Compact SnO2/Mesoporous TiO2 Bilayer Electron Transport Layer for Perovskite Solar Cells Fabricated at Low Process Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the SnO2 Layer
2.2. Fabrication of the Bilayer ETL
2.3. Fabrication of the PSC
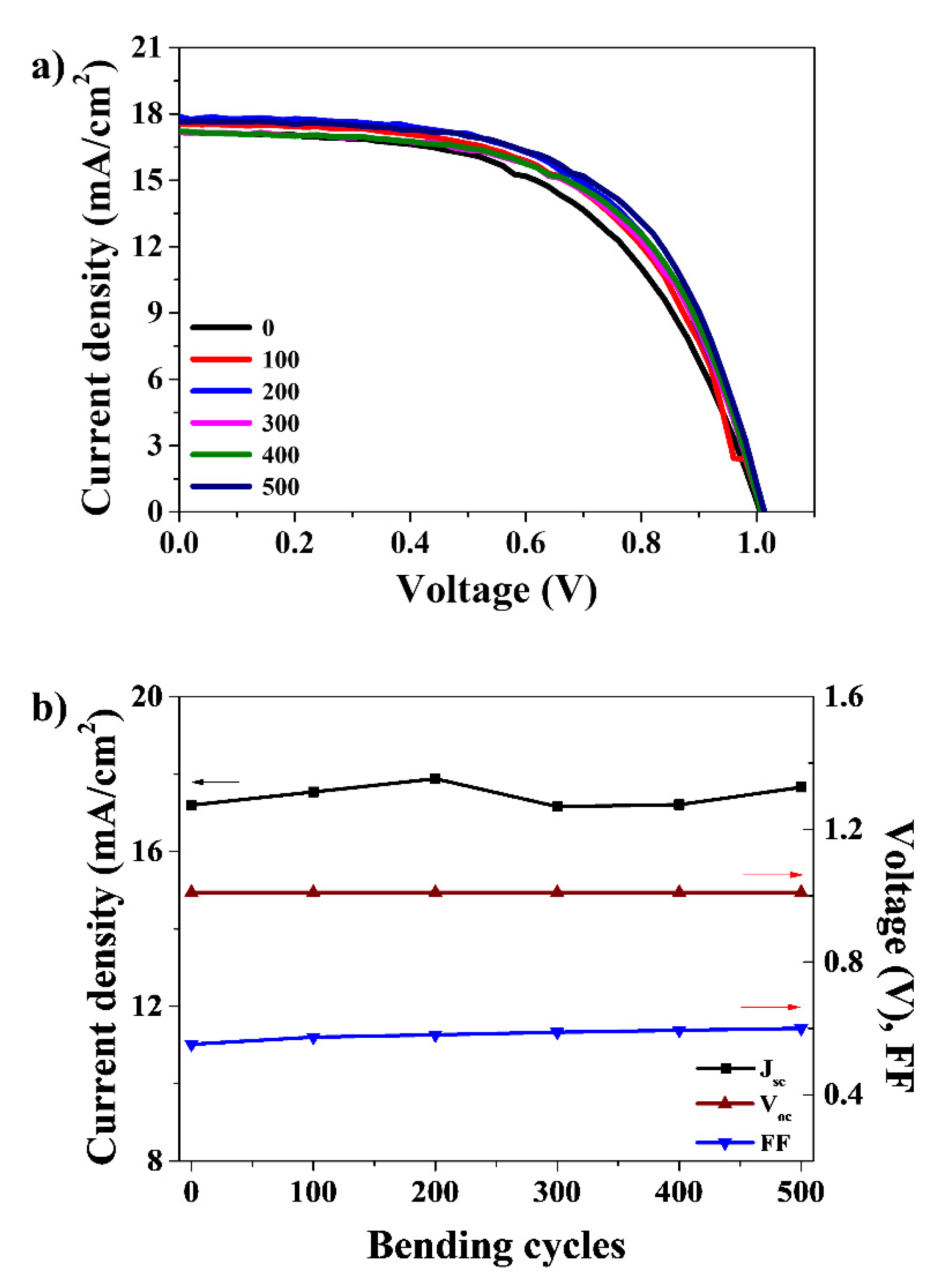
2.4. Fabrication of the Flexible PSC
2.5. Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovoltaics: Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Hu, Y.; Niu, T.; Liu, Y.; Zhou, Y.; Xia, Y.; Ran, C.; Wu, Z.; Song, L.; Müller-Buschbaum, P.; Chen, Y.; et al. Flexible Perovskite Solar Cells with High Power-Per-Weight: Progress, Application, and Perspectives. ACS Energy Lett. 2021, 6, 2917–2943. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, G.-Z.; Zhang, Y.; Yang, N.; Li, Y.; Chen, Q. Development of encapsulation strategies towards the commercialization of perovskite solar cells. Energy Environ. Sci. 2021, 15, 13–55. [Google Scholar] [CrossRef]
- Dai, T.; Cao, Q.; Yang, L.; Aldamasy, M.; Li, M.; Liang, Q.; Lu, H.; Dong, Y.; Yang, Y. Strategies for High-Performance Large-Area Perovskite Solar Cells toward Commercialization. Crystals 2021, 11, 295. [Google Scholar] [CrossRef]
