Recent Progress of Atomic Layer Technology in Spintronics: Mechanism, Materials and Prospects
Abstract
:1. Introduction
2. Atomic Layer Deposition Technique
2.1. Molecular Beam Epitaxy (MBE)
2.2. Magnetron Sputtering
2.3. Chemical Vapor Deposition (CVD)
3. Mechanism of Atomic Layer Etching
4. Materials for ALE
4.1. Insulators
4.1.1. Oxide Materials
4.1.2. Nitride Materials
4.2. Semiconductors
4.3. Metals
4.3.1. Signal Element Metals
4.3.2. Alloys
4.4. Two-Dimensional (2D) van der Waals Materials
5. The Connection between ALD and ALE
6. Prospects and Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Greene, J.E. Review Article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017. J. Vac. Sci. Technol. Vac. Surf. Films 2017, 35, 05C204. [Google Scholar] [CrossRef] [Green Version]
- Joyce, B.A. Molecular beam epitaxy. Rep. Prog. Phys. 1985, 48, 1637–1697. [Google Scholar] [CrossRef]
- Nunn, W.; Truttmann, T.K.; Jalan, B. A review of molecular-beam epitaxy of wide bandgap complex oxide semiconductors. J. Mater. Res. 2021, 36, 4846–4864. [Google Scholar] [CrossRef]
- Arthur, J.R. Interaction of Ga and As2 Molecular Beams with GaAs Surfaces. J. Appl. Phys. 1968, 39, 4032–4034. [Google Scholar] [CrossRef]
- Yuasa, S.; Nagahama, T.; Fukushima, A.; Suzuki, Y.; Ando, K. Giant room-temperature magnetoresistance in single-crystal Fe/MgO/Fe magnetic tunnel junctions. Nat. Mater. 2004, 3, 868–871. [Google Scholar] [CrossRef]
- O’Hara, D.J.; Zhu, T.; Trout, A.H.; Ahmed, A.S.; Luo, Y.K.; Lee, C.H.; Brenner, M.R.; Rajan, S.; Gupta, J.A.; McComb, D.W.; et al. Room Temperature Intrinsic Ferromagnetism in Epitaxial Manganese Selenide Films in the Monolayer Limit. Nano Lett. 2018, 18, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Q.; Xing, J.; Liu, N.; Guo, Y.; Liu, Z.; Zhao, J. Recent progress on 2D magnets: Fundamental mechanism, structural design and modification. Appl. Phys. Rev. 2021, 8, 031305. [Google Scholar] [CrossRef]
- Evidence of Spin Frustration in a Vanadium Diselenide Monolayer Magnet. Adv. Mater. 2019, 31, 1901185.1–1901185.8.
- Zhang, S.; Wu, H.; Yang, L.; Zhang, G.; Xie, Y.; Zhang, L.; Zhang, W.; Chang, H. Two-dimensional magnetic atomic crystals. Mater. Horizons 2022, 9, 559–576. [Google Scholar] [CrossRef]
- Chua, R.; Zhou, J.; Yu, X.; Yu, W.; Gou, J.; Zhu, R.; Zhang, L.; Liu, M.; Breese, M.B.H.; Chen, W.; et al. Room Temperature Ferromagnetism of Monolayer Chromium Telluride with Perpendicular Magnetic Anisotropy. Adv. Mater. 2021, 33, 2103360. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Febvrier, A.L.; Landlv, L.; Liersch, T.; Sandmark, D.; Eklund, P. An upgraded ultra-high vacuum magnetron-sputtering system for high-versatility and software-controlled deposition. Vacuum 2021, 187, 110137. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Grove, W.R. VII. On the electro-chemical polarity of gases. Philos. Trans. R. Soc. Lond. 1852, 142, 87–101. [Google Scholar]
- Kylián, O.; Shelemin, A.; Solař, P.; Pleskunov, P.; Nikitin, D.; Kuzminova, A.; Štefaníková, R.; Kúš, P.; Cieslar, M.; Hanuš, J.; et al. Magnetron Sputtering of Polymeric Targets: From Thin Films to Heterogeneous Metal/Plasma Polymer Nanoparticles. Materials 2019, 12, 2366. [Google Scholar] [CrossRef] [Green Version]
- Schiller, S.; Goedicke, K.; Reschke, J.; Kirchhoff, V.; Milde, F. Pulsed magnetron sputter technology. Surf. Coat. Technol. 1993, 61, 331–337. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Yin, X.; Fang, L.; Meng, X.; Yin, A. TiN Films Deposited on Uranium by High Power Pulsed Magnetron Sputtering under Low Temperature. Materials 2018, 11, 1400. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.I.; Liu, K.T.; Wu, F.B.; Duh, J.G. Mo–Ru coatings on tungsten carbide by direct current magnetron sputtering. Thin Solid Films 2006, 515, 2207–2212. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, J.; Jiang, D.; Shan, C.; Zhang, Z.; Yao, B.; Zhao, D.; Shen, D. Ultraviolet photodetector based on a MgZnO film grown by radio-frequency magnetron sputtering. ACS Appl. Mater. Interfaces 2009, 1, 2428–2430. [Google Scholar] [CrossRef] [PubMed]
- Lundin, D.; Minea, T.; Gudmundsson, J.T. High Power Impulse Magnetron Sputtering: Fundamentals, Technologies, Challenges and Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yu, Y.-L.; Xu, X.Q.; Zhang, T.-H.; Ma, Y. Investigation on the microstructural and mechanical properties of a Polytetrafluoroethylene thin film by radio frequency magnetron sputtering. Thin Solid Films 2020, 712, 138302. [Google Scholar] [CrossRef]
- Tan, X.Q.; Liu, J.Y.; Niu, J.R.; Liu, J.Y.; Tian, J.Y. Recent Progress in Magnetron Sputtering Technology Used on Fabrics. Materials 2018, 11, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, S.; Wang, Y.; Wang, T.; Shang, S.; Ren, W. Magnetron-sputtering deposited molybdenum carbide MXene thin films as a saturable absorber for passively Q-switched lasers. J. Mater. Chem. C 2020, 8, 1608–1613. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Q.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Zhang, F.; Asiri, A.M.; Sun, X. Magnetron sputtering enabled sustainable synthesis of nanomaterials for energy electrocatalysis. Green Chem. 2021, 23, 2834–2867. [Google Scholar] [CrossRef]
- Zhao, J.; Baibuz, E.; Vernieres, J.; Grammatikopoulos, P.; Jansson, V.; Nagel, M.; Steinhauer, S.; Sowwan, M.; Kuronen, A.; Nordlund, K.; et al. Formation mechanism of Fe nanocubes by magnetron sputtering inert gas condensation. ACS Nano 2016, 10, 4684–4694. [Google Scholar] [CrossRef]
