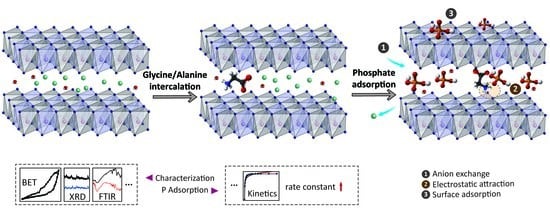
Glycine- and Alanine-Intercalated Layered Double Hydroxides as Highly Efficient Adsorbents for Phosphate with Kinetic Advantages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cl-LDH, Gly-Cl-LDH and Ala-Cl-LDH
2.3. Characterization of Cl-LDH, Gly-Cl-LDH and Ala-Cl-LDH
2.4. Adsorption Experiments
2.5. Data Analyses
3. Results and Discussions
3.1. Characterization of LDH
3.1.1. FESEM Images and Surface Properties
3.1.2. Chemical Composition Analysis
3.1.3. PXRD Analysis
3.1.4. FTIR Spectroscopy
3.2. Effect of Solution pH
3.3. Effect of Coexisting Anions
3.4. Effect of Contact Time and Adsorption Kinetics
3.5. Adsorption Isotherms
3.6. Suggested Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, F.; Zhao, T.; Shen, Q.; Fang, D.; Kuang, L.; Jiang, L.; Ding, S. Systematic Screening of Layered Double Hydroxides for Phosphate Removal and Mechanism Insight. Appl. Clay Sci. 2019, 174, 159–169. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. O19–5 Insights into the Modeling of Adsorption Isotherm Systems. Chem Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Moharami, S.; Jalali, M. Removal of Phosphorus from Aqueous Solution by Iranian Natural Adsorbents. Chem. Eng. J. 2013, 223, 328–339. [Google Scholar] [CrossRef]
- Drizo, A.; Frost, C.A.; Grace, J.; Smith, K.A. Physico-Chemical Screening of Phosphate-Removing Substrates for Use in Constructed Wetland Systems. Water Res. 1999, 33, 3595–3602. [Google Scholar] [CrossRef]
- Fang, D.; Huang, L.; Fang, Z.; Zhang, Q.; Shen, Q.; Li, Y.; Xu, X.; Ji, F. Evaluation of Porous Calcium Silicate Hydrate Derived from Carbide Slag for Removing Phosphate from Wastewater. Chem. Eng. J. 2018, 354, 1–11. [Google Scholar] [CrossRef]
- Lǚ, J.; Liu, H.; Liu, R.; Zhao, X.; Sun, L.; Qu, J. Adsorptive Removal of Phosphate by a Nanostructured Fe–Al–Mn Trimetal Oxide Adsorbent. Powder Technol. 2013, 233, 146–154. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Z.; Shan, W.; Mei, Z.; Wang, W. Adsorption Behavior of Phosphate on Lanthanum(III)-Coordinated Diamino-Functionalized 3D Hybrid Mesoporous Silicates Material. J. Hazard. Mater. 2011, 186, 76–83. [Google Scholar] [CrossRef]
- Hassan, M.H.; Stanton, R.; Secora, J.; Trivedi, D.J.; Andreescu, S. Ultrafast Removal of Phosphate from Eutrophic Waters Using a Cerium-Based Metal-Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 52788–52796. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Tang, N.; Wang, Y.; Yang, X.; Wang, S. Highly Efficient Capture of Phosphate from Water via Cerium-Doped Metal-Organic Frameworks. J. Clean. Prod. 2020, 265, 121782. [Google Scholar] [CrossRef]
- Lundehøj, L.; Jensen, H.; Wybrandt, L.; Nielsen, U.; Christensen, M.; Quist-Jensen, C. Layered Double Hydroxides for Phosphorus Recovery from Acidified and Non-Acidified Dewatered Sludge. Water Res. 2019, 153, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Tsigdinos, G.A.; Pinnavaia, T.J. Pillaring of Layered Double Hydroxides (LDH’s) by Polyoxometalate Anions. J. Am. Chem. Soc. 1988, 110, 3653–3654. [Google Scholar] [CrossRef]