- Cai, L.; Liang, L.; Wu, J.; Ding, B.; Gao, L.; Fan, B. Large area perovskite solar cell module. J. Semicond. 2017, 38, 014006. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Noh, M.F.M.; Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ludin, N.A.; Yusoff, A.R.B.M.; Jang, J.; Teridi, M.A.M. The architecture of the electron transport layer for a perovskite solar cell. J. Mater. Chem. C 2018, 6, 682–712. [Google Scholar] [CrossRef]
- Anaraki, E.H.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Grätzel, M.; Hagfeldt, A.; et al. Highly efficient and stable planar perovskite solar cells by solution-processed tin oxide. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar] [CrossRef]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-Sensitized Solar Cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Il Seok, S.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the Application of SnO2 in Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Nam, J.; Kim, J.-H.; Kim, C.S.; Kwon, J.-D.; Jo, S. Surface Engineering of Low-Temperature Processed Mesoporous TiO2 via Oxygen Plasma for Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 12648–12655. [Google Scholar] [CrossRef]
- Song, J.; Zheng, E.; Wang, X.-F.; Tian, W.; Miyasaka, T. Low-temperature-processed ZnO–SnO2 nanocomposite for efficient planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Yang, G.; Qin, M.; Zheng, X.; Lei, H.; Chen, C.; Chen, Z.; Guo, Y.; Han, H.; Zhao, X.; et al. MgO Nanoparticle Modified Anode for Highly Efficient SnO2 -Based Planar Perovskite Solar Cells. Adv. Sci. 2017, 4, 1700031. [Google Scholar] [CrossRef]
- Han, G.S.; Chung, H.S.; Kim, D.H.; Kim, B.J.; Lee, J.-W.; Park, N.-G.; Cho, I.S.; Lee, J.-K.; Lee, S.; Jung, H.S. Epitaxial 1D electron transport layers for high-performance perovskite solar cells. Nanoscale 2015, 7, 15284–15290. [Google Scholar] [CrossRef]
- Kogo, A.; Ikegami, M.; Miyasaka, T. A SnOx–brookite TiO2 bilayer electron collector for hysteresis-less high efficiency plastic perovskite solar cells fabricated at low process temperature. Chem. Commun. 2016, 52, 8119–8122. [Google Scholar] [CrossRef]