- Hejazi, S.; Altomare, M.; Mohajernia, S.; Schmuki, P. Composition Gradients in Sputtered Ti–Au Alloys: Site-Selective Au Decoration of Anodic TiO2 Nanotubes for Photocatalytic H2 Evolution. ACS Appl. Nano Mater. 2019, 2, 4018–4025. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, Q.; Su, S.; Zhang, S.; Ma, J.; Shen, L.; Yu, Q.; Zhao, L.; Liu, Y.; Kang, F.; et al. Constructing multifunctional interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li metal by magnetron sputtering for highly stable solid-state lithium metal batteries. Adv. Energy Mater. 2019, 9, 1901604. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Thorwarth, K.; Niesen, B.; Liardet, L.; Patscheider, J.; Ballif, C.; Hu, X. Solar hydrogen production by amorphous silicon photocathodes coated with a magnetron sputter deposited Mo2C catalyst. J. Am. Chem. Soc. 2015, 137, 7035–7038. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zhou, C.; Zhou, H.; Chang, L.; Wang, Y.; Liu, Y.; Bilek, M.M.; Liu, Z. High entropy nitride (HEN) thin films of AlCoCrCu0.5FeNi deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2020, 402, 126327. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Yao, X.; Leng, Y.; Xu, X.; Peng, A.; Wang, L.; Xue, Q. Si/aC Nanocomposites with a Multiple Buffer Structure via One-Step Magnetron Sputtering for Ultrahigh-Stability Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2019, 11, 45726–45736. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ohshima, N.; Goto, K.; Yamamoto, H.; Morita, T.; Kinoshita, K.; Ishijima, T.; Toyoda, H. Effect of O-ion beam irradiation during RF-magnetron sputtering on characteristics of CoFeB–MgO magnetic tunnel junctions. Jpn. J. Appl. Phys. 2011, 50, 023001. [Google Scholar] [CrossRef]
- Watanabe, K.; Jinnai, B.; Fukami, S.; Sato, H.; Ohno, H. Shape anisotropy revisited in single-digit nanometer magnetic tunnel junctions. Nat. Commun. 2018, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Bainsla, L.; Suzuki, K.Z.; Tsujikawa, M.; Tsuchiura, H.; Shirai, M.; Mizukami, S. Magnetic tunnel junctions with an equiatomic quaternary CoFeMnSi Heusler alloy electrode. Appl. Phys. Lett. 2018, 112, 052403. [Google Scholar] [CrossRef]
- Julliere, M. Tunneling between ferromagnetic films. Phys. Lett. A 1975, 54, 225–226. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tezuka, N. Giant magnetic tunneling effect in Fe/Al2O3/Fe junction. J. Magn. Magn. Mater. 1995, 139, L231–L234. [Google Scholar] [CrossRef]
- Moodera, J.S.; Kinder, L.R.; Wong, T.M.; Meservey, R. Large magnetoresistance at room temperature in ferromagnetic thin film tunnel junctions. Phys. Rev. Lett. 1995, 74, 3273. [Google Scholar] [CrossRef]
- Parkin, S.S.; Kaiser, C.; Panchula, A.; Rice, P.M.; Hughes, B.; Samant, M.; Yang, S.H. Giant tunnelling magnetoresistance at room temperature with MgO (100) tunnel barriers. Nat. Mater. 2004, 3, 862–867. [Google Scholar] [CrossRef]
- Djayaprawira, D.D.; Tsunekawa, K.; Nagai, M.; Maehara, H.; Yamagata, S.; Watanabe, N.; Yuasa, S.; Suzuki, Y.; Ando, K. 230% room-temperature magnetoresistance in CoFeB/ MgO/ CoFeB magnetic tunnel junctions. Appl. Phys. Lett. 2005, 86, 092502. [Google Scholar] [CrossRef]
- Ohtsu, Y.; Nakashima, T.; Tanaka, R.; Schulze, J. Characteristics of a rotational windmill-shaped radio frequency magnetron sputtering plasma for effective target utilization. Vacuum 2020, 181, 109593. [Google Scholar] [CrossRef]
- Powell, C.F.; Oxley, J.H.; Blocher, J.M.; Klerer, J. Vapor Deposition. J. Electrochem. Soc. 1966, 113, 266C. [Google Scholar] [CrossRef]
- Antson, J. United States Patent (19). Geothermics 1977, 14, 595–599. [Google Scholar]
- Leskelä, M.; Ritala, M. Minireviews Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chem. Int. Ed. 2003, 42, 15548–15554. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Gavalas, G.R. Preparation of H2 Permselective Silica Membranes by Alternating Reactant Vapor Deposition. Ind. Eng. Chem. Res. 1995, 34, 168–176. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Tanaka, N.; Nomura, M.; Kubo, S. Hydrogen production tests by hydrogen iodide decomposition membrane reactor equipped with silica-based ceramics membrane. Int. J. Hydrogen Energy 2017, 42, 29091–29100. [Google Scholar] [CrossRef]
- Daub, M.; Knez, M.; Goesele, U.; Nielsch, K. Ferromagnetic nanotubes by atomic layer deposition in anodic alumina membranes. J. Appl. Phys. 2007, 101, 2005–2008. [Google Scholar] [CrossRef]
- Cobden, D.H. Molecular electronics: Nanowires begin to shine. Nature 2001, 409, 32–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, W.; Jiang, Z.; Yang, S.; Jing, W.; Shi, P.; Wu, X.; Ye, Z.G. Low-temperature remote plasma-enhanced atomic layer deposition of graphene and characterization of its atomic-level structure. J. Mater. Chem. C 2014, 2, 7570–7574. [Google Scholar] [CrossRef]
- Cao, Y.; Rodan-Legrain, D.; Rubies-Bigorda, O.; Park, J.M.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Tunable correlated states and spin-polarized phases in twisted bilayer–bilayer graphene. Nature 2020, 583, 215–220. [Google Scholar] [CrossRef]
- Rozen, A.; Park, J.M.; Zondiner, U.; Cao, Y.; Rodan-Legrain, D.; Taniguchi, T.; Watanabe, K.; Oreg, Y.; Stern, A.; Berg, E.; et al. Entropic evidence for a Pomeranchuk effect in magic-angle graphene. Nature 2021, 592, 214–219. [Google Scholar] [CrossRef]
- Park, J.M.; Cao, Y.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Flavour Hund’s coupling, Chern gaps and charge diffusivity in moiré graphene. Nature 2021, 592, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.E.; Chen, C.C. Morphological and Photoelectrochemical Properties of ALD TiO2 Films. J. Electrochem. Soc. 2008, 155, D604. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Liu, J.; Fu, L.; Li, J. Quantum spin Hall effect in two-dimensional transition metal dichalcogenides. Science 2014, 346, 1344–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–79. [Google Scholar] [CrossRef]