- Pauling, L. The Principles Determining the Structure of Complex Ionic Crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of Layered Double Hydroxides for Removal of Oxyanions: A Review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Mandel, K.; Drenkova-Tuhtan, A.; Hutter, F.; Gellermann, C.; Steinmetz, H.; Sextl, G. Layered Double Hydroxide Ion Exchangers on Superparamagnetic Microparticles for Recovery of Phosphate from Waste Water. J. Mater. Chem. A 2012, 1, 1840–1848. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate Removal Performances of Layered Double Hydroxides (LDH) Embedded Polyvinyl Alcohol/Lanthanum Alginate Hydrogels. Chem. Eng. J. 2021, 430, 132754. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Yu, J.; Shu, Z. Novel Hollow Microspheres of Hierarchical Zinc–Aluminum Layered Double Hydroxides and their Enhanced Adsorption Capacity for Phosphate in Water. J. Hazard. Mater. 2011, 192, 1114–1121. [Google Scholar] [CrossRef]
- Yu, Q.; Zheng, Y.; Wang, Y.; Shen, L.; Wang, H.; Zheng, Y.; He, N.; Li, Q. Highly Selective Adsorption of Phosphate by Pyromellitic Acid Intercalated ZnAl-LDHs: Assembling Hydrogen Bond Acceptor Sites. Chem. Eng. J. 2014, 260, 809–817. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Q.; Li, H.; Wang, P.; Islam, S.M.; Gu, Q.; Yang, X.; Kanatzidis, M.G. Highly Selective and Efficient Heavy Metal Capture with Polysulfide Intercalated Layered Double Hydroxides. J. Mater. Chem. A 2014, 2, 10280–10289. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Z.; Sun, D.; Wu, T.; Li, Y. Enhanced Adsorption Capacity of Dyes by Surfactant-Modified Layered Double Hydroxides from Aqueous Solution. J. Ind. Eng. Chem. 2017, 49, 208–218. [Google Scholar] [CrossRef]
- Nakayama, H.; Hirami, S.; Tsuhako, M. Selective Adsorption of Mercury Ion by Mercaptocarboxylic Acid Intercalated Mg–Al Layered Double Hydroxide. J. Colloid Interface Sci. 2007, 315, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent Progress in Layered Double Hydroxides (LDH)-Containing Hybrids as Adsorbents for Water Remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Yan, D.; Lu, J.; Wei, M.; Evans, D.G.; Duan, X. Sulforhodamine B Intercalated Layered Double Hydroxide Thin Film with Polarized Photoluminescence. J. Phys. Chem. B 2009, 113, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Salinas, E.; Ono, Y. Intercalation Chemistry of a MgAl Layered Double Hydroxide Ion-Exchanged with Complex MCl2−4 (M Ni, Co) Ions from Organic Media. Microporous Mater. 1993, 1, 33–42. [Google Scholar] [CrossRef]
- Chibwe, K.; Jones, W. Synthesis of Polyoxometalate Pillared Layered Double Hydroxides via Calcined Precursors. Chem. Mater. 1989, 1, 489–490. [Google Scholar] [CrossRef]
- Choy, J.-H.; Kwak, S.-Y.; Park, J.-S.; Jeong, Y.-J.; Portier, J. Intercalative Nanohybrids of Nucleoside Monophosphates and DNA in Layered Metal Hydroxide. J. Am. Chem. Soc. 1999, 121, 1399–1400. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Highly Selective and Efficient Removal of Arsenic (V), Chromium (VI) and Selenium (VI) Oxyanions by Layered Double Hydroxide Intercalated with Zwitterionic Glycine. J. Hazard. Mater. 2017, 339, 239–247. [Google Scholar] [CrossRef]
