Effective Adsorption of Congo Red from Aqueous Solution Using Fe/Al Di-Metal Nanostructured Composite Synthesised from Fe(III) and Al(III) Recovered from Real Acid Mine Drainage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Sample Collection
2.2. Synthesis of PDFe/Al from AMD
2.3. Preparation of CR Dye Stock Solutions
2.4. Batch Experiments
2.5. Characterisation of Aqueous Samples
2.6. Characterisation of Solid Samples
2.7. Point of Zero Charge (PZC)
2.8. Adsorption Capacity and Removal Efficiency
2.8.1. Adsorption Capacity
2.8.2. Removal Efficiency
2.9. Desorption Study
3. Results
3.1. Characterisation of PDFe/Al before and after CR Dye Adsorption
3.1.1. FTIR Functional Groups
3.1.2. XRD Mineralogical Composition
3.1.3. SEM Morphology
3.1.4. EDX Elemental Mapping
3.1.5. TGA Thermal Stability
3.1.6. BET Surface Area and Porosity
3.2. Batch Adsorption Experiments
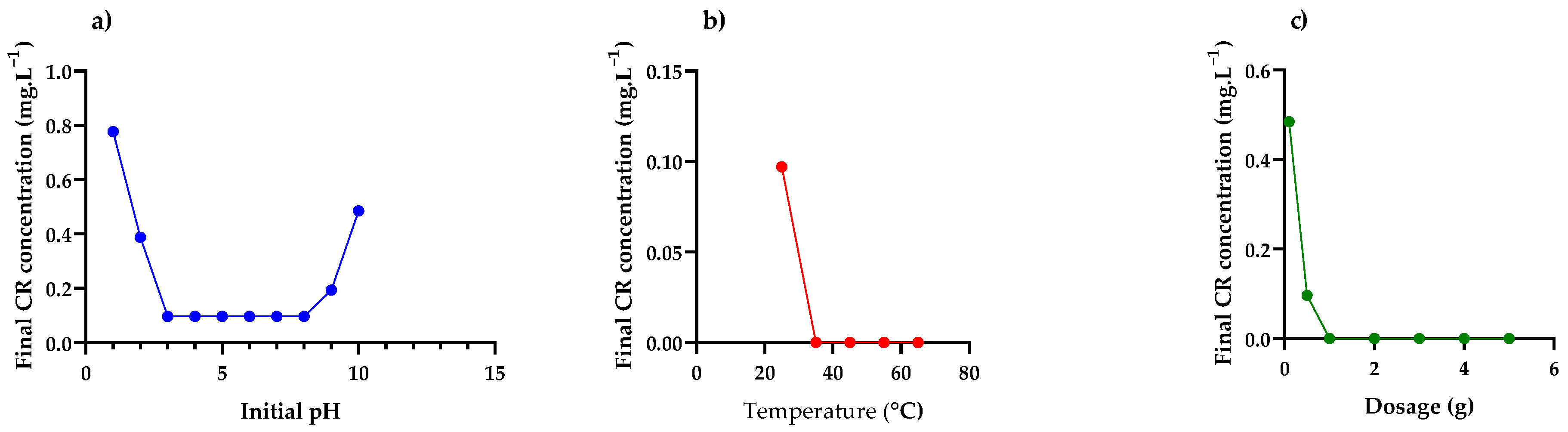
Effect of Initial pH, Temperature and Adsorbent Dosage
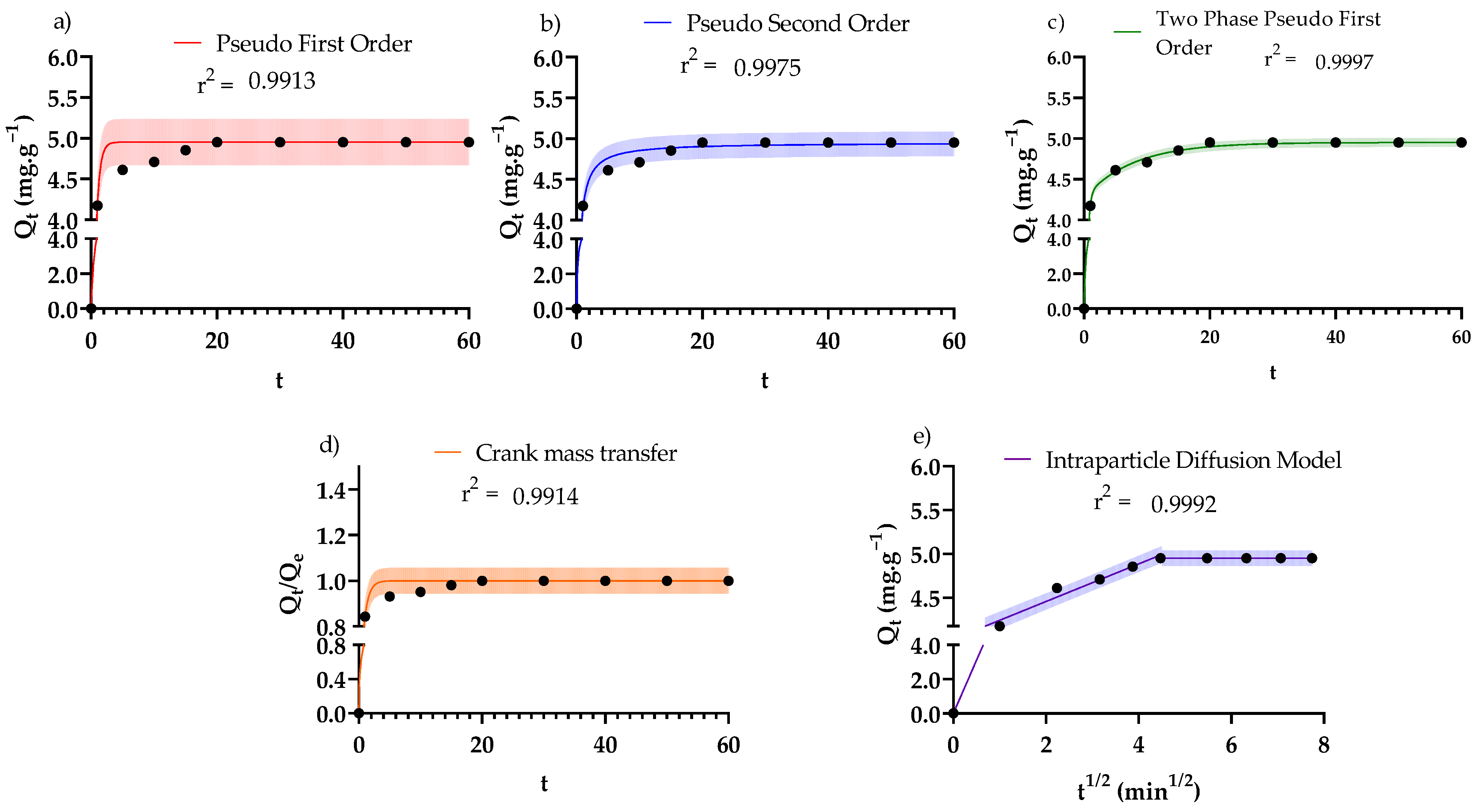
3.3. Adsorption Kinetics