- Mircea, D.; Adrian, D.; Daniela, D. 2D Materials Nanoelectronics: New Concepts, Fabrication, Characterization From Microwaves up to Optical Spectrum. Phys. Status Solidi 2019, 216, 1800724.1–1800724.15. [Google Scholar]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Muellen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent Exfoliation of Transition Metal Dichalcogenides: Dispersibility of Exfoliated Nanosheets Varies Only Weakly between Compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J.; et al. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Calderon, B.; Han, Y.; Cueva, P.; Jungwirth, N.R.; Alsalman, H.A.; Hwang, J.; Fuchs, G.D.; Muller, D.A.; Spencer, M.G. Chemical Vapor Deposition Growth of Large Single-Crystal Mono-, Bi-, Tri-Layer Hexagonal Boron Nitride and Their Interlayer Stacking. ACS Nano 2017, 11, 12057–12066. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Li, L.J. Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques. Chem. Soc. Rev. 2015, 44, 2744–2756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Vega, V.; García, J.; Zierold, R.; Nielsch, K.; Prida, V.M.; Hernando, B.; Benavente, J. Changes in morphology and ionic transport induced by ALD SiO2 coating of nanoporous alumina membranes. ACS Appl. Mater. Interfaces 2013, 5, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; Demarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.T.; Stair, P.C.; Snurr, R.Q.; et al. Vapor-phase metalation by atomic layer deposition in a metal-organic framework. J. Am. Chem. Soc. 2013, 135, 10294–10297. [Google Scholar] [CrossRef]
- Leus, K.; Dendooven, J.; Tahir, N.; Ramachandran, R.K.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Goeman, J.L.; Van der Eycken, J.; Detavernier, C.; et al. Atomic layer deposition of pt nanoparticles within the cages of MIL-101: A mild and recyclable hydrogenation catalyst. Nanomaterials 2016, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Meledina, M.; Turner, S.; Filippousi, M.; Leus, K.; Lobato, I.; Ramachandran, R.K.; Dendooven, J.; Detavernier, C.; Van Der Voort, P.; Van Tendeloo, G. Direct Imaging of ALD Deposited Pt Nanoclusters inside the Giant Pores of MIL-101. Part. Part. Syst. Charact. 2016, 33, 382–387. [Google Scholar] [CrossRef]
- Lee, S.M.; Pippel, E.; Moutanabbir, O.; Gunkel, I.; Thurn-Albrecht, T.; Knez, M. Improved mechanical stability of dried collagen membrane after metal infiltration. ACS Appl. Mater. Interfaces 2010, 2, 2436–2441. [Google Scholar] [CrossRef]
- Peters, A.W.; Li, Z.; Farha, O.K.; Hupp, J.T. Atomically Precise Growth of Catalytically Active Cobalt Sulfide on Flat Surfaces and within a Metal-Organic Framework via Atomic Layer Deposition. ACS Nano 2015, 9, 8484–8490. [Google Scholar] [CrossRef] [Green Version]
- Mårlid, B.; Ottosson, M.; Pettersson, U.; Larsson, K.; Carlsson, J.O. Atomic layer deposition of BN thin films. Thin Solid Films 2002, 402, 167–171. [Google Scholar] [CrossRef]
- Weimer, M.A.; Weimer, A.W.; Park, W. Theory of conduction in ultrafast metal-insulator varistors. J. Appl. Phys. 2008, 104, 1–8. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Weimer, A.W.; George, S.M. Atomic layer deposition of boron nitride using sequential exposures of BCl3 and NH3. Thin Solid Films 2002, 413, 16–25. [Google Scholar] [CrossRef]
- Snure, M.; Paduano, Q.; Hamilton, M.; Shoaf, J.; Matthew Mann, J. Optical characterization of nanocrystalline boron nitride thin films grown by atomic layer deposition. Thin Solid Films 2014, 571, 51–55. [Google Scholar] [CrossRef]
- Browning, R.; Padigi, P.; Solanki, R.; Tweet, D.J.; Schuele, P.; Evans, D. Atomic layer deposition of MoS2 thin films. Mater. Res. Express 2015, 2, 35006. [Google Scholar] [CrossRef]
- Valdivia, A.; Tweet, D.J.; Conley, J.F. Atomic layer deposition of two dimensional MoS2 on 150 mm substrates. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 021515. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.F.; Chai, Z.M.; Lu, X.C.; He, D.N. A direct atomic layer deposition method for growth of ultra-thin lubricant tungsten disulfide films. Sci. China Technol. Sci. 2017, 60, 51–57. [Google Scholar] [CrossRef]
- Petrochenko, P.E.; Kumar, G.; Fu, W.; Zhang, Q.; Zheng, J.; Liang, C.; Goering, P.L.; Narayan, R.J. Nanoporous aluminum oxide membranes coated with atomic layer deposition-grown titanium dioxide for biomedical applications: An in vitro evaluation. J. Biomed. Nanotechnol. 2015, 11, 2275–2285. [Google Scholar] [CrossRef]
- Tong, J.; Lei, X.; Fang, J.; Han, M.; Huang, K. Remarkable O2 permeation through a mixed conducting carbon capture membrane functionalized by atomic layer deposition. J. Mater. Chem. A 2016, 4, 1828–1837. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Fan, Y.; Xing, W.; Wang, Y. Modification of ceramic membranes for pore structure tailoring: The atomic layer deposition route. J. Membr. Sci. 2012, 397–398, 17–23. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, J.; Jee, Y.; Huang, K. Stabilizing a high-temperature electrochemical silver-carbonate CO2 capture membrane by atomic layer deposition of a ZrO2 overcoat. Chem. Commun. 2016, 52, 9817–9820. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.B.; Dlubak, B.; Weatherup, R.S.; Yang, H.; Deranlot, C.; Bouzehouane, K.; Petroff, F.; Anane, A.; Hofmann, S.; Robertson, J.; et al. Sub-nanometer atomic layer deposition for spintronics in magnetic tunnel junctions based on graphene spin-filtering membranes. ACS Nano 2014, 8, 7890–7895. [Google Scholar] [CrossRef] [PubMed]
