Impact of Albumin Pre-Coating on Gold Nanoparticles Uptake at Single-Cell Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AuNRs
2.3. Pre-Coating AuNRs with BSA
2.4. Cell Culture
3. Results
3.1. Characterization of AuNRs
3.2. AuNRs Agglomeration and Sedimentation after BSA Pre-Coating
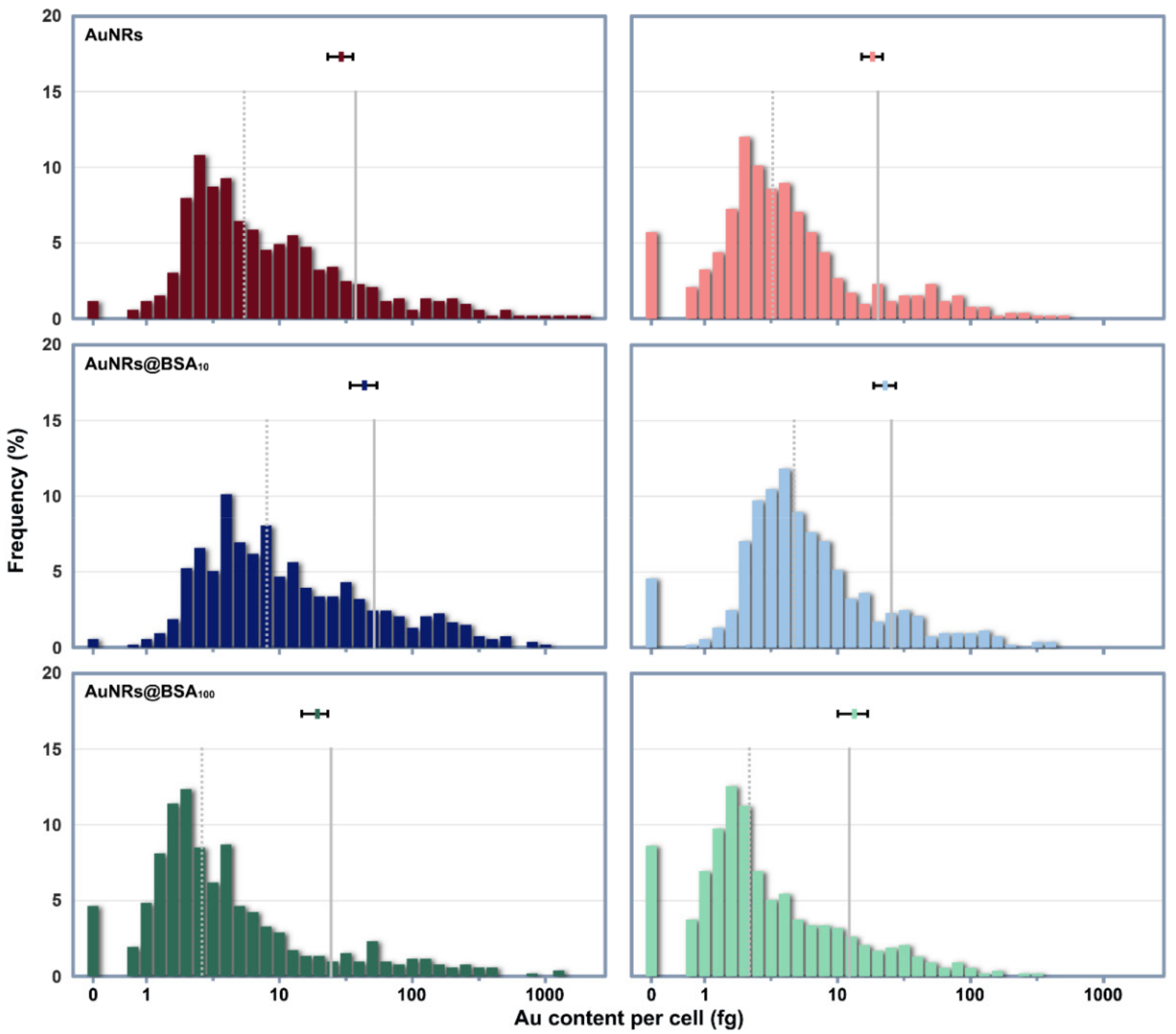
3.3. Cellular Uptake of AuNRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Xuan, Z.; Li, J.; Liu, Q.; Yi, F.; Wang, S.; Lu, W. Artificial Structural Colors and Applications. Innovation 2021, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Su, W.; Tan, M. Endogenous Fluorescence Carbon Dots Derived from Food Items. Innovation 2020, 1, 100009. [Google Scholar] [CrossRef]
- Ovais, M.; Guo, M.; Chen, C. Tailoring Nanomaterials for Targeting Tumor-Associated Macrophages. Adv. Mater. 2019, 31, 1808303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tang, Y.; Suo, X.; Zhang, C.; Dou, Y.; Chang, J. A dual-targeted multifunctional nanoformulation for potential prevention and therapy of Alzheimer’s disease. Innovation 2021, 2, 2. [Google Scholar] [CrossRef]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A Decade of the Protein Corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2019, 31, 1805740. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, J.-Q.; Ashby, C.R.; Zeng, L.; Fan, Y.-F.; Chen, Z.-S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef]
- Zafar, M.; Ijaz, M.; Iqbal, T. Efficient Au nanostructures for NIR-responsive controlled drug delivery systems. Chem. Pap. 2021, 75, 2277–2293. [Google Scholar] [CrossRef]
- Perera, G.S.; Yang, G.; Nettles, C.B.; Perez, F.; Hollis, T.K.; Zhang, D. Counterion Effects on Electrolyte Interactions with Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 23604–23612. [Google Scholar] [CrossRef]
- Johnston, B.D.; Kreyling, W.G.; Pfeiffer, C.; Schäffler, M.; Sarioglu, H.; Ristig, S.; Hirn, S.; Haberl, N.; Thalhammer, S.; Hauck, S.M.; et al. Colloidal Stability and Surface Chemistry Are Key Factors for the Composition of the Protein Corona of Inorganic Gold Nanoparticles. Adv. Funct. Mater. 2017, 27, 1701956. [Google Scholar] [CrossRef]
- Carl, N.; Prévost, S.; Fitzgerald, J.P.S.; Karg, M. Salt-induced cluster formation of gold nanoparticles followed by stopped-flow SAXS, DLS and extinction spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 16348–16357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Prince, E.; Narayanan, P.; Liu, K.; Nie, Z.; Kumacheva, E. Colloidal stability of nanoparticles stabilized with mixed ligands in solvents with varying polarity. Chem. Commun. 2020, 56, 8131–8134. [Google Scholar] [CrossRef]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic Light Scattering as a Powerful Tool for Gold Nanoparticle Bioconjugation and Biomolecular Binding Studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef]