- Hong, J.; Zhu, Z.; Lu, H.; Qiu, Y. Synthesis and Arsenic Adsorption Performances of Ferric-Based Layered Double Hydroxide with α-Alanine Intercalation. Chem. Eng. J. 2014, 252, 267–274. [Google Scholar] [CrossRef]
- Tran, H.N.; Lin, C.-C.; Chao, H.-P. Amino Acids-Intercalated Mg/Al Layered Double Hydroxides as Dual-Electronic Adsorbent for Effective Removal of Cationic and Oxyanionic Metal Ions. Sep. Purif. Technol. 2018, 192, 36–45. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Xu, Y.; Quill, K.; Simon, A.J.; Shettle, K. Laboratory Study of Boron Removal by Mg/Al Double-Layered Hydroxides. Ind. Eng. Chem. Res. 2007, 46, 4577–4583. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, X.; Zhu, B.; Jiang, C.; Le, Y.; Yu, J. Superb Adsorption Capacity of Hierarchical Calcined Ni/Mg/Al Layered Double Hydroxides for Congo Red and Cr (VI) ions. J. Hazard. Mater. 2017, 321, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidis, K.S.; Peleka, E.; Komvokis, V.G.; Mavros, P.P. Iron-Modified Hydrotalcite-Like Materials as Highly Efficient Phosphate Sorbents. J. Colloid Interface Sci. 2010, 342, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of Phosphate by Layered Double Hydroxides in Aqueous Solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Subramanian, T.; Dhakshinamoorthy, A.; Pitchumani, K. Amino Acid Intercalated Layered Double Hydroxide Catalyzed Chemoselective Methylation of Phenols and Thiophenols with Dimethyl Carbonate. Tetrahedron Lett. 2013, 54, 7167–7170. [Google Scholar] [CrossRef]
- Nakayama, H.; Wada, N.; Tsuhako, M. Intercalation of Amino Acids and Peptides into Mg–Al Layered Double Hydroxide by Reconstruction Method. Int. J. Pharm. 2004, 269, 469–478. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Synthesis and Application of LDH Intercalated Cellulose Nanocomposite for Separation of Rare Earth Elements (REEs). Chem. Eng. J. 2017, 309, 130–139. [Google Scholar] [CrossRef]
- Cai, P.; Zheng, H.; Wang, C.; Ma, H.; Hu, J.; Pu, Y.; Liang, P. Competitive Adsorption Characteristics of Fluoride and Phosphate on Calcined Mg–Al–CO3 Layered Double Hydroxides. J. Hazard. Mater. 2012, 213–214, 100–108. [Google Scholar] [CrossRef]
- Manos, E.; Ding, N.; Kanatzidis, M.G. Layered Metal Sulfides: Exceptionally Selective Agents for Radioactive Strontium Removal. Proc. Natl. Acad. Sci. USA 2008, 105, 3696–3699. [Google Scholar] [CrossRef] [Green Version]
- Novillo, C.; Guaya, D.; Avendaño, A.A.-P.; Armijos, C.; Cortina, J.L.; Cota, I. Evaluation of Phosphate Removal Capacity of Mg/Al Layered Double Hydroxides from Aqueous Solutions. Fuel 2014, 138, 72–79. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of Second-Order Models for Adsorption Systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Gao, S.; Zhang, G.; Zhang, X. Enhanced Adsorption of Phosphate from Aqueous Solution by Nanostructured Iron (III)–Copper (II) Binary Oxides. Chem. Eng. J. 2014, 235, 124–131. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of Phosphate from Water by a Fe–Mn Binary Oxide Adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Lee, C.-G.; Park, J.-A.; Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Kinetic, Equilibrium and Thermodynamic Studies for Phosphate Adsorption to Magnetic Iron Oxide Nanoparticles. Chem. Eng. J. 2014, 236, 341–347. [Google Scholar] [CrossRef]