- Mantovan, R.; Vangelista, S.; Kutrzeba-Kotowska, B.; Lamperti, A.; Manca, N.; Pellegrino, L.; Fanciulli, M. Fe3-δO4/MgO/Co magnetic tunnel junctions synthesized by full in situ atomic layer and chemical vapour deposition. J. Phys. D Appl. Phys. 2014, 47, 102002. [Google Scholar] [CrossRef]
- Soumyanarayanan, A.; Reyren, N.; Fert, A.; Panagopoulos, C. Emergent phenomena induced by spin–orbit coupling at surfaces and interfaces. Nature 2016, 539, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellman, F.; Hoffmann, A.; Tserkovnyak, Y.; Beach, G.S.; Fullerton, E.E.; Leighton, C.; Macdonald, A.H.; Ralph, D.C.; Arena, D.A.; Dürr, H.A.; et al. Interface-induced phenomena in magnetism. Rev. Mod. Phys. 2017, 89, 025006. [Google Scholar] [CrossRef] [PubMed]
- Longo, E.; Mantovan, R.; Cecchini, R.; Overbeek, M.D.; Longo, M.; Trevisi, G.; Lazzarini, L.; Tallarida, G.; Fanciulli, M.; Winter, C.H.; et al. ALD growth of ultra-thin Co layers on the topological insulator Sb2Te3. Nano Res. 2020, 13, 570–575. [Google Scholar] [CrossRef]
- Longo, E.; Wiemer, C.; Belli, M.; Cecchini, R.; Longo, M.; Cantoni, M.; Rinaldi, C.; Overbeek, M.D.; Winter, C.H.; Gubbiotti, G.; et al. Ferromagnetic resonance of Co thin films grown by atomic layer deposition on the Sb2Te3 topological insulator. J. Magn. Magn. Mater. 2020, 509, 166885. [Google Scholar] [CrossRef]
- Bubber, R.; Mao, M.; Schneider, T.; Hegde, H.; Sin, K.; Funada, S.; Shi, S. ALCVD AlOx barrier layers for magnetic tunnel junction applications. IEEE Trans. Magn. 2002, 38, 2724–2726. [Google Scholar] [CrossRef]
- Mao, M.; Bubber, R.; Schneider, T. ALD for data storage applications. ECS Trans. 2006, 1, 37. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J. Magnetic tunnel junctions with Al2O3 tunnel barriers prepared by atomic layer deposition. Appl. Phys. Lett. 2013, 102, 202401. [Google Scholar] [CrossRef]
- Knechten, C.A.M. Plasma Oxidation for Magnetic Tunnel Junctions; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2004. [Google Scholar]
- Mantovan, R.; Georgieva, M.; Perego, M.; Lu, H.; Cocco, S.; Zenkevich, A.; Scarel, G.; Fanciulli, M. Atomic layer deposition of magnetic thin films. Acta Phys. Pol. Ser. A 2007, 112, 1271. [Google Scholar] [CrossRef]
- Mantovan, R.; Vangelista, S.; Kutrzeba-Kotowska, B.; Cocco, S.; Lamperti, A.; Tallarida, G.; Mameli, D.; Fanciulli, M. Synthesis of magnetic tunnel junctions with full in situ atomic layer and chemical vapor deposition processes. Thin Solid Films 2012, 520, 4820–4822. [Google Scholar] [CrossRef]
- Mantovan, R.; Lamperti, A.; Georgieva, M.; Tallarida, G.; Fanciulli, M. CVD synthesis of polycrystalline magnetite thin films: Structural, magnetic and magnetotransport properties. J. Phys. D Appl. Phys. 2010, 43, 065002. [Google Scholar] [CrossRef] [Green Version]
- Fabretti, S.; Zierold, R.; Nielsch, K.; Voigt, C.; Ronning, C.; Peretzki, P.; Seibt, M.; Thomas, A. Temperature and bias-voltage dependence of atomic-layer-deposited HfO2-based magnetic tunnel junctions. Appl. Phys. Lett. 2014, 105, 132405. [Google Scholar] [CrossRef]
- Wilt, J.; Goul, R.; Acharya, J.; Sakidja, R.; Wu, J.Z. In situ atomic layer deposition and electron tunneling characterization of monolayer Al2O3 on Fe for magnetic tunnel junctions. AIP Adv. 2018, 8, 125218. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Z.; Acharya, J.; Goul, R. In vacuo atomic layer deposition and electron tunneling characterization of ultrathin dielectric films for metal/insulator/metal tunnel junctions. J. Vac. Sci. Technol. A Vac. Surf. Films 2020, 38, 040802. [Google Scholar] [CrossRef]
- Wilt, J.; Sakidja, R.; Goul, R.; Wu, J.Z. Effect of an interfacial layer on electron tunneling through atomically thin Al2O3 tunnel barriers. ACS Appl. Mater. Interfaces 2017, 9, 37468–37475. [Google Scholar] [CrossRef]
- Acharya, J.; Goul, R.; Wu, J. High Tunneling Magnetoresistance in Magnetic Tunnel Junctions with Subnanometer Thick Al2O3 Tunnel Barriers Fabricated Using Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2020, 13, 15738–15745. [Google Scholar] [CrossRef]
- Creighton, J.R.; Ho, P. Introduction to Chemical Vapor Deposition (CVD); ASM International: Almere, The Netherlands, 2001; Volume 2, pp. 1–22. [Google Scholar]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, L.; Wu, B.; Ding, F.; Liu, Y. Theoretical Study of Chemical Vapor Deposition Synthesis of Graphene and Beyond: Challenges and Perspectives. J. Phys. Chem. Lett. 2021, 12, 7942–7963. [Google Scholar] [CrossRef]
- Yoder, M.N. Atomic Layer Etching; Department of the Navy: Washington, DC, USA, 1988. [Google Scholar]
- Janiak, F.; Dyksik, M.; Motyka, M.; Ryczko, K.; Misiewicz, J.; Kosiel, K.; Bugajski, M. Advanced optical characterization of AlGaAs/GaAs superlattices for active regions in quantum cascade lasers. Opt. Quantum Electron. 2015, 47, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Maki, P.A.; Ehrlich, D.J. Laser bilayer etching of GaAs surfaces. Appl. Phys. Lett. 1989, 55, 91–93. [Google Scholar] [CrossRef]
- Sakaue, H.; Asami, K.; Ichihara, T.; Ishizuka, S.; Kawamura, K.; Horiike, Y. Digital Process for advanced VLSI’s and Surface Reaction Study. MRS Proc. 1991, 222, 195. [Google Scholar] [CrossRef]
- Lee, Y.; George, S.M. Atomic Layer Etching of Al2O3 Using Sequential, Self-Limiting Thermal Reactions with Sn(acac)2 and Hydrogen Fluoride. ACS Nano 2015, 9, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ma, H.; Yuan, G.; Zhang, W. Research Progress in Atomic Layer Etching Technology. Micro/Nano Electron. Intell. Manuf. 2019, 1, 47–56. [Google Scholar]