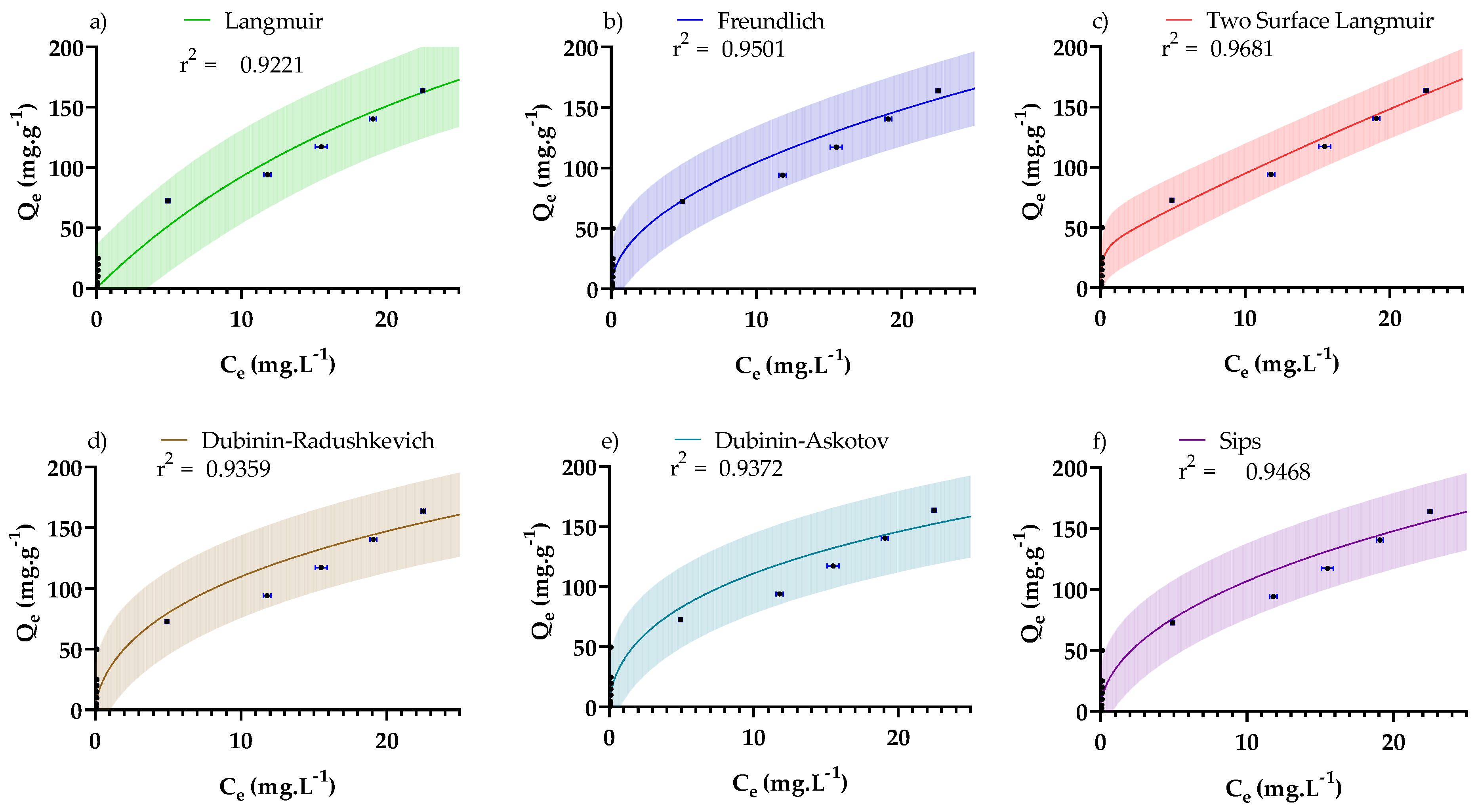
3.4. Adsorption Isotherms
| Isotherm | Non-Linear Form * | Fitted Parameters * | R2/RMSE |
|---|---|---|---|
| Langmuir [55,65,66] | 0.922/ 17.7 mg·g−1 | ||
| Freundlich [66] | 0.950/ 14.2 mg·g−1 | ||
| Two-surface Langmuir [65] | 0.968 11.34 mg·g−1 | ||
| Dubinin–Radushkevich [66] | 0.936/ 16.1 mg·g−1 | ||
| Dubinin–Astokov [66] | 0.937/ 15.9 mg·g−1 | ||
| Sips [67] | 0.947/14.6 mg·g−1 |
3.5. Comparison of PDFe/Al with Other Adsorbents
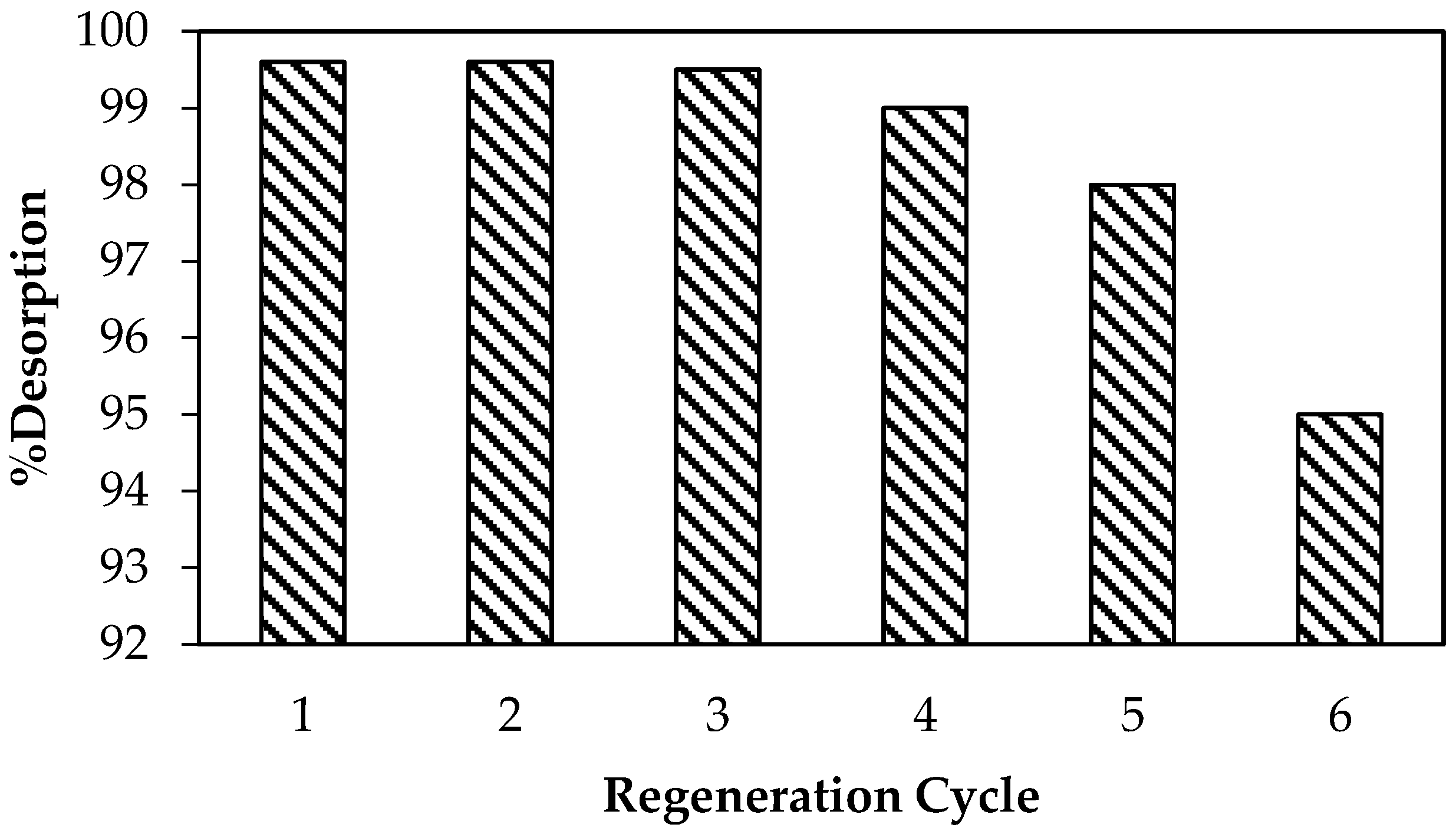