- Tang, H.; Cao, Q.; He, Z.; Wang, S.; Han, J.; Li, T.; Gao, B.; Yang, J.; Deng, D.; Li, X. SnO2–Carbon Nanotubes Hybrid Electron Transport Layer for Efficient and Hysteresis-Free Planar Perovskite Solar Cells. Sol. RRL 2020, 4, 1900415. [Google Scholar] [CrossRef]
- Yang, G.; Wang, C.; Lei, H.; Zheng, X.; Qin, P.; Xiong, L.; Zhao, X.; Yan, Y.; Fang, G. Interface engineering in planar perovskite solar cells: Energy level alignment, perovskite morphology control and high performance achievement. J. Mater. Chem. A 2017, 5, 1658–1666. [Google Scholar] [CrossRef]
- Xiong, Z.; Lan, L.; Wang, Y.; Lu, C.; Qin, S.; Chen, S.; Zhou, L.; Zhu, C.; Li, S.; Meng, L.; et al. Multifunctional Polymer Framework Modified SnO2 Enabling a Photostable α-FAPbI3 Perovskite Solar Cell with Efficiency Exceeding 23%. ACS Energy Lett. 2021, 6, 3824–3830. [Google Scholar] [CrossRef]
- Kogo, A.; Sanehira, Y.; Numata, Y.; Ikegami, M.; Miyasaka, T. Amorphous Metal Oxide Blocking Layers for Highly Efficient Low-Temperature Brookite TiO2-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 2224–2229. [Google Scholar] [CrossRef]