- Lee, Y.; George, S.M. Thermal Atomic Layer Etching of Titanium Nitride Using Sequential, Self-Limiting Reactions: Oxidation to TiO2 and Fluorination to Volatile TiF4. Chem. Mater. 2017, 29, 8202–8210. [Google Scholar] [CrossRef]
- Lee, Y.; DuMont, J.W.; George, S.M. Atomic layer etching of HfO2 using sequential, self-limiting thermal reactions with Sn(acac)2 and HF. ECS J. Solid State Sci. Technol. 2015, 4, N5013. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, P.C.; Parsons, G.N. Thermal Selective Vapor Etching of TiO2: Chemical Vapor Etching via WF6 and Self-Limiting Atomic Layer Etching Using WF6 and BCl3. Chem. Mater. 2017, 29, 6653–6665. [Google Scholar] [CrossRef]
- Jeng, S.J.; Natzle, W.C.; Yu, C. Device and Method for Accurate Etching and Removal of Thin Film. U.S. Patent 5,282,925, 1 February 1994. [Google Scholar]
- Johnson, N.R.; George, S.M. WO3 and W thermal atomic layer etching using “conversion-fluorination” and “oxidation-conversion-fluorination” mechanisms. ACS Appl. Mater. Interfaces 2017, 9, 34435–34447. [Google Scholar] [CrossRef]
- Thermal Atomic Layer Etching of SiO2 by a “Conversion-Etch” Mechanism Using Sequential Reactions of Trimethylaluminum and Hydrogen Fluoride. ACS Appl. Mater. Interfaces 2017, 9, 10296–10307. [CrossRef]
- Kanarik, K.J.; Tan, S.; Yang, W.; Kim, T.; Lill, T.; Kabansky, A.; Hudson, E.A.; Ohba, T.; Nojiri, K.; Yu, J.; et al. Predicting synergy in atomic layer etching. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 05C302. [Google Scholar] [CrossRef]
- Effect of crystallinity on thermal atomic layer etching of hafnium oxide, zirconium oxide, and hafnium zirconium oxide. J. Vac. Sci. Technol. A 2020, 38, 022608. [CrossRef]
- Lee, Y.; Dumont, J.W.; George, S.M. Atomic Layer Etching of AlF3 Using Sequential, Self-Limiting Thermal Reactions with Sn(acac)2 and Hydrogen Fluoride. J. Phys. Chem. C 2015, 119, 25385–25393. [Google Scholar] [CrossRef]
- Sharma, V.; Elliott, S.D.; Blomberg, T.; Haukka, S.; Givens, M.E.; Tuominen, M.; Ritala, M. Thermal Atomic Layer Etching of Aluminum Oxide (Al2O3) Using Sequential Exposures of Niobium Pentafluoride (NbF5) and Carbon Tetrachloride (CCl4): A Combined Experimental and Density Functional Theory Study of the Etch Mechanism. Chem. Mater. 2021, 33, 2883–2893. [Google Scholar] [CrossRef]
- Lee, Y.; Dumont, J.W.; George, S.M. Trimethylaluminum as the Metal Precursor for the Atomic Layer Etching of Al2O3 Using Sequential, Self-Limiting Thermal Reactions. Chem. Mater. 2016, 28, 2994–3003. [Google Scholar] [CrossRef]
- Lee, Y.; Huffman, C.; George, S.M. Selectivity in Thermal Atomic Layer Etching Using Sequential, Self-Limiting Fluorination and Ligand-Exchange Reactions. Chem. Mater. 2016, 28, 7657–7665. [Google Scholar] [CrossRef]
- Zywotko, D.R.; George, S.M. Thermal Atomic Layer Etching of ZnO by a “Conversion-Etch” Mechanism Using Sequential Exposures of Hydrogen Fluoride and Trimethylaluminum. Chem. Mater. 2017, 29, 1183–1191. [Google Scholar] [CrossRef]
- Lee, Y.; Johnson, N.R.; George, S.M. Thermal Atomic Layer Etching of Gallium Oxide Using Sequential Exposures of HF and Various Metal Precursors. Chem. Mater. 2020, 32, 5937–5948. [Google Scholar] [CrossRef]
- Matsuura, T.; Honda, Y.; Murota, J. Atomic-order layer-by-layer role-share etching of silicon nitride using an electron cyclotron resonance plasma. Appl. Phys. Lett. 1999, 74, 3573–3575. [Google Scholar] [CrossRef]
- Johnson, N.R.; Sun, H.; Sharma, K.; George, S.M. Thermal atomic layer etching of crystalline aluminum nitride using sequential, self-limiting hydrogen fluoride and Sn(acac)2 reactions and enhancement by H2 and Ar plasmas. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 050603. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; George, S.M. Thermal atomic layer etching of silicon nitride using an oxidation and “conversion etch” mechanism. J. Vac. Sci. Technol. A 2020, 38, 022607. [Google Scholar] [CrossRef]
- Kanarik, K.J.; Lill, T.; Hudson, E.A.; Sriraman, S.; Tan, S.; Marks, J.; Vahedi, V.; Gottscho, R.A. Overview of atomic layer etching in the semiconductor industry. J. Vac. Sci. Technol. A Vac. Surf. Films 2015, 33, 020802. [Google Scholar] [CrossRef] [Green Version]
- Sakaue, H.; Iseda, S.; Asami, K.; Yamamoto, J.; Hirose, M.; Horiike, Y. Atomic Layer Controlled Digital Etching of Silicon. Jpn. J. Appl. Phys. 1990, 29, 2648–2652. [Google Scholar] [CrossRef]
- Athavale, S.D.; Economou, D.J. Realization of atomic layer etching of silicon. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1996, 14, 3702–3705. [Google Scholar] [CrossRef]
- Bozada, C.A.; Quach, T.K.; Nakano, K.; Desalvo, G.C.; Via, G.D.; Dettmer, R.W.; Havasy, C.K.; Sewell, J.S.; Ebel, J.L.; Gillespie, J.K. Field Effect Transistor Process with Semiconductor Mask, Single Layer Integrated Metal, and Dual Etch Stops. U.S. Patent 5,940,694, 17 August 1999. [Google Scholar]
- Ohba, T.; Yang, W.; Tan, S.; Kanarik, K.J.; Nojiri, K. Atomic layer etching of GaN and AlGaN using directional plasma-enhanced approach. Jpn. J. Appl. Phys. 2017, 56, 06HB06. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.I.; Dong, C.S.; Jang, S.O.; Yong, S.C. Development of Silicon Carbide Atomic Layer Etching Technology. Thin Solid Films 2020, 707, 138084. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; George, S.M. Thermal Atomic Layer Etching of Silicon Using O2, HF, and Al(CH3)3 as the Reactants. Chem. Mater. 2018, 30, 8465–8475. [Google Scholar] [CrossRef]