- Pan, B.; Han, F.; Nie, G.; Wu, B.; He, K.; Lu, L. New Strategy to Enhance Phosphate Removal from Water by Hydrous Manganese Oxide. Environ. Sci. Technol. 2014, 48, 5101–5107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, Z.; Shan, W.; Chen, Z.; Mei, Z.; Lei, Y.; Wang, W. Adsorption Behavior of Phosphate on Lanthanum (III) Doped Mesoporous Silicates Material. J. Environ. Sci. 2010, 22, 507–511. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Wang, L. Preferable Adsorption of Phosphate Using Lanthanum-Incorporated Porous Zeolite: Characteristics and Mechanism. Appl. Surf. Sci. 2017, 426, 995–1004. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Pan, G.; Lundehøj, L.; Nielsen, U.G.; Shi, Y.; Hansen, H.C.B. Phosphate Capture by Ultrathin MgAl Layered Double Hydroxide Nanoparticles. Appl. Clay Sci. 2019, 177, 82–90. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 786. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms, and its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Z.; Ji, W.; Xu, J.; Zhang, X. Insights to Perfluorooctanoic Acid Adsorption Micro-Mechanism over Fe-Based Metal Organic Frameworks: Combining Computational Calculation with Response Surface Methodology. J. Hazard. Mater. 2020, 395, 122686. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.-G.; Yang, Y.-M.; Yu, S.-J.; Shan, R.-R.; Yu, H.-Q.; Zhu, B.-C.; Du, B. Adsorptive Removal of Phosphate by Mg–Al and Zn–Al Layered Double Hydroxides: Kinetics, Isotherms and Mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Kuzawa, K.; Jung, Y.-J.; Kiso, Y.; Yamada, T.; Nagai, M.; Lee, T.-G. Phosphate Removal and Recovery with a Synthetic Hydrotalcite as an Adsorbent. Chemosphere 2006, 62, 45–52. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, M.J.; Nam, S.Y.; Moon, H.C. Phosphorus Removal by Hydrotalcite-Like Compounds (htlcs). Water Sci. Technol. 1996, 34, 161–168. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.; Jiang, J.-Q. Study on the Sorption–Desorption–Regeneration Performance of Ca-, Mg- and CaMg-Based Layered Double Hydroxides for Removing Phosphate from Water. Chem. Eng. J. 2014, 246, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.; Lakzian, A. Adsorption–Desorption Characteristics of Nitrate, Phosphate and Sulfate on Mg–Al Layered Double Hydroxide. Appl. Clay Sci. 2013, 80–81, 305–312. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and Solid Diffusion Models for Fixed-Bed Adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Q.; Liu, J.; Feng, Y.; Shih, K. Phosphorus Recovery through Adsorption by Layered Double Hydroxide Nano-Composites and Transfer into a Struvite-Like Fertilizer. Water Res. 2018, 145, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, X.; Liu, J. Adsorptive Removal of Phosphate from Aqueous Solutions Using Iron Oxide Tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Du, C.; Zhang, L.; Zhuang, Y.; Xu, M. Removal of Phosphate in Aqueous Solutions by the Aluminum Salt Slag Derived from the Scrap Aluminum Melting Process. Desalination Water Treat. 2015, 57, 11291–11299. [Google Scholar] [CrossRef]
