acta 2013, 95, 251–259. [Google Scholar]
- Xu, F.; Zheng, G.; Wu, D.; Liang, Y.; Li, Z.; Fu, R. Improving electrochemical performance of polyaniline by introducing carbon aerogel as filler. Phys. Chem. Chem. Phys. 2010, 12, 3270–3275. [Google Scholar] [CrossRef]
- Sapner, V.S.; Chavan, P.P.; Digraskar, R.V.; Narwade, S.S.; Mulik, B.B.; Mali, S.M.; Sathe, B.R. Tyramine Functionalized Graphene: Metal-Free Electrochemical Non-Enzymatic Biosensing of Hydrogen Peroxide. Chem. Electrol. Chem. 2018, 5, 3191–3197. [Google Scholar] [CrossRef]


















| Sample | Pore Volume | Pore Size | Surface Area | Correlation |
|---|---|---|---|---|
| (m3/g) (BJH) | (Ao) (BJH) | (m2/g) (BET) | Coefficient (R2) | |
| GO | 0.02 | 16.4 | 116.5 | 0.96 |
| N-rGO | 0.02 | 16.4 | 111.3 | 0.98 |
| N-rGO-Ni/Ag | 0.14 | 17.7 | 621.3 | 0.98 |
| N-rGO-Ni/Co | 0.12 | 16.4 | 528.2 | 0.98 |
| Sample | Onset Potential | Tafel Slope | Mass Activity |
|---|---|---|---|
| (V vs. RHE) | (mV/Decade) | (mA/mg) | |
| N-rGO-Ni/Co | 1.063 | 35 | 54.9 |
| N-rGO-Ni/Ag | 1.063 | 76 | 6.2 |
| Catalysts | ORR (V vs. RHE) | OER (V vs. RHE) | ∆E Ej=10 − Ej=−1.2 | Ref. | ||
|---|---|---|---|---|---|---|
| Onset Pot. | Ej2=−1mA/cm | Onset Pot. | Ej2=10mA/cm | (V vs. RHE) | ||
| N-rGO-Ni/Ag | 1.146 | 0.28 | 1.063 | 1.52 | 1.23 | Present work |
| N-rGO-Ni/Co | 1.146 | 0.17 | 1.063 | 1.61 | 1.12 | Present work |
| NiCo2O4/G | 1.126 | 0.62 | 1.796 | 1.75 | 1.13 | [42] |
| 20 wt% Pt/C | 1.226 | 0.84 | 1.956 | 1.83 | 0.99 | [34] |
| Voltage (V) | Rct (Ω/cm2) | Rs (Ω/cm2) |
|---|---|---|
| 0.8 | 2.978 | 6.44 × 10−1 |
| 1 | 1.739 | 7.39 × 10−1 |
| 1.2 | 1.200 | 8.84 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, M.; Bilal, S.; Shah, A.u.H.A. Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells. Nanomaterials 2022, 12, 585. https://doi.org/10.3390/nano12040585
Arif M, Bilal S, Shah AuHA. Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells. Nanomaterials. 2022; 12(4):585. https://doi.org/10.3390/nano12040585
Chicago/Turabian StyleArif, Muhammad, Salma Bilal, and Anwar ul Haq Ali Shah. 2022. "Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells" Nanomaterials 12, no. 4: 585. https://doi.org/10.3390/nano12040585
APA StyleArif, M., Bilal, S., & Shah, A. u. H. A. (2022). Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells. Nanomaterials, 12(4), 585. https://doi.org/10.3390/nano12040585