3.6. Regeneration Study
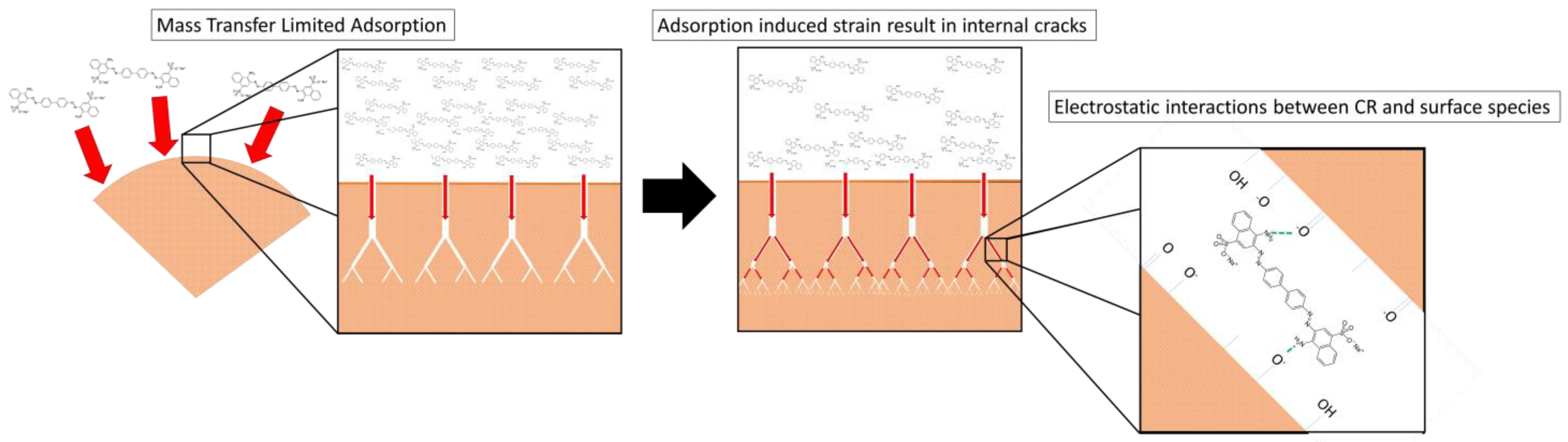
3.7. Adsorption Mechanism
4. Practical Implications of This Study
- Column tests will be carried out to assess the developed materials’ ability to remove CR continuously.
- A techno-economic analysis of the suggested material should be performed to determine the technology’s economic viability.
- The doping of the material should be considered to boost its adsorption capacity, hence increasing the proposed materials’ adsorption efficiency.