- Nandakumar, A.; Wei, W.; Siddiqui, G.; Tang, H.; Li, Y.; Kakinen, A.; Wan, X.; Koppel, K.; Lin, S.; Davis, T.P.; et al. Dynamic Protein Corona of Gold Nanoparticles with an Evolving Morphology. ACS Appl. Mater. Interfaces 2021, 13, 58238–58251. [Google Scholar] [CrossRef]
- Kuschnerus, I.; Lau, M.; Giri, K.; Bedford, N.; Biazik, J.; Ruan, J.; Garcia-Bennett, A. Effect of a protein corona on the fibrinogen induced cellular oxidative stress of gold nanoparticles. Nanoscale 2020, 12, 5898–5905. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, J.; Pan, J.; Jiang, X.; Ji, Y.; Li, Y.; Qu, Y.; Zhao, Y.; Wu, X.; Chen, C. Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: Understanding the reduced damage in cell membranes. J. Am. Chem. Soc. 2013, 135, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.P.; Gifford, G.B.; Chen, F.; Benasutti, H.; Wang, G.; Groman, E.V.; Scheinman, R.; Saba, L.; Moghimi, S.M.; Simberg, D. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat. Nanotechnol. 2019, 14, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein Corona of Nanoparticles: Distinct Proteins Regulate the Cellular Uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Mirshafiee, V.; Kim, R.; Park, S.; Mahmoudi, M.; Kraft, M.L. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials 2016, 75, 295–304. [Google Scholar] [CrossRef]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, 1801451. [Google Scholar] [CrossRef]
- Yang, Y.-S.S.; Atukorale, P.U.; Moynihan, K.D.; Bekdemir, A.; Rakhra, K.; Tang, L.; Stellacci, F.; Irvine, D.J. High-throughput quantitation of inorganic nanoparticle biodistribution at the single-cell level using mass cytometry. Nat. Commun. 2017, 8, 14069. [Google Scholar] [CrossRef]
- Malysheva, A.; Ivask, A.; Doolette, C.L.; Voelcker, N.H.; Lombi, E. Cellular binding, uptake and biotransformation of silver nanoparticles in human T lymphocytes. Nat. Nanotechnol. 2021, 16, 926–932. [Google Scholar] [CrossRef]
- Rashkow, J.T.; Patel, S.C.; Tappero, R.; Sitharaman, B. Quantification of single-cell nanoparticle concentrations and the distribution of these concentrations in cell population. J. R. Soc. Interface 2014, 11, 20131152. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, M.; Wang, B.; Zheng, L.; Chen, H.; Chai, Z.; Feng, W. Interrogating the variation of element masses and distribution patterns in single cells using ICP-MS with a high efficiency cell introduction system. Anal. Bioanal. Chem. 2017, 409, 1415–1423. [Google Scholar] [CrossRef]
- Cho, E.C.; Zhang, Q.; Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011, 6, 385–391. [Google Scholar] [CrossRef]