- Liu, T.; Wu, K.; Zeng, L. Removal of Phosphorus by a Composite Metal Oxide Adsorbent Derived from Manganese Ore Tailings. J. Hazard. Mater. 2012, 217–218, 29–35. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, P.; Ning, P.; Su, Y. Enhanced Adsorption Removal of Phosphate from Water by Mixed Lanthanum/Aluminum Pillared Montmorillonite. Chem. Eng. J. 2009, 151, 141–148. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Liu, Q.; Wang, L. Simultaneous Removal of Ammonium and Phosphate by Alkaline-Activated and Lanthanum-Impregnated Zeolite. Chemosphere 2016, 164, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nur, T.; Johir, M.; Loganathan, P.; Nguyen, T.; Vigneswaran, S.; Kandasamy, J. Phosphate Removal from Water Using an Iron Oxide Impregnated Strong Base Anion Exchange Resin. J. Ind. Eng. Chem. 2013, 20, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Jia, L.; He, Y.; Zhang, B.; Kirumba, G.; Xie, J. Adsorptive Removal of Phosphorus from Aqueous Solution Using Sponge Iron and Zeolite. J. Colloid Interface Sci. 2013, 402, 246–252. [Google Scholar] [CrossRef]
- Khitous, M.; Salem, Z.; Halliche, D. Removal of Phosphate from Industrial Wastewater Using Uncalcined MgAl-NO3 Layered Double Hydroxide: Batch Study and Modeling. Desalination Water Treat. 2015, 57, 15920–15931. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.; Jiang, J.-Q. Strategic Phosphate Removal/Recovery by a Re-Usable Mg–Fe–Cl Layered Double Hydroxide. Process Saf. Environ. Prot. 2017, 107, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Drenkova-Tuhtan, A.; Schneider, M.; Mandel, K.; Meyer, C.; Gellermann, C.; Sextl, G.; Steinmetz, H. Influence of Cation Building Blocks of Metal Hydroxide Precipitates on their Adsorption and Desorption Capacity for Phosphate in Wastewater—A Screening Study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 145–153. [Google Scholar] [CrossRef]
- Aisawa, S.; Kudo, H.; Hoshi, T.; Takahashi, S.; Hirahara, H.; Umetsu, Y.; Narita, E. Intercalation Behavior of Amino Acids into Zn–Al-Layered Double Hydroxide by Calcination–Rehydration Reaction. J. Solid State Chem. 2004, 177, 3987–3994. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.; Pinto, F.G.; Vergutz, L.; Tronto, J.; da Costa, L.M. Layered Double Hydroxides: New Technology in Phosphate Fertilizers Based on Nanostructured Materials. ACS Sustain. Chem. Eng. 2016, 5, 399–409. [Google Scholar] [CrossRef]
- Luengo, C.V.; Volpe, M.A.; Avena, M.J. High Sorption of Phosphate on Mg-Al Layered Double Hydroxides: Kinetics and Equilibrium. J. Environ. Chem. Eng. 2017, 5, 4656–4662. [Google Scholar] [CrossRef]
- Moraes, P.I.R.; Tavares, S.R.; Vaiss, V.S.; Leitão, A.A. Investigation on Sustainable Phosphate Release in Agriculture: Structural and Thermodynamic Study of Stability, Dehydration and Anionic Exchange of Mg-Al-HPO4 Layered Double Hydroxide by DFT Calculations. Appl. Clay Sci. 2018, 162, 428–434. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Yang, Y.; Zhang, Y.; Lei, X.; Yuan, D. Application of FeMgMn Layered Double Hydroxides for Phosphate Anions Adsorptive Removal from Water. Appl. Clay Sci. 2020, 200, 105903. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils 1. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Fang, D.; Zhuang, X.; Huang, L.; Zhang, Q.; Shen, Q.; Jiang, L.; Xu, X.; Ji, F. Developing the new kinetics model based on the adsorption process: From fitting to comparison and prediction. Sci. Total Environ. 2020, 725, 138490. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh. Fiz. Chim. 1941, 15, 296–332. [Google Scholar]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]







| Sample | Metal Ratio in Precursor | Determined Chemical Composition | Wt %, Found (calcd.) | |||
|---|---|---|---|---|---|---|
| Mg | Al | C | N | |||
| Cl-LDH | Mg0.67Al0.33 | Mg0.67Al0.33(OH)2Cl0.33·0.28H2O | 18.1 ± 0.13 | 9.80 ± 0.06 | - | - |
| (21.4) | (11.7) | - | - | |||
| Gly-Cl-LDH | Mg0.67Al0.33 | Mg0.67Al0.33(OH)2Gly0.03Cl0.30·0.28H2O | 17.5 ± 0.08 | 9.60 ± 0.10 | 1.14 ± 0.03 | 0.55 ± 0.01 |
| (21.4) | (11.7) | (0.95) | (0.55) | |||
| Ala-Cl-LDH | Mg0.67Al0.33 | Mg0.67Al0.33(OH)2Ala0.03Cl0.30·0.21H2O | 17.6 ± 0.10 | 9.60 ± 0.06 | 1.46 ± 0.05 | 0.55 ± 0.01 |
| (22.0) | (11.4) | (1.43) | (0.56) | |||
| Sample | (003) (°) | d003 (Å) | Interlayer Space (Å) | (110) (°) | d110 (Å) | a (Å) |
|---|---|---|---|---|---|---|
| Cl-LDH | 11.69 | 7.57 | 2.77 | 61.17 | 1.52 | 3.03 |
| Gly-Cl-LDH | 11.48 | 7.71 | 2.91 | 60.86 | 1.52 | 3.05 |
| Ala-Cl-LDH | 11.37 | 7.78 | 2.98 | 60.82 | 1.52 | 3.05 |
| Kinetics Model | Cl-LDH | Gly-Cl-LDH | Ala-Cl-LDH | |
|---|---|---|---|---|
| Pseudo-first-order model | qe (mg/g) | 38.6 | 37.6 | 38.2 |
| k1 | 0.565 | 0.683 | 1.444 | |
| R2 | 0.967 | 0.952 | 0.973 | |
| RSS | 53.061 | 70.959 | 37.262 | |
| Pseudo-second-order model | qe (mg/g) | 40.4 | 39.4 | 39.4 |
| k2 | 0.025 | 0.032 | 0.080 | |
| R2 | 0.998 | 0.996 | 0.997 | |
| RSS | 2.876 | 6.235 | 4.151 | |
| Elovich model | α (mg·g−1) | 5.54 × 103 | 1.99 × 104 | 1.28 × 109 |
| β (mg·g−1) | 0.283 | 0.324 | 0.618 | |
| R2 | 0.965 | 0.973 | 0.989 | |
| RSS | 0.958 | 39.801 | 15.574 | |
| Avrami model | qe (mg/g) | 39.7 | 39.3 | 39.5 |
| k | 0.766 | 0.910 | 1.502 | |
| n | 0.549 | 0.452 | 0.342 | |
| R2 | 1.000 | 1.000 | 0.999 | |
| RSS | 0.702 | 0.502 | 1.142 | |
| Intrinsic model | ξeq | 0.971 | 1.016 | 1.012 |
| kint | 3.368 | 4.102 | 10.170 | |
| R2 | 0.998 | 0.996 | 0.997 | |
| RSS | 0.724 | 1.606 | 1.095 | |
| ξini | 0.636 | 0.713 | 0.683 | |
| Material Type | Adsorbent | Parameters of Intrinsic Model | Reference | |||
|---|---|---|---|---|---|---|
| ξini | kint | ρ | ξeq | |||
| Metal oxide and hydroxide (excluding LDH) | Fe1–Cu2 binary oxide | 0.71 | 0.561 | 0.2 | 1.32 | [42] |
| Fe6–Mn1 binary oxide | 0.75 | 0.303 | 0.2 | 1.15 | [43] | |
| Iron oxide | 0.66 | 0.120 | 0.6 | 1.05 | [44] | |
| HMO@NS | 0.71 | 0.041 | 0.5 | 0.29 | [45] | |
| Functionalized mesoporous silica | La25M41 | 0.74 | 0.002 | 1.0 | 2.49 | [46] |
| Industrial by-products | P-CSH | 0.68 | 0.006 | 5.0 | 1.33 | [6] |
| Modified clay minerals | La-Z | 0.58 | 0.016 | 2.0 | 1.02 | [47] |
| Metal organic framework | 0.75Ce-UiO-66-NH2 | 0.59 | 1.340 | 0.4 | 1.05 | [10] |
| Ce-BDC | 0.61 | 1.385 | 0.5 | 1.00 | [9] | |
| Layered double hydroxides (LDH) | LDHns-U25 | 0.51 | 1.773 | 1.0 | 1.16 | [48] |