- A life-cycle analysis of the proposed system will be used to determine the environmental sustainability of the technology.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Foteinis, S.; Chatzisymeon, E. Co-treatment of acid mine drainage and municipal wastewater effluents: Emphasis on the fate and partitioning of chemical contaminants. J. Hazard. Mater. 2022, 421, 126677. [Google Scholar] [CrossRef] [PubMed]
- Jooste, S.; Thirion, C. An ecological risk assessment for a South African acid mine drainage. Water Sci. Technol. 1999, 39, 297–303. [Google Scholar] [CrossRef]
- Waters, A.S.; Webster-Brown, J.G. Assessing aluminium toxicity in streams affected by acid mine drainage. Water Sci. Technol. 2013, 67, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Kumar, S.P.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D.D.; Tran, H.N.; Woo, S.H. Enhanced adsorption of congo red from aqueous solution using chitosan/hematite nanocomposite hydrogel capsule fabricated via anionic surfactant gelation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126911. [Google Scholar] [CrossRef]
- Masindi, V.; Osman, M.S.; Mbhele, R.N.; Rikhotso, R. Fate of pollutants post treatment of acid mine drainage with basic oxygen furnace slag: Validation of experimental results with a geochemical model. J. Clean. Prod. 2018, 172, 2899–2909. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, X.; Ge, J.; Liu, F.; Zhao, W.; Wu, C. Influence of mining activities on groundwater hydrochemistry and heavy metal migration using a self-organizing map (SOM). J. Clean. Prod. 2020, 257, 120664. [Google Scholar] [CrossRef]
- Bologo, V.; Maree, J.P.; Carlsson, F. Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water. Water SA 2012, 38, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States–An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Cook, A.; Finkelman, R.B.; Fourie, A. Mineral and Fuel Extraction: Health Consequences. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 408–414. ISBN 978-0-444-63952-3. [Google Scholar] [CrossRef]
- Holmes, S. Department of Water Affairs and Forestry. In South African Water Quality Guidelines, Field Guide, 1st ed.; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996; Volume 8. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Water Quality Standards Handbook: Chapter 3: Water Quality Criteria; EPA Office of Water, Office of Science and Technology: Washington, DC, USA, 2017.
- Akinwekomi, V.; Maree, J.P.; Zvinowanda, C.; Masindi, V. Synthesis of magnetite from iron-rich mine water using sodium carbonate. J. Environ. Chem. Eng. 2017, 5, 2699–2707. [Google Scholar] [CrossRef]
- Akinwekomi, V.; Maree, J.P.; Masindi, V.; Zvinowanda, C.; Osman, M.S.; Foteinis, S.; Mpenyana-Monyatsi, L.; Chatzisymeon, E. Beneficiation of acid mine drainage (AMD): A viable option for the synthesis of goethite, hematite, magnetite, and gypsum–Gearing towards a circular economy concept. Miner. Eng. 2020, 148, 106204. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Nleya, Y.; Simate, G.S.; Ndlovu, S. Sustainability assessment of the recovery and utilisation of acid from acid mine drainage. J. Clean. Prod. 2016, 113, 17–27. [Google Scholar] [CrossRef]
- Wei, X.; Viadero, R.C., Jr.; Bhojappa, S. Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants. Water Res. 2008, 42, 3275–3284. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.I.; Mamba, B.B. Synthesis and application of hematite nanoparticles for acid mine drainage treatment. J. Environ. Chem. Eng. 2018, 6, 1865–1874. [Google Scholar] [CrossRef]