- Park, S.D.; Oh, C.K.; Bae, J.W.; Yeom, G.Y.; Kim, T.W.; Song, J.I.; Jang, J.H. Atomic layer etching of InP using a low angle forward reflected Ne neutral beam. Appl. Phys. Lett. 2006, 89, 043109. [Google Scholar] [CrossRef] [Green Version]
- Aoyagi, Y.; Shinmura, K.; Kawasaki, K.; Tanaka, T.; Gamo, K.; Namba, S.; Nakamoto, I. Molecular layer etching of GaAs. Appl. Phys. Lett. 1992, 60, 968–970. [Google Scholar] [CrossRef]
- Kauppinen, C.; Khan, S.A.; Sundqvist, J.; Suyatin, D.B.; Suihkonen, S.; Kauppinen, E.I.; Sopanen, M. Atomic layer etching of gallium nitride (0001). J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 060603. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.R.; Hite, J.K.; Mastro, M.A.; Eddy, C.R.; George, S.M. Thermal atomic layer etching of crystalline GaN using sequential exposures of XeF2 and BCl3. Appl. Phys. Lett. 2019, 114, 243103. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, D.S.; Mun, M.K.; Lee, W.O.; Kim, K.S.; Yeom, G.Y. Atomic layer etching of InGaAs by controlled ion beam. J. Phys. D Appl. Phys. 2017, 50, 254007. [Google Scholar] [CrossRef]
- Lu, W.; Lee, Y.; Gertsch, J.C.; Murdzek, J.A.; Cavanagh, A.S.; Kong, L.; Del Alamo, J.A.; George, S.M. In Situ Thermal Atomic Layer Etching for Sub-5 nm InGaAs Multigate MOSFETs. Nano Lett. 2019, 19, 5159–5166. [Google Scholar] [CrossRef] [PubMed]
- Fukumizu, H.; Sekine, M.; Hori, M.; Kanomaru, K.; Kikuchi, T. Atomic layer etching of AlGaN using Cl2 and Ar gas chemistry and UV damage evaluation. J. Vac. Sci. Technol. A 2019, 37, 021002. [Google Scholar] [CrossRef]
- Konh, M.; Janotti, A.; Teplyakov, A. Molecular Mechanism of Thermal Dry Etching of Iron in a Two-Step Atomic Layer Etching Process: Chlorination Followed by Exposure to Acetylacetone. Phys. Chem. C 2021, 125, 7142–7154. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, D.S.; Lee, W.O.; Kim, J.E.; Yeom, G.Y. Atomic layer etching of chrome using ion beams. Nanotechnology 2018, 30, 085303. [Google Scholar] [CrossRef] [Green Version]
- Coffey, B.M.; Nallan, H.C.; Engstrom, J.R.; Lam, C.H.; Ekerdt, J.G. Vacuum Ultraviolet-Enhanced Oxidation—A Route to the Atomic Layer Etching of Palladium Metal. Chem. Mater. 2020, 32, 6035–6042. [Google Scholar] [CrossRef]
- Altieri, N.D.; Chen, J.K.C.; Minardi, L.; Chang, J.P. Review Article: Plasma–surface interactions at the atomic scale for patterning metals. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 05C203. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Kikuchi, Y.; Nozawa, T.; Samukawa, S. A new metallic complex reaction etching for transition metals by a low-temperature neutral beam process. J. Phys. D Appl. Phys. 2014, 47, 322002. [Google Scholar] [CrossRef]
- Lin, X.; Chen, M.; Janotti, A.; Opila, R. In situ XPS study on atomic layer etching of Fe thin film using Cl2 and acetylacetone. J. Vac. Sci. Technol. A 2018, 36, 051401. [Google Scholar] [CrossRef]
- Konh, M.; He, C.; Lin, X.; Guo, X.; Pallem, V.; Opila, R.L.; Teplyakov, A.V.; Wang, Z.; Yuan, B. Molecular mechanisms of atomic layer etching of cobalt with sequential exposure to molecular chlorine and diketones. J. Vac. Sci. Technol. A 2019, 37, 021004. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.C.; Altieri, N.D.; Kim, T.; Chen, E.; Lill, T.; Shen, M.; Chang, J.P. Directional etch of magnetic and noble metals. II. Organic chemical vapor etch. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 05C305. [Google Scholar] [CrossRef]
- Xie, W.; Lemaire, P.C.; Parsons, G.N. Thermally Driven Self-Limiting Atomic Layer Etching of Metallic Tungsten Using WF6 and O2. ACS Appl. Mater. Interfaces 2018, 10, 9147–9154. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, J.E.; Lee, W.O.; Park, J.W.; Gill, Y.J.; Jeong, B.H.; Yeom, G.Y. Anisotropic atomic layer etching of W using fluorine radicals/oxygen ion beam. Plasma Process. Polym. 2019, 16, 1–8. [Google Scholar] [CrossRef]
- Mohimi, E.; Chu, X.I.; Trinh, B.B.; Babar, S.; Girolami, G.S.; Abelson, J.R. Thermal Atomic Layer Etching of Copper by Sequential Steps Involving Oxidation and Exposure to Hexafluoroacetylacetone. ECS J. Solid State Sci. Technol. 2018, 7, P491–P495. [Google Scholar] [CrossRef]
- Toyoda, N.; Ogawa, A. Atomic layer etching of Cu film using gas cluster ion beam. J. Phys. D Appl. Phys. 2017, 50, 184003. [Google Scholar] [CrossRef]
- Coffey, B.M.; Nallan, H.C.; Ekerdt, J.G. Vacuum ultraviolet enhanced atomic layer etching of ruthenium films. J. Vac. Sci. Technol. A 2021, 39, 012601. [Google Scholar] [CrossRef]
- Sang, X.; Chen, E.; Chang, J.P. Patterning nickel for extreme ultraviolet lithography mask application I. Atomic layer etch processing. J. Vac. Sci. Technol. A 2020, 38, 042603. [Google Scholar] [CrossRef]
- Basher, A.H.; Krsti, M.; Fink, K.; Ito, T.; Hamaguchi, S. Formation and desorption of nickel hexafluoroacetylacetonate Ni(hfac)2 on a nickel oxide surface in atomic layer etching processes. J. Vac. Sci. Technol. A 2020, 38, 052602. [Google Scholar] [CrossRef]
- Kuo, Y.; Lee, S. A Novel Plasma-Based Copper Dry Etching Method. Jpn. J. Appl. Phys. 2000, 39, L188–L190. [Google Scholar] [CrossRef]
- Umezaki, T.; Gunji, I.; Takeda, Y.; Mori, I. Thermal dry-etching of nickel using oxygen and 1,1,1,5,5,5 - hexafluoro-2,4-pentanedione (Hhfac). In Proceedings of the 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), Sendai, Japan, 22–25 August 2016. [Google Scholar]
- Jung, K.B.; Marburger, J.; Sharifi, F.; Park, Y.D.; Pearton, S.J. Long term stability of dry etched magnetoresistive random access memory elements. J. Vac. Sci. Technol. A Vac. Surf. Films 2000, 18, 268–272. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhou, N.; Wang, G.; Zhang, Q.; Du, A.; Zhang, Y.; Gao, J.; Kong, Z.; Lin, H.; et al. A novel dry selective isotropic atomic layer etching of SiGe for manufacturing vertical nanowire array with diameter less than 20 nm. Materials 2020, 13, 771. [Google Scholar] [CrossRef] [Green Version]