| Cl-LDH | 0.67 | 3.368 | 0.5 | 0.97 | This work | |
| Gly-Cl-LDH | 0.71 | 4.102 | 0.5 | 1.02 | ||
| Ala-Cl-LDH | 0.68 | 10.17 | 0.5 | 1.01 | ||
| LDH Type | Langmuir | Freundlich | Temkin | Dubinin–Radushkevish | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm1 | B2 | R2 | KF 3 | n | R2 | Kt | B | R2 | qm1 | BD4 | R2 | |
| Cl-LDH | 63.2 | 2.16 | 0.930 | 32.90 | 5.42 | 0.871 | 100.11 | 7.81 | 0.914 | 59.8 | 0.05 | 0.876 |
| Gly-Cl-LDH | 55.8 | 9.13 | 0.958 | 31.92 | 6.21 | 0.918 | 347.15 | 6.07 | 0.962 | 55.4 | 0.02 | 0.954 |
| Ala-Cl-LDH | 58.2 | 8.60 | 0.973 | 34.10 | 6.50 | 0.881 | 368.51 | 6.30 | 0.942 | 57.7 | 0.02 | 0.968 |
| Adsorbent | Specific Surface Area (m2/g) | qmax1 (mg-P/g) | pH | Equilibrium Time (h) | Temperature (°C) | Reference |
|---|---|---|---|---|---|---|
| Aluminum salt slag | 16.7 | 2.3–3.5 | 7.0 | 48 | 25 | [59] |
| Composite metal oxides | 307.2 | 26.3 | 6.0 | 24 | 25 | [60] |
| Iron oxide tailings | 47.9 | 8.2 | 6.6 | >24 | 20–21 | [58] |
| Bentonite | 85.0 | 0.37 | 5.45 | 24 | 25 | [4] |
| Kaolinite | 3.7 | 0.62 | 5.45 | - | 25 | |
| Zeolite | 13.8 | 0.63 | 5.45 | - | 25 | |
| Bauxite | 6.8 | 0.61 | - | 24 | 21 | [5] |
| La/Al-pillared bentonite | 13 | 5.0 | 12 | 25 | [61] | |
| Alkaline and La-modified zeolite | - | 9.1 | 7.0 | 4 | 39.85 | [62] |
| Purolite FerrIX A33E resin | 48.0 | 7.2–7.6 | 48 | 24 | [63] | |
| Sponge iron | ≥80 | 1.1 | - | 8 | 25 | [64] |
| Zn-Al-CO3-LDH | 135 | 68.4 | - | 1 | 25 | [51] |
| Mg-Al-CO3-LDH | 104 | 31.3 | - | 1 | 25 | |
| Calcined Mg-Cl-NO3-LDH | 210.0 | 44.0 | 6.0 | 4 | 29.85 | [33] |
| Mg-Al-NO3-LDH | 5.7 | 64.1 | 6.0 | 2 | 20 | [65] |
| Zn-Al-PMA-Cl-LDH | - | 57.1 | - | 4 | 20 | [18] |
| Calcined Mg-Fe-Cl-LDH | - | 9.8 | 7.0 | 2 | 25 | [66] |
| Zn-Al- NO3-LDH | 135 | 68.4 | - | 0.67 | 25 | [51] |
| Mg-Al-NO3-LDH | 104 | 31.3 | - | 1 | 25 | |
| Zn- Fe-Zr-Cl-LDH | 115 | 20.3 | 7.0–8.0 | 1 | 25 | [67] |
| Cl-LDH | 13.4 | 63.2 | 5.5 | 0.5 | 25 | This study |
| Gly-Cl-LDH | 19.0 | 55.8 | 5.5 | 0.5 | 25 | |
| Ala-Cl-LDH | 21.5 | 58.2 | 5.5 | 0.5 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Ji, F.; Jiang, L.; Shen, Q.; Mao, Y.; Liu, C. Glycine- and Alanine-Intercalated Layered Double Hydroxides as Highly Efficient Adsorbents for Phosphate with Kinetic Advantages. Nanomaterials 2022, 12, 586. https://doi.org/10.3390/nano12040586
Zhang Q, Ji F, Jiang L, Shen Q, Mao Y, Liu C. Glycine- and Alanine-Intercalated Layered Double Hydroxides as Highly Efficient Adsorbents for Phosphate with Kinetic Advantages. Nanomaterials. 2022; 12(4):586. https://doi.org/10.3390/nano12040586
Chicago/Turabian StyleZhang, Qian, Fangying Ji, Lei Jiang, Qiushi Shen, Yuanxiang Mao, and Caocong Liu. 2022. "Glycine- and Alanine-Intercalated Layered Double Hydroxides as Highly Efficient Adsorbents for Phosphate with Kinetic Advantages" Nanomaterials 12, no. 4: 586. https://doi.org/10.3390/nano12040586
APA StyleZhang, Q., Ji, F., Jiang, L., Shen, Q., Mao, Y., & Liu, C. (2022). Glycine- and Alanine-Intercalated Layered Double Hydroxides as Highly Efficient Adsorbents for Phosphate with Kinetic Advantages. Nanomaterials, 12(4), 586. https://doi.org/10.3390/nano12040586