- Muedi, K.L.; Brink, H.G.; Masindi, V.; Maree, J.P. Effective removal of arsenate from wastewater using aluminium enriched ferric oxide-hydroxide recovered from authentic acid mine drainage. J. Hazard. Mater. 2021, 414, 125491. [Google Scholar] [CrossRef]
- Wei, X.; Viadero, R.C. Adsorption and Precoat Filtration Studies of Synthetic Dye Removal by Acid Mine Drainage Sludge. J. Environ. Eng. 2007, 133, 633–640. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review. J. Environ. Manag. 2017, 191, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.P. Chemistry of dyeing. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 150–183. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. Adsorption of dyes onto palygorskite and its composites: A review. J. Environ. Chem. Eng. 2016, 4, 1274–1294. [Google Scholar] [CrossRef]
- Hu, M.; Yan, X.; Hu, X.; Zhang, J.; Feng, R.; Zhou, M. Ultra-high adsorption capacity of MgO/SiO2 composites with rough surfaces for Congo red removal from water. J. Colloid Interface Sci. 2018, 510, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, X.; Hu, M.; Hu, X.; Zhou, M. Adsorption of Congo red from aqueous solution using ZnO-modified SiO2 nanospheres with rough surfaces. J. Mol. Liq. 2018, 249, 772–778. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J. 2012, 187, 79–88. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B.; Dai, S.; Vimonses, V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J. Ind. Eng. Chem. 2015, 21, 653–661. [Google Scholar] [CrossRef]
- Sasmal, D.; Maity, J.; Kolya, H.; Tripathy, T. Study of congo red dye removal from its aqueous solution using sulfated acrylamide and N, N-dimethyl acrylamide grafted amylopectin. J. Water Process Eng. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Miandad, R.; Kumar, R.; Barakat, M.A.; Basheer, C.; Aburiazaiza, A.S.; Nizami, A.S.; Rehan, M. Untapped conversion of plastic waste char into carbon-metal LDOs for the adsorption of Congo red. J. Colloid Interface Sci. 2018, 511, 402–410. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Kampoosaen, C.; Kengnok, O. Adsorption characteristics of Congo red on carbonized leonardite. J. Clean. Prod. 2016, 134, 506–514. [Google Scholar] [CrossRef]
- Abdel-Khalek, M.A.; Abdel Rahman, M.K.; Francis, A.A. Exploring the adsorption behavior of cationic and anionic dyes on industrial waste shells of egg. J. Environ. Chem. Eng. 2017, 5, 319–327. [Google Scholar] [CrossRef]
- Dhal, J.P.; Sethi, M.; Mishra, B.G.; Hota, G. MgO nanomaterials with different morphologies and their sorption capacity for removal of toxic dyes. Mater. Lett. 2015, 141, 267–271. [Google Scholar] [CrossRef]
- Hou, R.; Gao, Y.; Zhu, H.; Yang, G.; Liu, W.; Huo, Y.; Xie, Z.; Li, H. Coupling system of Ag/BiOBr photocatalysis and direct contact membrane distillation for complete purification of N-containing dye wastewater. Chem. Eng. J. 2017, 317, 386–393. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z. A loose nano-filtration membrane prepared by coating HPAN UF membrane with modified PEI for dye reuse and desalination. J. Membr. Sci. 2017, 524, 214–224. [Google Scholar] [CrossRef]
- Dotto, G.L.; Lima, E.C.; Pinto, L.A.A. Biosorption of food dyes onto Spirulina platensis nanoparticles: Equilibrium isotherm and thermodynamic analysis. Bioresour. Technol. 2012, 103, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohy Eldin, M.S.; Aly, K.M.; Khan, Z.A.; Meky, A.E.; Saleh, T.S.; Elbogamy, A.S. Development of novel acid–base ions exchanger for basic dye removal: Phosphoric acid doped pyrazole-g-polyglycidyl methacrylate. Desalin. Water Treat. 2016, 57, 24047–24055. [Google Scholar] [CrossRef]
- Gao, B.-Y.; Yue, Q.-Y.; Wang, Y.; Zhou, W.-Z. Color removal from dye-containing wastewater by magnesium chloride. J. Environ. Manag. 2007, 82, 167–172. [Google Scholar] [CrossRef]