- Abdulagatov, A.I.; Sharma, V.; Murdzek, J.A.; Cavanagh, A.S.; George, S.M. Thermal atomic layer etching of germanium-rich SiGe using an oxidation and “conversion-etch” mechanism. J. Vac. Sci. Technol. A 2021, 39, 022602. [Google Scholar] [CrossRef]
- Ikeda, S.; Miura, K.; Yamamoto, H.; Mizunuma, K.; Gan, H.D.; Endo, M.; Kanai, S.; Hayakawa, J.; Matsukura, F.; Ohno, H. A perpendicular-anisotropy CoFeB-MgO magnetic tunnel junction. Nat. Mater. 2010, 9, 721–724. [Google Scholar] [CrossRef]
- Sugiura, K.; Takahashi, S.; Amano, M.; Kajiyama, T.; Iwayama, M.; Asao, Y.; Shimomura, N.; Kishi, T.; Ikegawa, S.; Yoda, H. Ion Beam Etching Technology for High-Density Spin Transfer Torque Magnetic Random Access Memory. Jpn. J. Appl. Phys. 2009, 48, 08HD02. [Google Scholar] [CrossRef]
- Altieri, N.D.; Chen, K.C.; Chang, J.P. Controlling surface chemical states for selective patterning of CoFeB. J. Vac. Sci. Technol. A Vac. Surf. Films 2019, 37, 011303. [Google Scholar] [CrossRef]
- Konh, M.; Wang, Y.; Chen, H.; Bhatt, S.; Xiao, J.Q.; Teplyakov, A.V. Selectivity in atomically precise etching: Thermal atomic layer etching of a CoFeB alloy and its protection by MgO. Appl. Surf. Sci. 2022, 575, 151751. [Google Scholar] [CrossRef]
- Kim, K.S.; Ji, Y.J.; Nam, Y.; Kim, K.H.; Singh, E.; Lee, J.Y.; Yeom, G.Y. Atomic layer etching of graphene through controlled ion beam for graphene-based electronics. Sci. Rep. 2017, 7, 2462. [Google Scholar] [CrossRef]
- Fischer, A.; Routzahn, A.; George, S.M.; Lill, T. Thermal atomic layer etching: A review. J. Vac. Sci. Technol. A 2021, 39, 030801. [Google Scholar] [CrossRef]
- Xiao, S.; Xiao, P.; Zhang, X.; Yan, D.; Gu, X.; Qin, F.; Ni, Z.; Han, Z.J.; Ostrikov, K.K. Atomic-layer soft plasma etching of MoS2. Sci. Rep. 2016, 6, 19945. [Google Scholar] [CrossRef] [Green Version]
- Sha, Y.; Xiao, S.; Zhang, X.; Qin, F.; Gu, X. Layer-by-layer thinning of MoSe2 by soft and reactive plasma etching. Appl. Surf. Sci. 2017, 411, 182–188. [Google Scholar] [CrossRef]
- Faraz, T.; Roozeboom, F.; Knoops, H.C.M.; Kessels, W.M.M. Atomic Layer Etching: What Can We Learn from Atomic Layer Deposition? ECS J. Solid State Sci. Technol. 2015, 4, N5023–N5032. [Google Scholar] [CrossRef] [Green Version]
- Coburn, J.W.; Winters, H.F. Ion- and electron-assisted gas-surface chemistry—An important effect in plasma etching. J. Appl. Phys. 1979, 50, 3189–3196. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Rojas, D.; Nguyen, V.H.; de la Huerta, C.M.; Jiménez, C.; Bellet, D. Spatial atomic layer deposition. In Chemical Vapor Deposition for Nanotechnology; IntechOpen Limited: London, UK, 2019; pp. 1–25. [Google Scholar]
- Nonobe, S.; Takahashi, N.; Nakamura, T. Preparation of HfO2 nano-films by atomic layer deposition using HfCl4 and O2 under atmospheric pressure. Solid State Sci. 2004, 6, 1217–1219. [Google Scholar] [CrossRef]
- Sharma, K.; Hall, R.A.; George, S.M. Spatial atomic layer deposition on flexible substrates using a modular rotating cylinder reactor. J. Vac. Sci. Technol. A Vac. Surf. Films 2015, 33, 01A132. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.H.; Freeman, D.; Nelson, S.F.; Cowdery-Corvan, P.J.; Irving, L.M. Stable ZnO thin film transistors by fast open air atomic layer deposition. Appl. Phys. Lett. 2008, 92, 2006–2009. [Google Scholar] [CrossRef]
- Ryan Fitzpatrick, P.; Gibbs, Z.M.; George, S.M. Evaluating operating conditions for continuous atmospheric atomic layer deposition using a multiple slit gas source head. J. Vac. Sci. Technol. A Vac. Surf. Films 2012, 30, 01A136. [Google Scholar] [CrossRef]
- van Ommen, J.R.; Kooijman, D.; de Niet, M.; Talebi, M.; Goulas, A. Continuous production of nanostructured particles using spatial atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Films 2015, 33, 021513. [Google Scholar] [CrossRef] [Green Version]
- Poodt, P.; Mameli, A.; Schulpen, J.; Kessels, W.M.M.E.; Roozeboom, F. Effect of reactor pressure on the conformal coating inside porous substrates by atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 021502. [Google Scholar] [CrossRef]
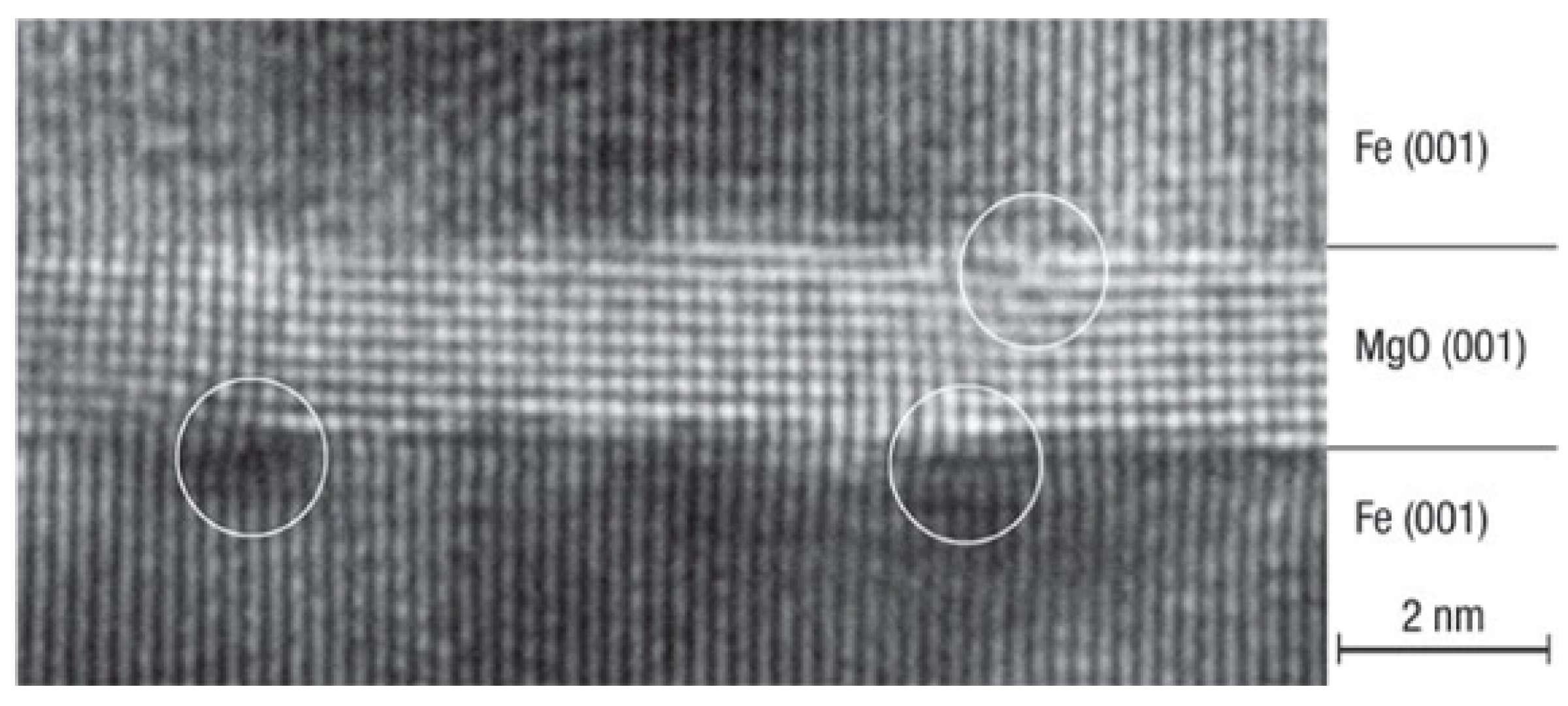
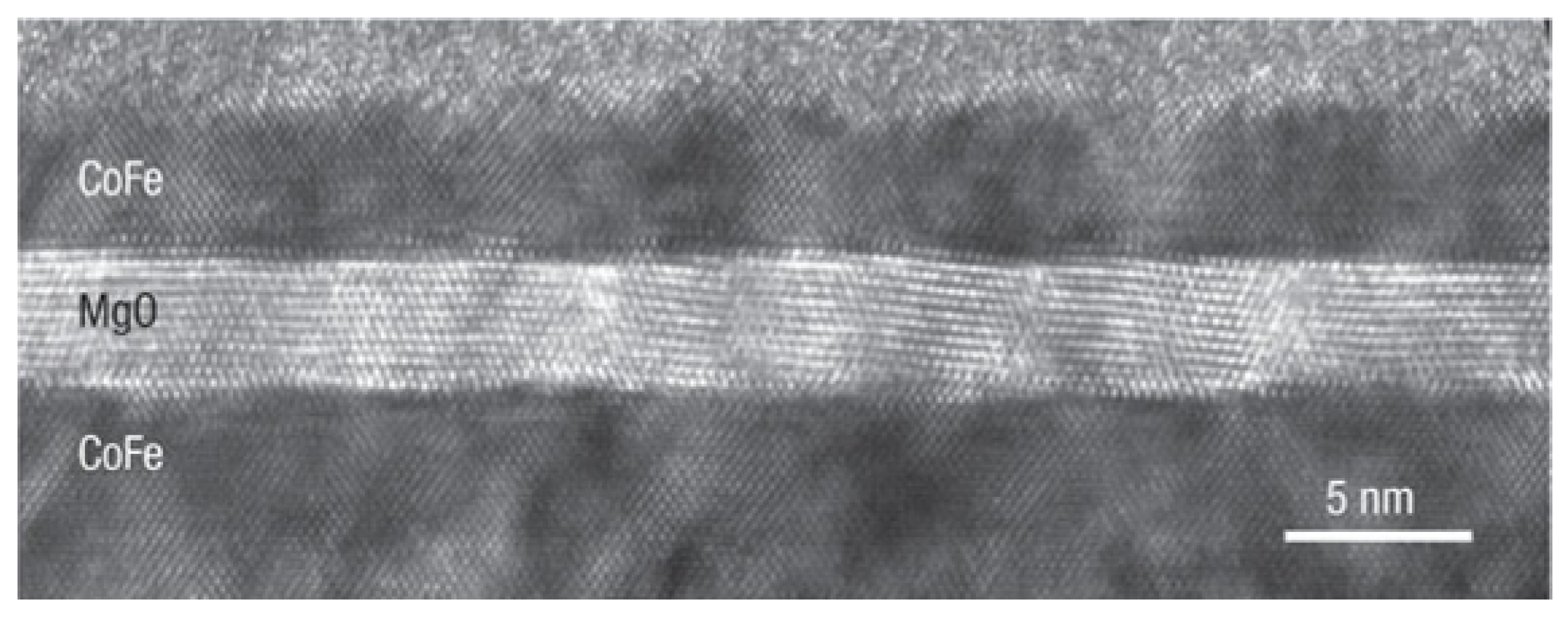
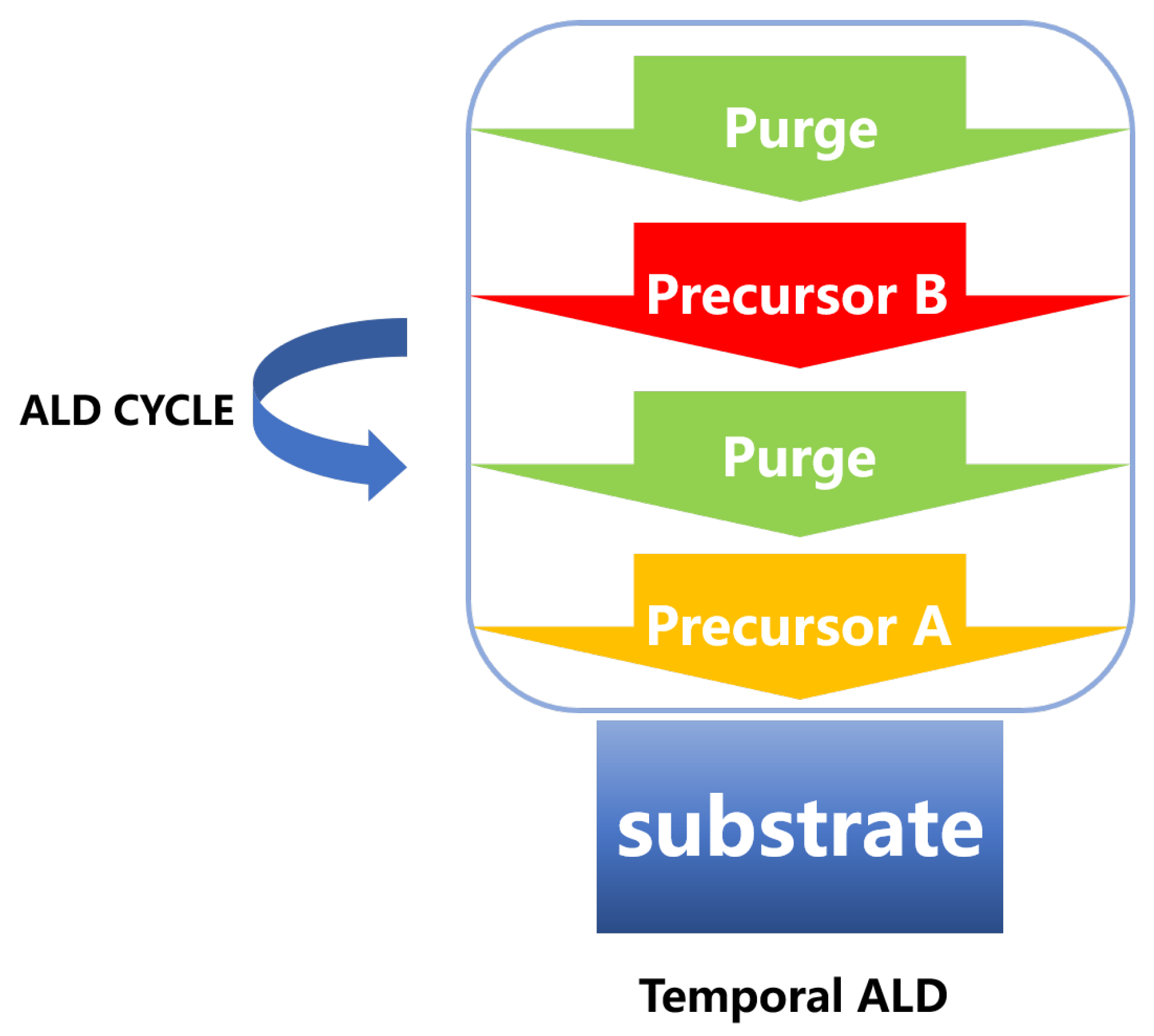
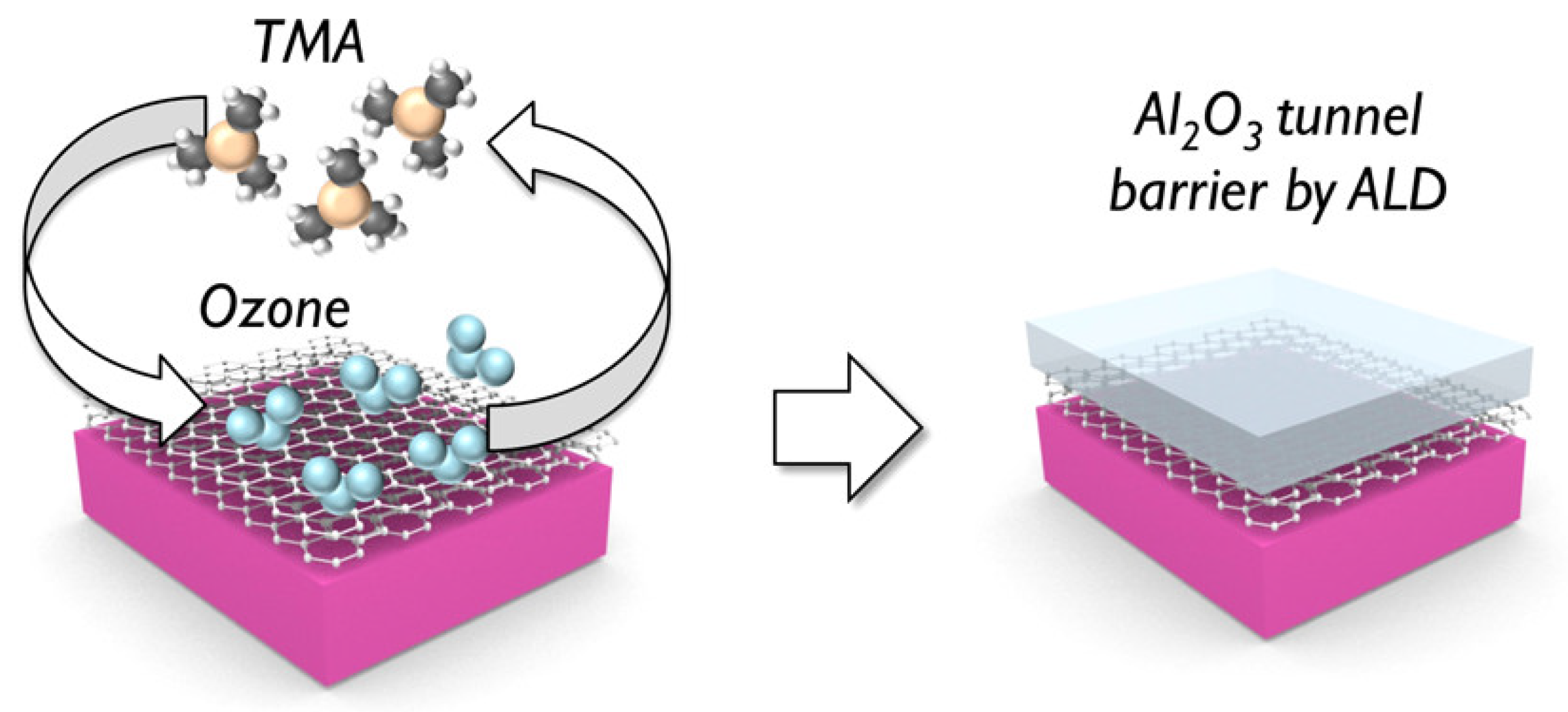
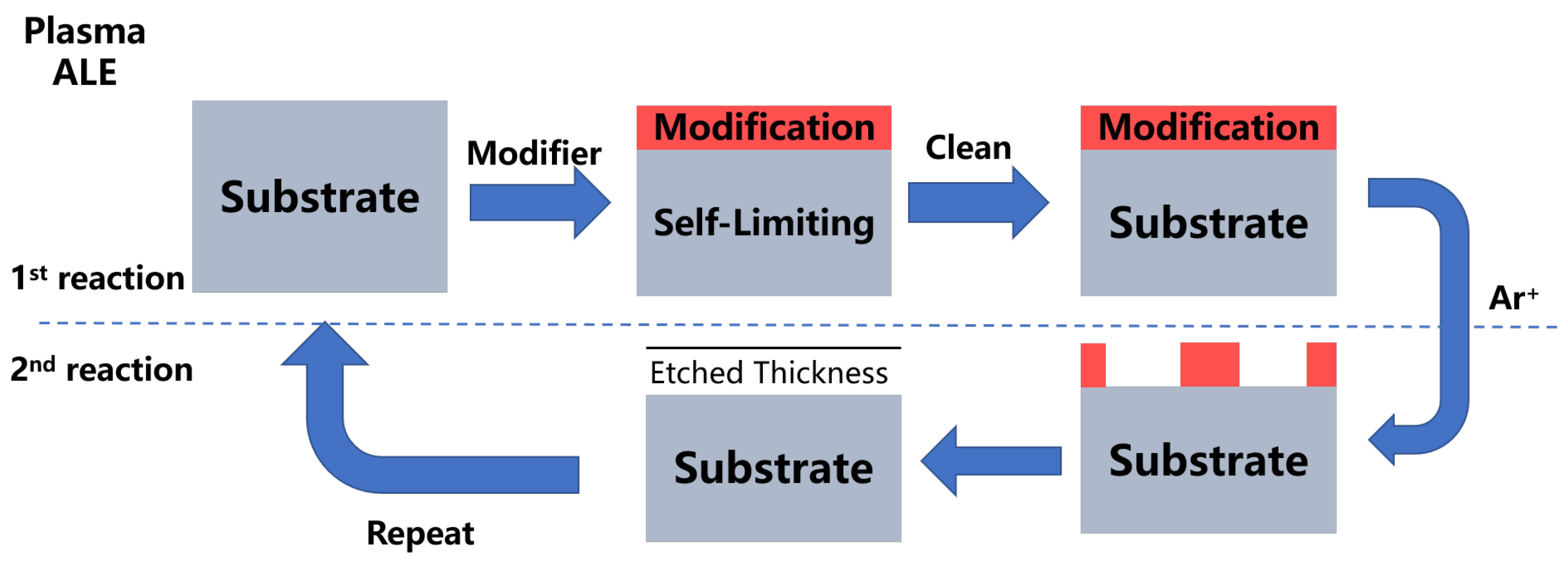
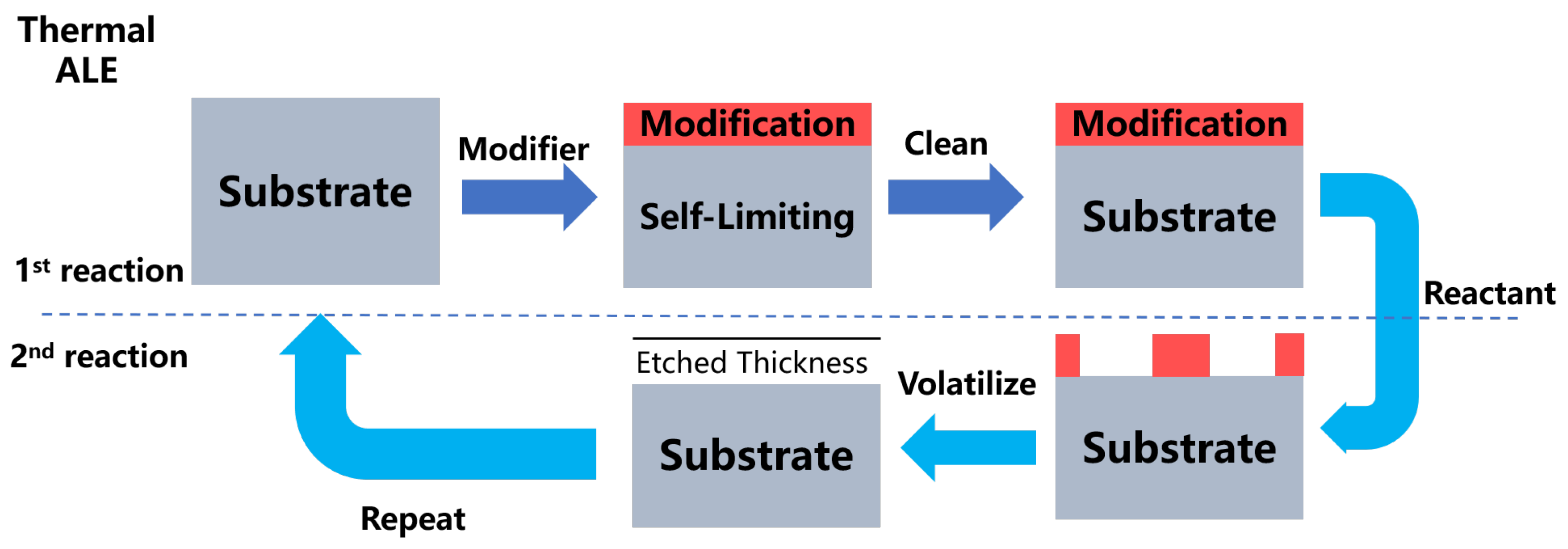
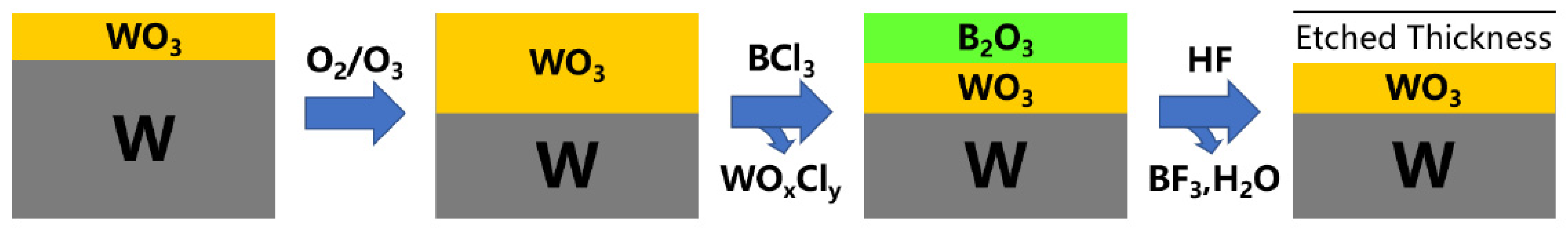
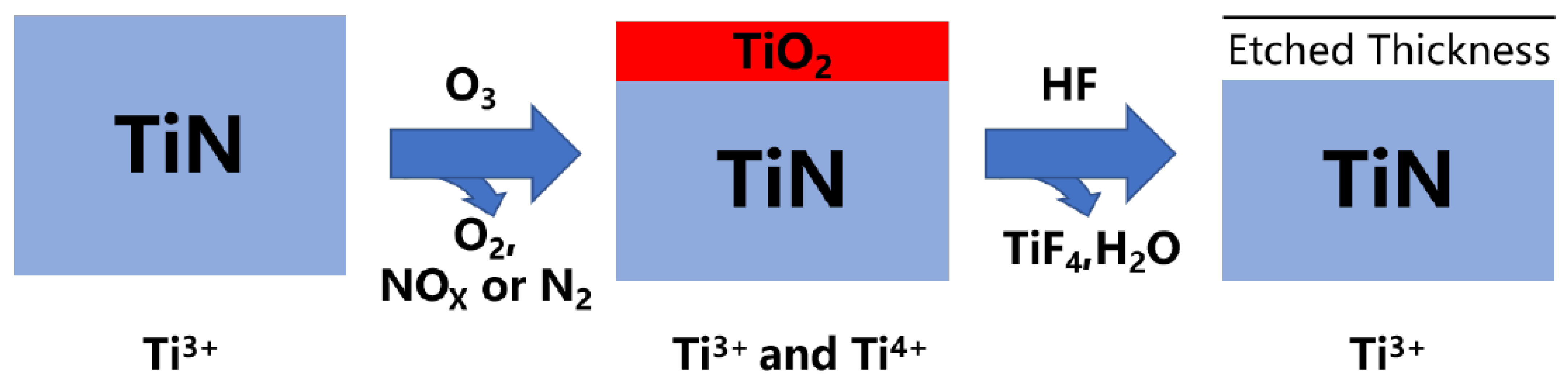

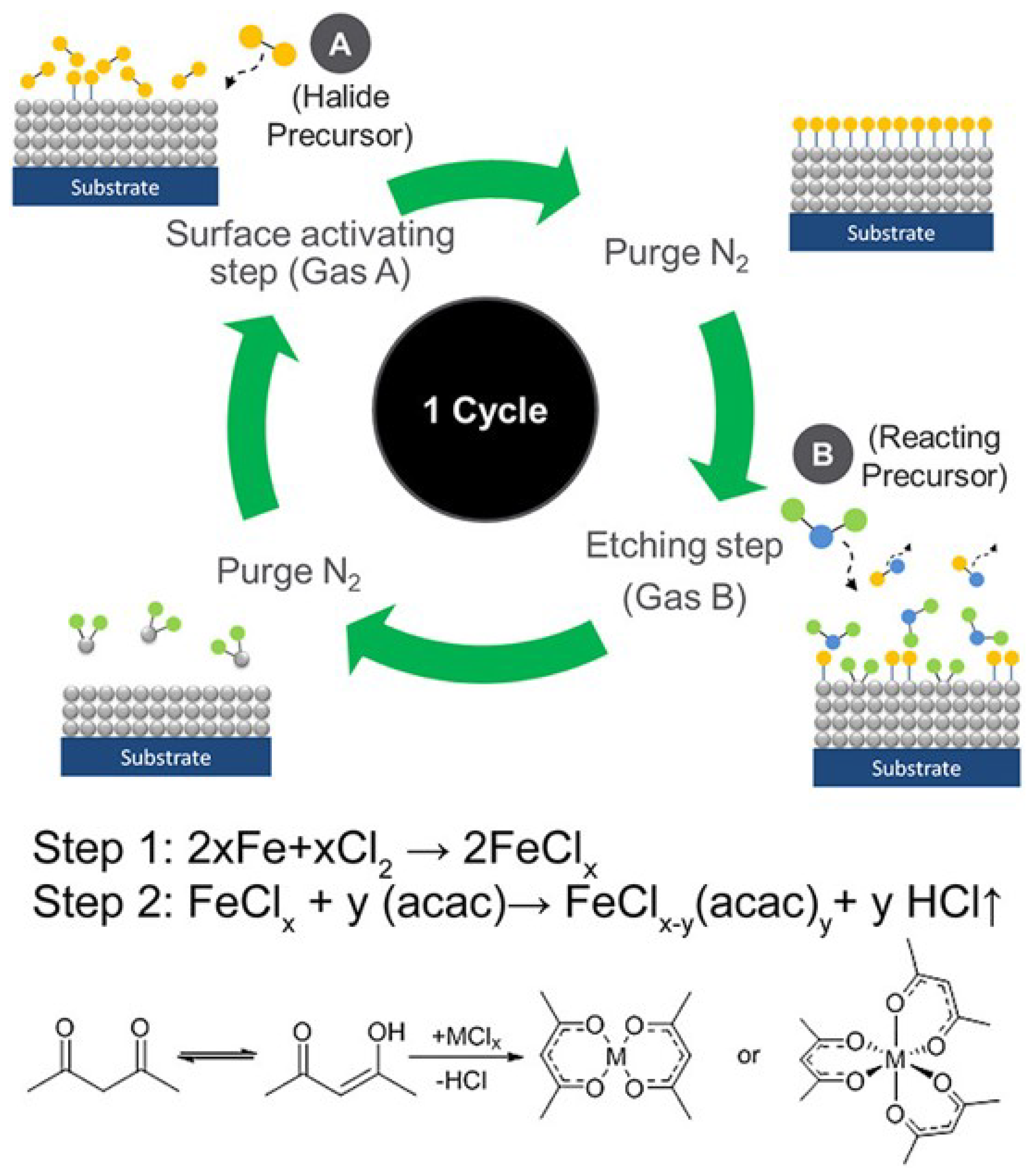
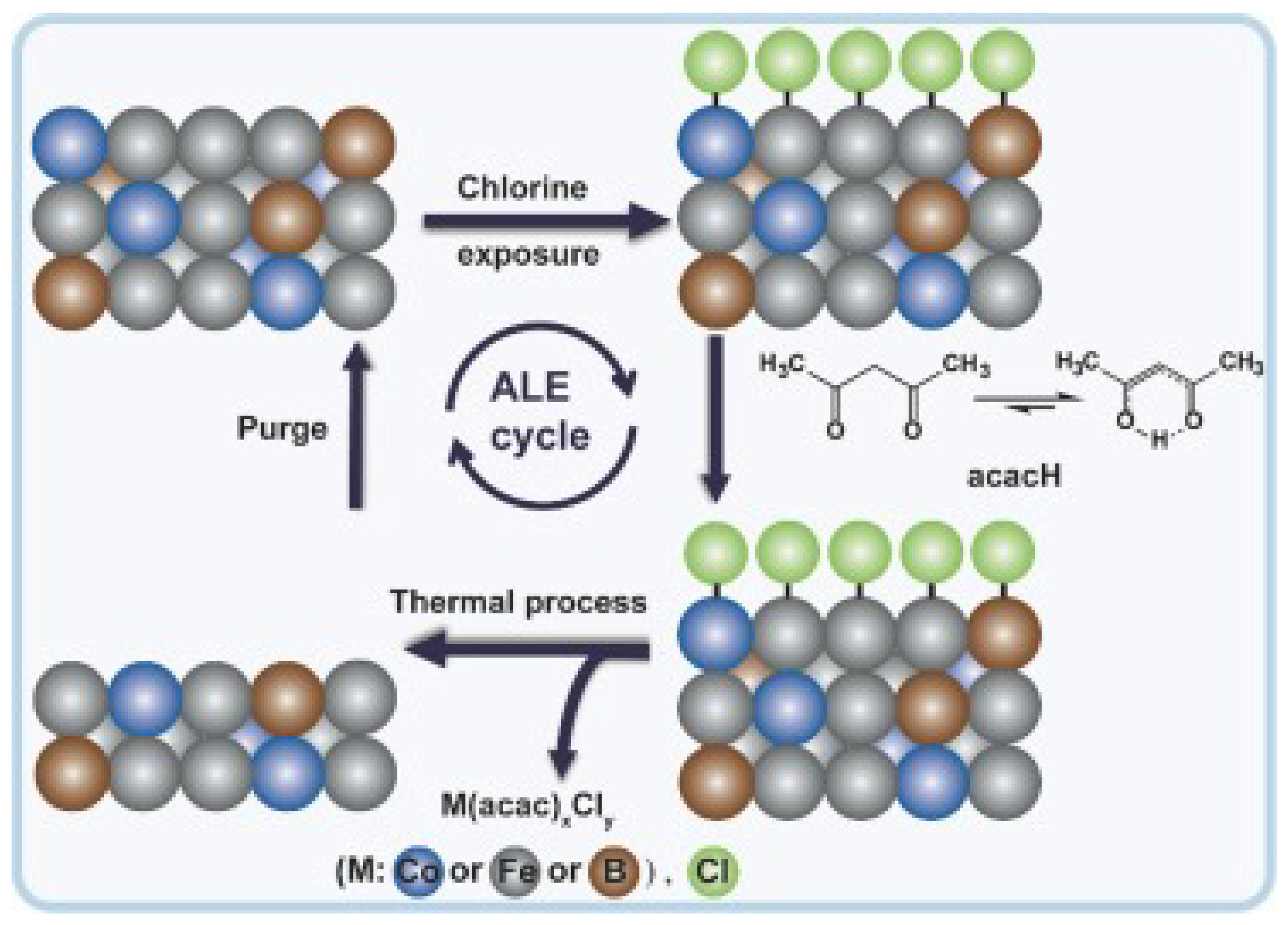
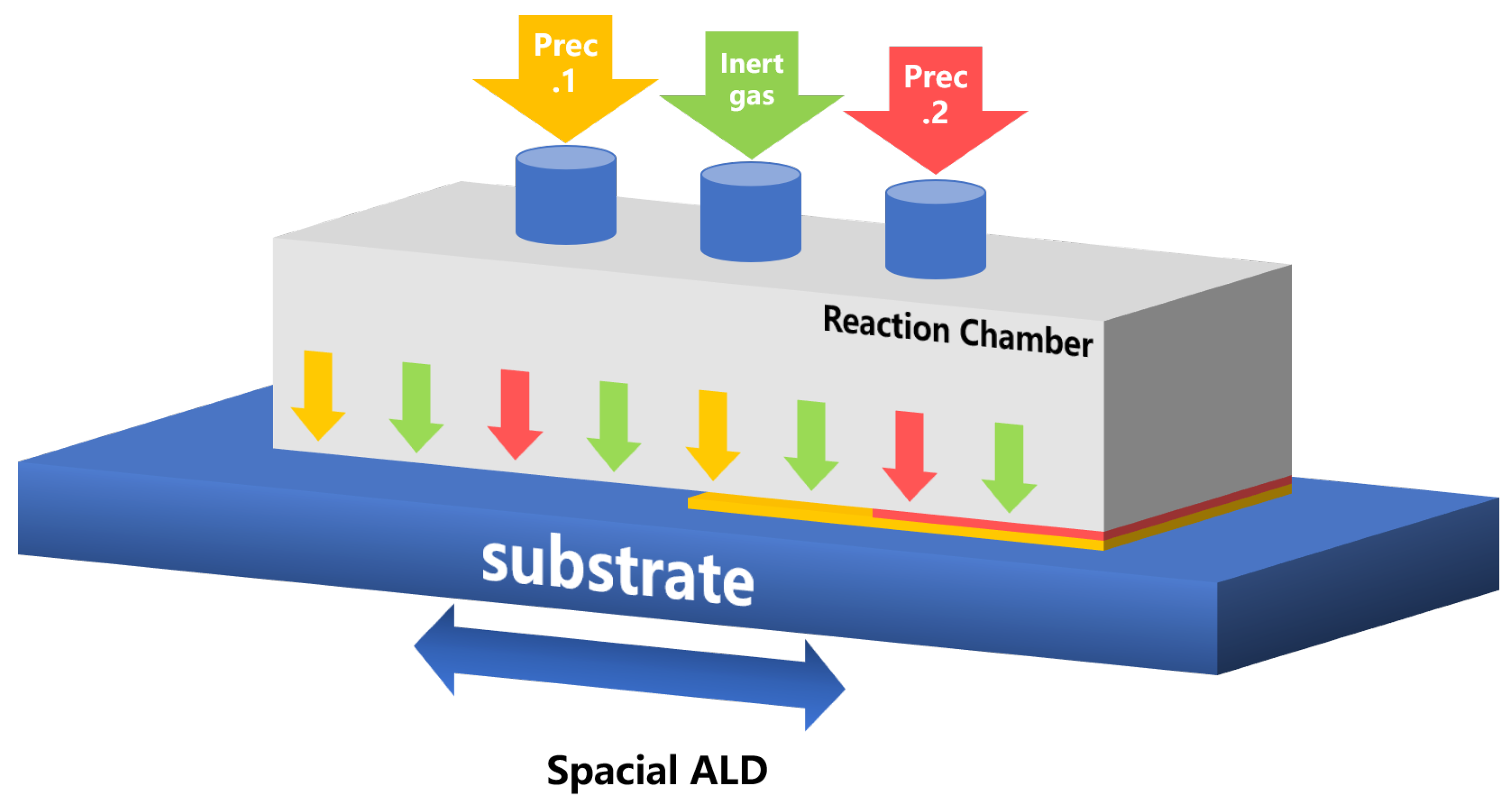
| Materials Deposited by ALD | Reactants of the ALD Process | Substrate | Application | Reference |
|---|---|---|---|---|
| Silica glass | Gas separation | [45] | ||
| AAO | Microfluidics | [66] | ||
| Ni, Co | , | AAO | Sensing | [47] |
| Al | TMA | MOFs | Catalysis, separation | [67] |
| Pt | MOF MIL-101 | Catalysis/Direct TEM imaging | [68,69] | |
| Natural collagen membranes | Improved mechanical properties | [70] | ||
| Natural collagen membranes | Improved mechanical properties | [70] | ||
| ZnO | Natural collagen membranes | Improved mechanical properties | [70] | |
| CoS | MOFs | Catalysis | [71] | |
| Graphene | plasma | Cu | High electron mobility, flexibility high optical transparency superior mechanical strength | [49] |
| BN | Silica | Wide band gap | [72] | |
| BN | high stability | [73,74] | ||
| BN | Sapphire or Si | excellent thermal conductivity | [75] | |