- Jung, K.-H.; Seo, J.-Y.; Lee, S.; Shin, H.; Park, N.-G. Solution-processed SnO2thin film for a hysteresis-free planar perovskite solar cell with a power conversion efficiency of 19.2%. J. Mater. Chem. A 2017, 5, 24790–24803. [Google Scholar] [CrossRef]
- Nam, J.; Nam, I.; Song, E.-J.; Kwon, J.-D.; Kim, J.; Kim, C.S.; Jo, S. Facile Interfacial Engineering of Mesoporous TiO2 for Low-Temperature Processed Perovskite Solar Cells. Nanomaterials 2019, 9, 1220. [Google Scholar] [CrossRef] [Green Version]
- Parida, B.; Singh, A.; Oh, M.; Jeon, M.; Kang, J.-W.; Kim, H. Effect of compact TiO2 layer on structural, optical, and performance characteristics of mesoporous perovskite solar cells. Mater. Today Commun. 2019, 18, 176–183. [Google Scholar] [CrossRef]
- Bu, T.; Wu, L.; Liu, X.; Yang, X.; Zhou, P.; Yu, X.; Qin, T.; Shi, J.; Wang, S.; Li, S.; et al. Synergic Interface Optimization with Green Solvent Engineering in Mixed Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700576. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Guo, J.; Wu, X.; Zhang, W.; Wu, S.; Gao, X.; Hu, X.; Wang, Q.; Zhou, G.; et al. Solvent-Assisted Low-Temperature Crystallization of SnO2 Electron-Transfer Layer for High-Efficiency Planar Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900557. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.-K.; Yang, Y.-G.; Hu, Y.; Feng, S.-L.; Wang, J.-M.; Gao, X.-Y.; Liao, L.-S. Copper Salts Doped Spiro-OMeTAD for High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1601156. [Google Scholar] [CrossRef]
- Hidayat, R.; Nurunnizar, A.A.; Fariz, A.; Herman; Rosa, E.S.; Shobih; Oizumi, T.; Fujii, A.; Ozaki, M. Revealing the charge carrier kinetics in perovskite solar cells affected by mesoscopic structures and defect states from simple transient photovoltage measurements. Sci. Rep. 2020, 10, 19197. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Peng, Y.; Gao, Y.; Shi, J.; Li, H.; Mo, X.; Huang, H.; Gao, Y.; Ding, L.; Yang, J. High-Performance Flexible Perovskite Solar Cells via Precise Control of Electron Transport Layer. Adv. Energy Mater. 2019, 9, 1901419. [Google Scholar] [CrossRef]
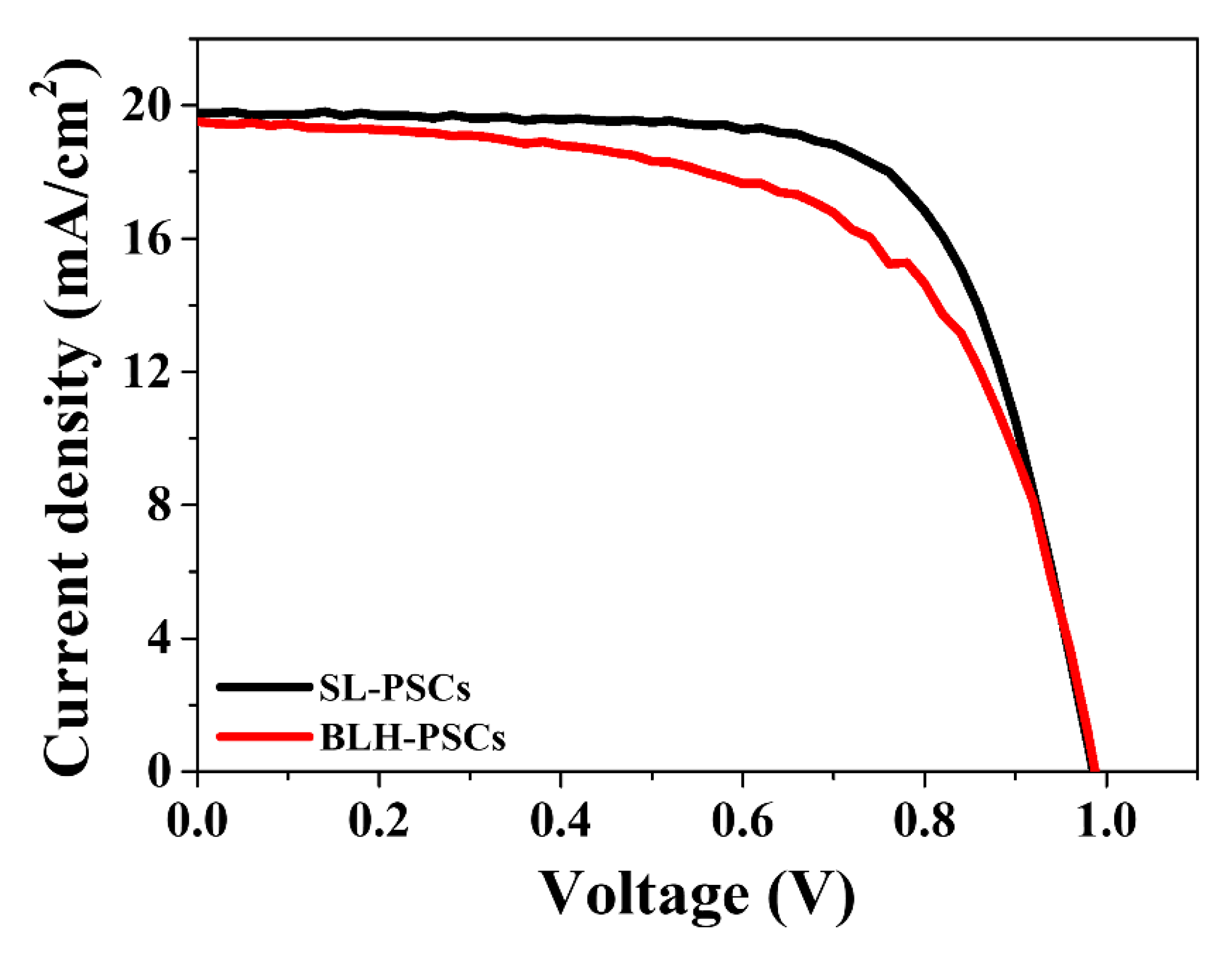
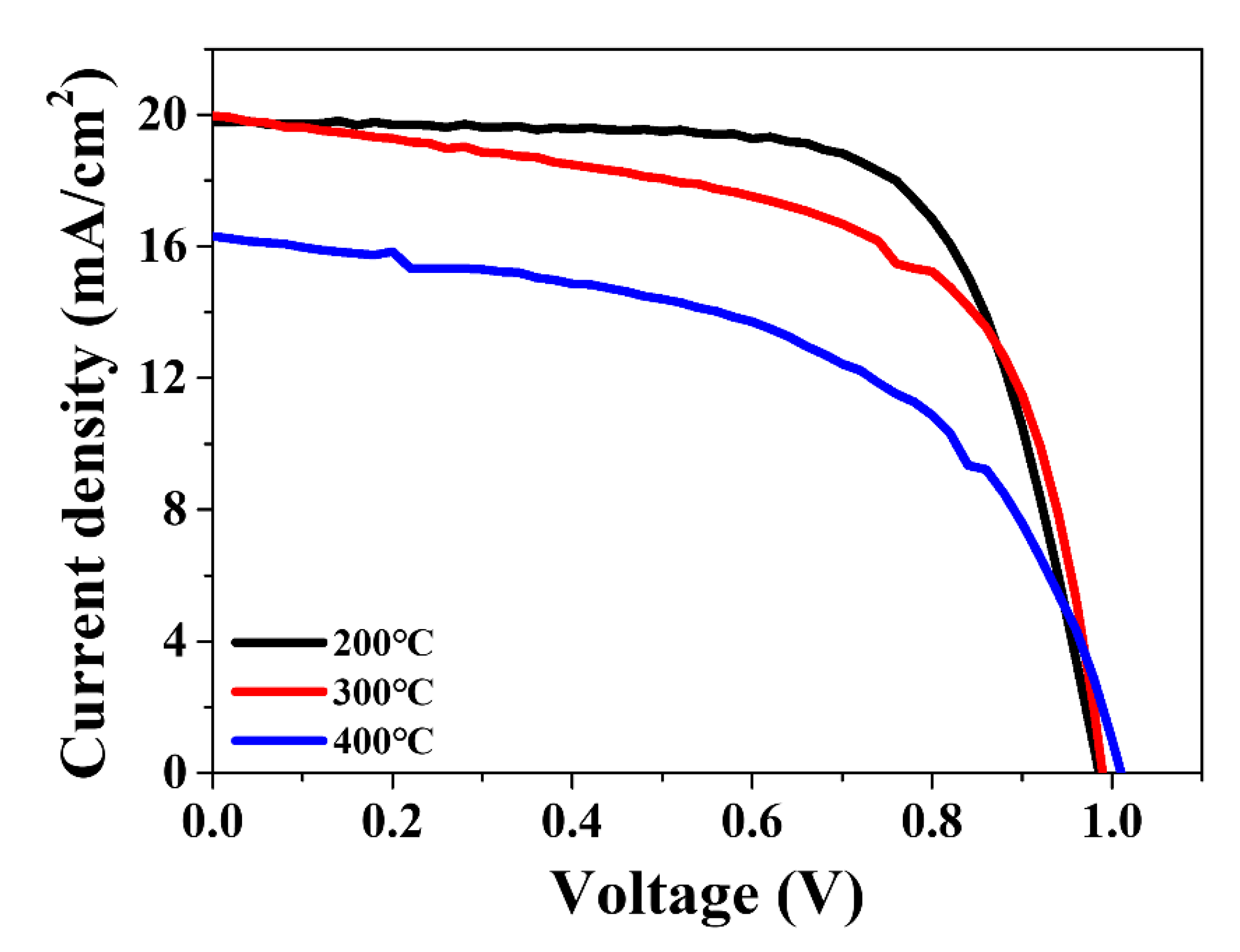

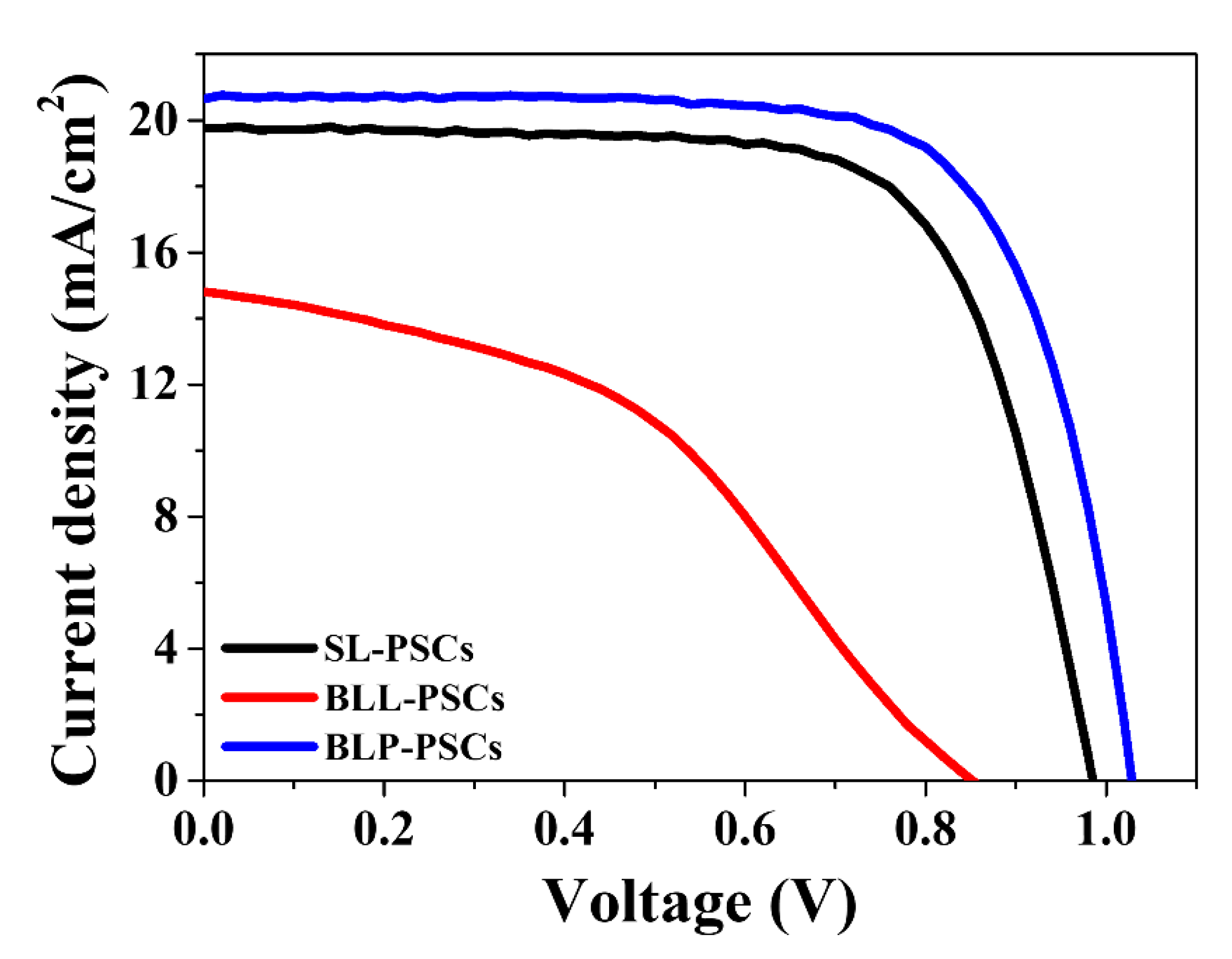
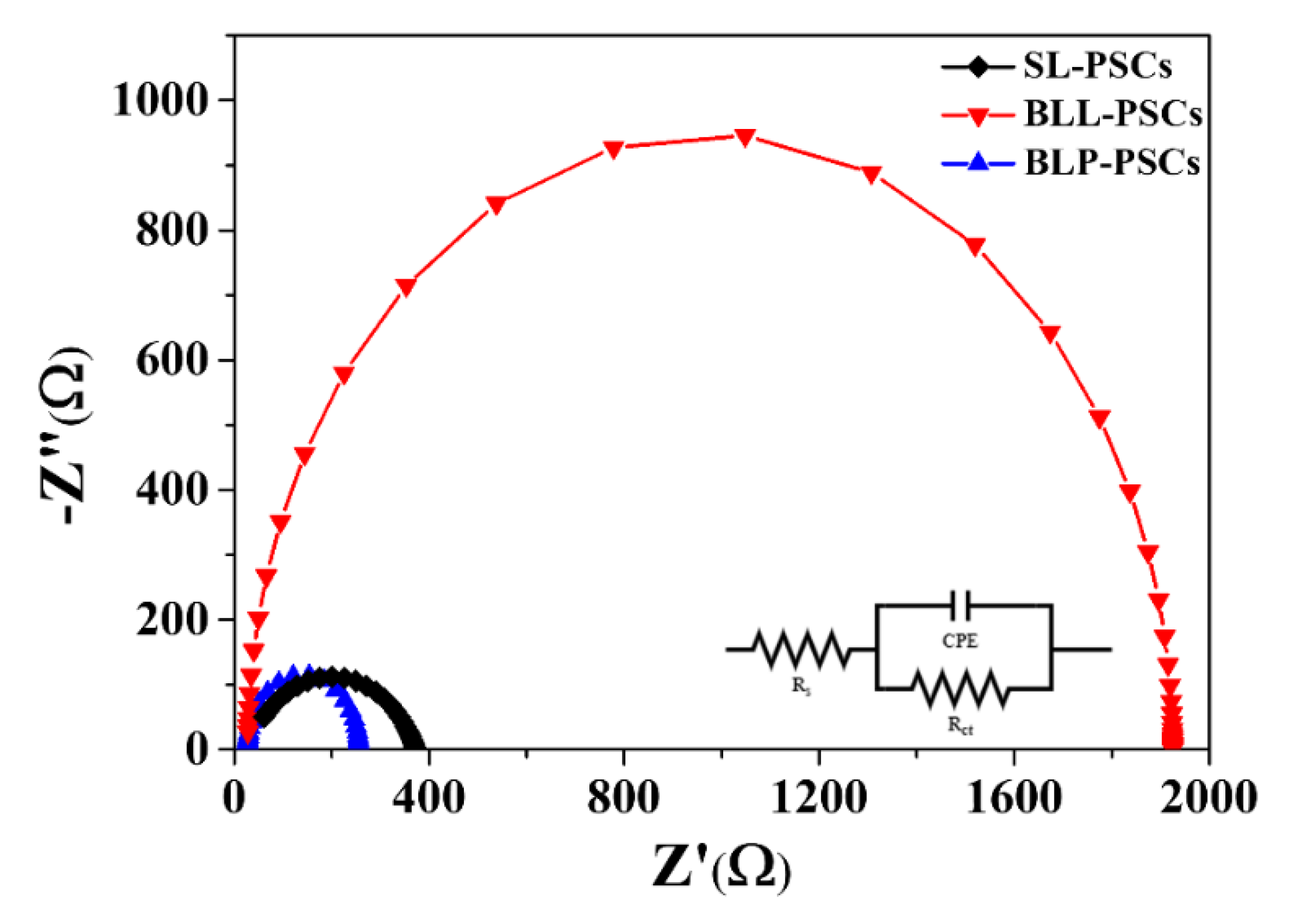
| Sample | JSC (mA/cm2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| SL-PSCs | 19.77 | 0.99 | 70.22 | 13.68 |