- Cai, H.; Ma, Y.; Wu, Z.; Ding, Y.; Zhang, P.; He, X.; Zhou, J.; Chai, Z.; Zhang, Z. Protein corona influences liver accumulation and hepatotoxicity of gold nanorods. NanoImpact 2016, 3, 40–46. [Google Scholar] [CrossRef]
- Dominguez-Medina, S.; Kisley, L.; Tauzin, L.J.; Hoggard, A.; Shuang, B.; DS Indrasekara, A.S.; Chen, S.; Wang, L.-Y.; Derry, P.J.; Liopo, A. Adsorption and unfolding of a single protein triggers nanoparticle aggregation. ACS Nano 2016, 10, 2103–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zook, J.M.; Rastogi, V.; MacCuspie, R.I.; Keene, A.M.; Fagan, J. Measuring Agglomerate Size Distribution and Dependence of Localized Surface Plasmon Resonance Absorbance on Gold Nanoparticle Agglomerate Size Using Analytical Ultracentrifugation. ACS Nano 2011, 5, 8070–8079. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjug. Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical-Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Röth, D.; Isoe, Y.; Hayashi, K.; Mochizuki, C.; Kalkum, M.; Nakamura, M. Protein corona components of polyethylene glycol-conjugated organosilica nanoparticles modulates macrophage uptake. Colloids Surf. B Biointerfaces 2021, 199, 111527. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [Green Version]
- Praetorius, A.; Tufenkji, N.; Goss, K.-U.; Scheringer, M.; von der Kammer, F.; Elimelech, M. The road to nowhere: Equilibrium partition coefficients for nanoparticles. Environ. Sci. Nano 2014, 1, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Baalousha, M.; Cornelis, G.; Kuhlbusch, T.A.J.; Lynch, I.; Nickel, C.; Peijnenburg, W.; van den Brink, N.W. Modeling nanomaterial fate and uptake in the environment: Current knowledge and future trends. Environ. Sci. Nano 2016, 3, 323–345. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wang, Y.; Wang, M.; Zheng, L.; Dai, W.; Jiao, C.; Song, Z.; Ma, Y.; Ding, Y.; Zhang, Z.; et al. Impact of Albumin Pre-Coating on Gold Nanoparticles Uptake at Single-Cell Level. Nanomaterials 2022, 12, 749. https://doi.org/10.3390/nano12050749
Li T, Wang Y, Wang M, Zheng L, Dai W, Jiao C, Song Z, Ma Y, Ding Y, Zhang Z, et al. Impact of Albumin Pre-Coating on Gold Nanoparticles Uptake at Single-Cell Level. Nanomaterials. 2022; 12(5):749. https://doi.org/10.3390/nano12050749
Chicago/Turabian StyleLi, Tao, Yun Wang, Meng Wang, Lingna Zheng, Wanqin Dai, Chunlei Jiao, Zhuda Song, Yuhui Ma, Yayun Ding, Zhiyong Zhang, and et al. 2022. "Impact of Albumin Pre-Coating on Gold Nanoparticles Uptake at Single-Cell Level" Nanomaterials 12, no. 5: 749. https://doi.org/10.3390/nano12050749
APA StyleLi, T., Wang, Y., Wang, M., Zheng, L., Dai, W., Jiao, C., Song, Z., Ma, Y., Ding, Y., Zhang, Z., Yang, F., & He, X. (2022). Impact of Albumin Pre-Coating on Gold Nanoparticles Uptake at Single-Cell Level. Nanomaterials, 12(5), 749. https://doi.org/10.3390/nano12050749