| Electronic and optical devices | [76] | |||
| and quartz | energy conversion, energy storage and solid-state lubrication | [77] | ||
| Solid lubricant | [78] | |||
| AAO | Biosensing | [79] | ||
| Porous silver/ Carbonate matrix | [80] | |||
| MF | Water filtration | [81] | ||
| Porous silver/ carbonate matrix | [82] |
| Materials | Etch Type | Surface Adsorption | Surface Removal | EPC (Å/cycle) | Reference |
|---|---|---|---|---|---|
| Thermal ALE | HF | TMA | 0.31 | [115] | |
| Plasma ALE | (50 eV) | 5 (Synergy = 80%) | [116] | ||
| Thermal ALE | HF (60 mTorr) | 4.18 | [114] | ||
| Thermal ALE | HF | 0.02 | [117] | ||
| Thermal ALE | 0.36 | [117] | |||
| Thermal ALE | HF | 0.61 | [118] | ||
| Thermal ALE | 1.4 | [119] | |||
| Thermal ALE | HF | TMA | 0.14/0.75 | [120] | |
| Thermal ALE | HF | 0.117 | [111] | ||
| Thermal ALE | HF | 0.77 | [121] | ||
| Thermal ALE | HF | 0.14 | [121] | ||
| Thermal ALE | HF | 0.117 | [121] | ||
| ZnO | Thermal ALE | HF | TMA | 2.19 | [122] |
| Thermal ALE | 0.6∼0.7 | [112] | |||
| Thermal ALE | HF | 4.19 | [114] | ||
| Thermal ALE | HF | 0.59–1.35 | [123] | ||
| Thermal ALE | HF | 1.2 | [123] | ||
| Thermal ALE | HF | 0.82 | [123] | ||
| Thermal ALE | HF | 0.85 | [123] | ||
| Thermal ALE | HF | 0.23 | [123] |
| Materials | Etch Type | Surface Adsorption | Surface Removal | EPC (Å/cycle) | Reference |
|---|---|---|---|---|---|
| TiN | Thermal ALE | HF | 0.2 | [110] | |
| Thermal ALE | HF | 0.15 | [110] | ||
| AIN | Thermal ALE | HF | 0.36 | [125] | |
| Thermal ALE | HF | plasma | 1.96 | [125] | |
| Thermal ALE | HF(0.65 Torr) + TMA(1.2 Torr) | 0.25 | [126] | ||
| Thermal ALE | HF(0.65 Torr) + TMA(1.2 Torr) | 0.25 | [126] | ||
| Thermal ALE | HF(0.65 Torr) + TMA(1.2 Torr) | 0.47 | [126] | ||
| Thermal ALE | HF(0.40 Torr) + TMA(1.2 Torr) | 0.38 | [126] | ||
| Thermal ALE | HF(0.65 Torr) + TMA(0.6 Torr) | 0.38 | [126] | ||
| Thermal ALE | HF(0.81 Torr) + TMA(1.2 Torr) | 0.5 | [126] |
| Materials | Etch Type | Surface Adsorption | Surface Removal | EPC (Å/cycle) | Reference |
|---|---|---|---|---|---|
| Si | Plasma ALE | 7 (Synergy = 90%) | [116] | ||
| Thermal ALE | TMA | 0.4 | [133] | ||
| Ge | Plasma ALE | 8 (Synergy = 66%) | [116] | ||
| C | Plasma ALE | (50 eV) | 3.1 (Synergy = 97%) | [116] | |
| Ga | Plasma ALE | Ar+ (70 eV) | 3.3 (Synergy = 91%) | [116] | |
| InP | Plasma ALE | Ne | [134] | ||
| GaAs | Plasma ALE | Ar | [135] | ||
| GaN | Plasma ALE | Ar | [136] | ||
| Thermal ALE | [137] | ||||
| Plasma ALE | 3.3 | [116] | |||
| InGaAs | Plasma ALE | Ar | [138] | ||
| Thermal ALE | HF-pyridine | [139] | |||
| AlGaN | Plasma ALE | Ar | [140] |
| Materials | Etch Type | Surface Adsorption | Surface Removal | EPC (Å/cycle) | Reference |
|---|---|---|---|---|---|
| Cr | Thermal ALE | 1.1 | [142] | ||
| Plasma ALE | Ar | 2 | [142] | ||
| Pd | Thermal ALE | VUV and | Formic acid | 2.81 | [143] |
| Plasma-Thermal ALE | 12 | [144] | |||
| Ta | Thermal ALE | Ethanol | [145] | ||
| Pt | Plasma-Thermal ALE | Formic acid | 5 | [144] | |
| Ethanol | [145] | ||||
| Fe | Thermal ALE | acac | 50 | [146] | |
| Plasma-Thermal ALE | Formic acid | 42 | [144] | ||
| Co | Thermal ALE | hfacH/acac | 16 | [147] | |
| Plasma-Thermal ALE | Formic acid | 28 | [148] | ||
| Plasma ALE | hfacH | 2 | [147] | ||
| W | Plasma ALE | 6.3 | [149] | ||
| Plasma ALE | / | 2.5 | [114] | ||
| Thermal ALE | 2.6 | [150] | |||
| Thermal ALE | Ar | 2.1 | [116] | ||
| Cu | Plasma-Thermal ALE | Formic acid | 37 | [144] | |
| Thermal ALE | / | hfac | 1 | [151] | |
| Thermal ALE | Acetic acid | - | 0.7 | [152] | |
| Ru | Thermal ALE | Ethanol | [145] | ||
| Plasma ALE | 30 s HCOOH (0.50 Torr) | 2.5 min Ar | 0.85 ± 0.15 | [153] | |
| Ru | Thermal ALE | HCOOH | 20 nm/min | [154] | |
| Thermal ALE | hfacH | [155] |
| Materials | Etch Type | Surface Adsorption | Surface Removal | EPC (Å/cycle) | Reference |
|---|---|---|---|---|---|
| Alloy etching | |||||
| Cu alloy | Plasma-Thermal ALE | Formic acid vapor (50 s) | 37 | [148] | |
| Pt alloy | Plasma-Thermal ALE | Formic acid vapor (50 s) | 5 | [148] | |
| Pd alloy | Plasma-Thermal ALE | Formic acid vapor (50 s) | 12 | [148] | |
| Co alloy | Plasma-Thermal ALE | Formic acid vapor (50 s) | 28 | [148] | |
| Fe alloy | Plasma-Thermal ALE | Formic acid vapor (50 s) | 42 | [148] | |
| CoFeB | Plasma ALE | [144] | |||
| Thermal ALE | Formic acid | 18 | [163] | ||
| Thermal ALE | chlorine | acacH | 1.5 | [164] | |
| SiGe | Thermal ALE | O | 2.3 | [159] | |
| Thermal ALE | O/ | HF TMA | 0.57/0.42 | [166] | |
| Two dimensional material etching | |||||
| Graphene | Plasma ALE | 7.3 | [165] | ||
| MoS | Thermal ALE | Cl radical (produced by ICP) | Ar | [165] | |
| Plasma ALE | plasma | 2.8–3.6 nm/min | [167] | ||
| MoSe | Plasma ALE | plasma | 0.18 Å/s | [168] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.; Li, Z.; Hu, S. Recent Progress of Atomic Layer Technology in Spintronics: Mechanism, Materials and Prospects. Nanomaterials 2022, 12, 661. https://doi.org/10.3390/nano12040661
Tsai Y, Li Z, Hu S. Recent Progress of Atomic Layer Technology in Spintronics: Mechanism, Materials and Prospects. Nanomaterials. 2022; 12(4):661. https://doi.org/10.3390/nano12040661
Chicago/Turabian StyleTsai, Yuanlu, Zhiteng Li, and Shaojie Hu. 2022. "Recent Progress of Atomic Layer Technology in Spintronics: Mechanism, Materials and Prospects" Nanomaterials 12, no. 4: 661. https://doi.org/10.3390/nano12040661
APA StyleTsai, Y., Li, Z., & Hu, S. (2022). Recent Progress of Atomic Layer Technology in Spintronics: Mechanism, Materials and Prospects. Nanomaterials, 12(4), 661. https://doi.org/10.3390/nano12040661