| BLL-PSCs | 14.81 | 0.85 | 43.01 | 5.43 |
| BLP-PSCs | 20.65 | 1.03 | 72.30 | 15.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, J.; Kim, C.-S.; Jo, S. Compact SnO2/Mesoporous TiO2 Bilayer Electron Transport Layer for Perovskite Solar Cells Fabricated at Low Process Temperature. Nanomaterials 2022, 12, 718. https://doi.org/10.3390/nano12040718
Lee J, Kim J, Kim C-S, Jo S. Compact SnO2/Mesoporous TiO2 Bilayer Electron Transport Layer for Perovskite Solar Cells Fabricated at Low Process Temperature. Nanomaterials. 2022; 12(4):718. https://doi.org/10.3390/nano12040718
Chicago/Turabian StyleLee, Junyeong, Jongbok Kim, Chang-Su Kim, and Sungjin Jo. 2022. "Compact SnO2/Mesoporous TiO2 Bilayer Electron Transport Layer for Perovskite Solar Cells Fabricated at Low Process Temperature" Nanomaterials 12, no. 4: 718. https://doi.org/10.3390/nano12040718
APA StyleLee, J., Kim, J., Kim, C.-S., & Jo, S. (2022). Compact SnO2/Mesoporous TiO2 Bilayer Electron Transport Layer for Perovskite Solar Cells Fabricated at Low Process Temperature. Nanomaterials, 12(4), 718. https://doi.org/10.3390/nano12040718