- Ziane, S.; Bessaha, F.; Marouf-Khelifa, K.; Khelifa, A. Single and binary adsorption of reactive black 5 and Congo red on modified dolomite: Performance and mechanism. J. Mol. Liq. 2018, 249, 1245–1253. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Sriram, G.; Uthappa, U.T.; Losic, D.; Kigga, M.; Jung, H.-Y.; Kurkuri, M.D. Mg–Al-Layered Double Hydroxide (LDH) Modified Diatoms for Highly Efficient Removal of Congo Red from Aqueous Solution. Appl. Sci. 2020, 10, 2285. [Google Scholar] [CrossRef] [Green Version]
- Dhal, J.; Mishra, B.; Hota, G. Ferrous oxalate, maghemite and hematite nanorods as efficient adsorbents for decontamination of Congo red dye from aqueous system. Int. J. Environ. Sci. Technol. 2014, 12, 1845–1856. [Google Scholar] [CrossRef]
- Bordoloi, S.; Nath, S.K.; Gogoi, S.; Dutta, R.K. Arsenic and iron removal from groundwater by oxidation-coagulation at optimized pH: Laboratory and field studies. J. Hazard. Mater. 2013, 260, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, B.; Zhang, L.; Yu, J.; Li, Y. Synthesis of MgNiCo LDH hollow structure derived from ZIF-67 as superb adsorbent for Congo red. J. Colloid Interface Sci. 2022, 612, 598–607. [Google Scholar] [CrossRef]
- Mei, L.; Liao, L.; Wang, Z.; Xu, C. Interactions between Phosphoric/Tannic Acid and Different Forms of FeOOH. Adv. Mater. Sci. Eng. 2015, 2015, 250836. [Google Scholar] [CrossRef] [Green Version]
- Mikhaylov, V.I.; Martakov, I.S.; Gerasimov, E.Y.; Sitnikov, P.A. Study of heteroaggregation and properties of sol-gel AlOOH-Fe3O4 composites. Heliyon 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Dolino, G.; Bellet, D.; Faivre, C. Adsorption strains in porous silicon. Phys. Rev. B-Condens. Matter Mater. Phys. 1996, 54, 17919–17929. [Google Scholar] [CrossRef]
- Gor, G.Y.; Huber, P.; Bernstein, N. Adsorption-induced deformation of nanoporous materials-A review. Appl. Phys. Rev. 2017, 4, 011303. [Google Scholar] [CrossRef] [Green Version]
- Gor, G.Y.; Bernstein, N. Revisiting Bangham’s law of adsorption-induced deformation: Changes of surface energy and surface stress. Phys. Chem. Chem. Phys. 2016, 18, 9788–9798. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Li, X.D.; Fang, Y.M.; Wu, S.Q.; Zhu, Z.Z. Adsorption of alkali, alkaline-earth, simple and 3d transition metal, and nonmetal atoms on monolayer MoS2. AIP Adv. 2015, 5, 57143. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, A.; Mishra, B.G.; Hota, G. Adsorptive removal of Congo red dye from wastewater by mixed iron oxide-alumina nanocomposites. Ceram. Int. 2013, 39, 5443–5451. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Jessup, R.E.; Suresh, P.; Rao, C. Nonequilibrium Sorption of Organic Chemicals: Elucidation of Rate-Limiting Processes. Environ. Sci. Technol. 1991, 25, 134–142. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Song, L.; Mashayekhi, H.; Chefetz, B.; Xing, B. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.; Van Noort, P.C.M.; Parsons, J.R.; Govers, H.A.J. Temperature dependence of slow adsorption and desorption kinetics of organic compounds in sediments. Environ. Sci. Technol. 1997, 31, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Nakagaki, M. The Diffusion of Dye Solutions. Bull. Chem. Soc. Jpn. 1950, 23, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Bhaumik, M.; Maity, A.; Brink, H.G. Zero valent nickel nanoparticles decorated polyaniline nanotubes for the efficient removal of Pb(II) from aqueous solution: Synthesis, characterization and mechanism investigation. Chem. Eng. J. 2021, 417, 127910. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.H. Dye adsorption characteristics of magnetite nanoparticles coated with a biopolymer poly(γ-glutamic acid). Bioresour. Technol. 2011, 102, 8868–8876. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sridhar, K.; Chen, B.H. Removal of polycyclic aromatic hydrocarbons from water by magnetic activated carbon nanocomposite from green tea waste. J. Hazard. Mater. 2021, 415, 125701. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Girods, P.; Dufour, A.; Fierro, V.; Rogaume, Y.; Rogaume, C.; Zoulalian, A.; Celzard, A. Activated carbons prepared from wood particleboard wastes: Characterisation and phenol adsorption capacities. J. Hazard. Mater. 2009, 166, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Osol, A.; Hoover, J.E. (Eds.) Remington’s Pharmaceutical Sciences, 15th ed.; Mack Publishing Co.: Easton, PA, USA, 1975. [Google Scholar]
- Jaroniec, M.; Deryło, A. Application of Dubinin—Radushkevich—type equation for describing bisolute adsorption from dilute aqueous solutions on activated carbon. J. Colloid Interface Sci. 1981, 84, 191–195. [Google Scholar] [CrossRef]
- Stoeckli, F.; López-Ramón, M.V.; Moreno-Castilla, C. Adsorption of phenolic compounds from aqueous solutions, by activated carbons, described by the dubinin-Astakhov equation. Langmuir 2001, 17, 3301–3306. [Google Scholar] [CrossRef]
- Malana, M.A.; Qureshi, R.B.; Ashiq, M.N. Adsorption Studies of Arsenic on Nano Aluminium Doped Manganese Copper Ferrite Polymer (MA, VA, AA) Composite: Kinetics and Mechanism. Chem. Eng. J. 2011, 172, 721–727. [Google Scholar] [CrossRef]
- Keren, Y.; Borisover, M.; Bukhanovsky, N. Sorption interactions of organic compounds with soils affected by agricultural olive mill wastewater. Chemosphere 2015, 138, 462–468. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guha, N.; Krishnan, S.; Singh, A.K.; Mathur, P.; Rai, D.K. Selective and Recyclable Congo Red Dye Adsorption by Spherical Fe3O4 Nanoparticles Functionalized with 1,2,4,5-Benzenetetracarboxylic Acid. Sci. Rep. 2020, 10, 111. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Zhang, S.; Zhang, X.; Han, R. Adsorption of congo red from solution by iron doped PVA-chitosan composite film. Desalin. Water Treat. 2020, 187, 378–389. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Zhang, H.; Baeyens, J. Adsorption of Congo red dye on FexCo3−xO4 nanoparticles. J. Environ. Manag. 2019, 238, 473–483. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Kim, D.S. Equilibrium isotherms, kinetics, and thermodynamics studies for congo red adsorption using calcium alginate beads impregnated with nano-goethite. Ecotoxicol. Environ. Saf. 2017, 141, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zourou, A.; Ntziouni, A.; Adamopoulos, N.; Roman, T.; Zhang, F.; Terrones, M.; Kordatos, K. Graphene oxide-CuFe2O4 nanohybrid material as an adsorbent of Congo red dye. Carbon Trends 2022, 7, 100147. [Google Scholar] [CrossRef]
- Adesina, A.O.; Elvis, O.A.; Mohallem, N.D.S.; Olusegun, S.J. Adsorption of Methylene blue and Congo red from aqueous solution using synthesized alumina–zirconia composite. Environ. Technol. 2021, 42, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Hamd, A.; Rady, D.; Shaban, M.; Elsayed, K.N.M.; Al Mohamadi, H.; Elzanaty, A.M.; Ahmed, S.A.; El-Sayed, R.; Soliman, N.K. Application of Nano Bio-clay Composite in a Scaling-up Study for Wastewater Treatment. Biointerface Res. Appl. Chem. 2021, 12, 6393–6414. [Google Scholar] [CrossRef]
- Barany, S.; Strelko, V. Laws and mechanism of adsorption of cations by different ion-exchange forms of silica gel. Adsorption 2013, 19, 769–776. [Google Scholar] [CrossRef]

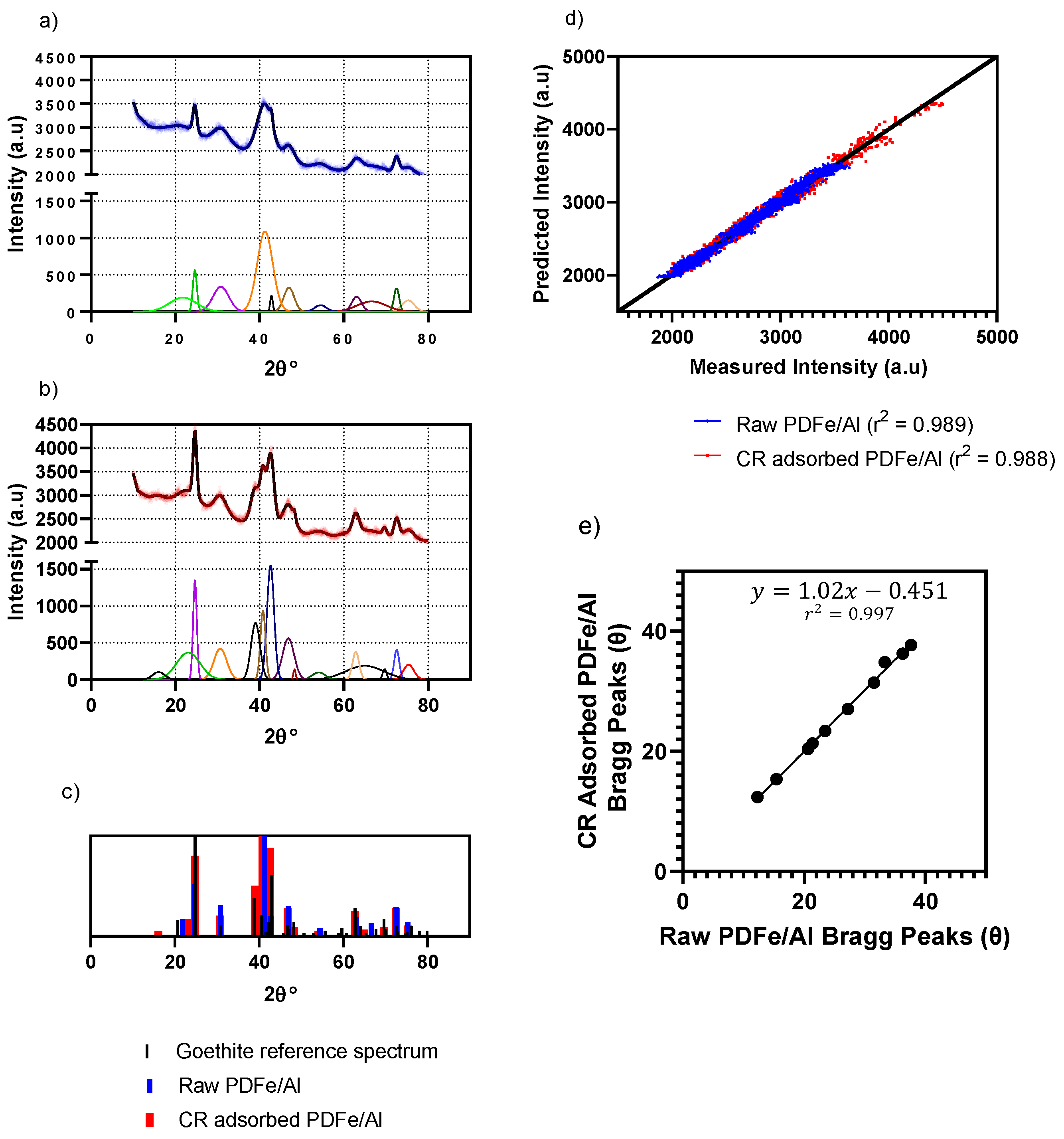

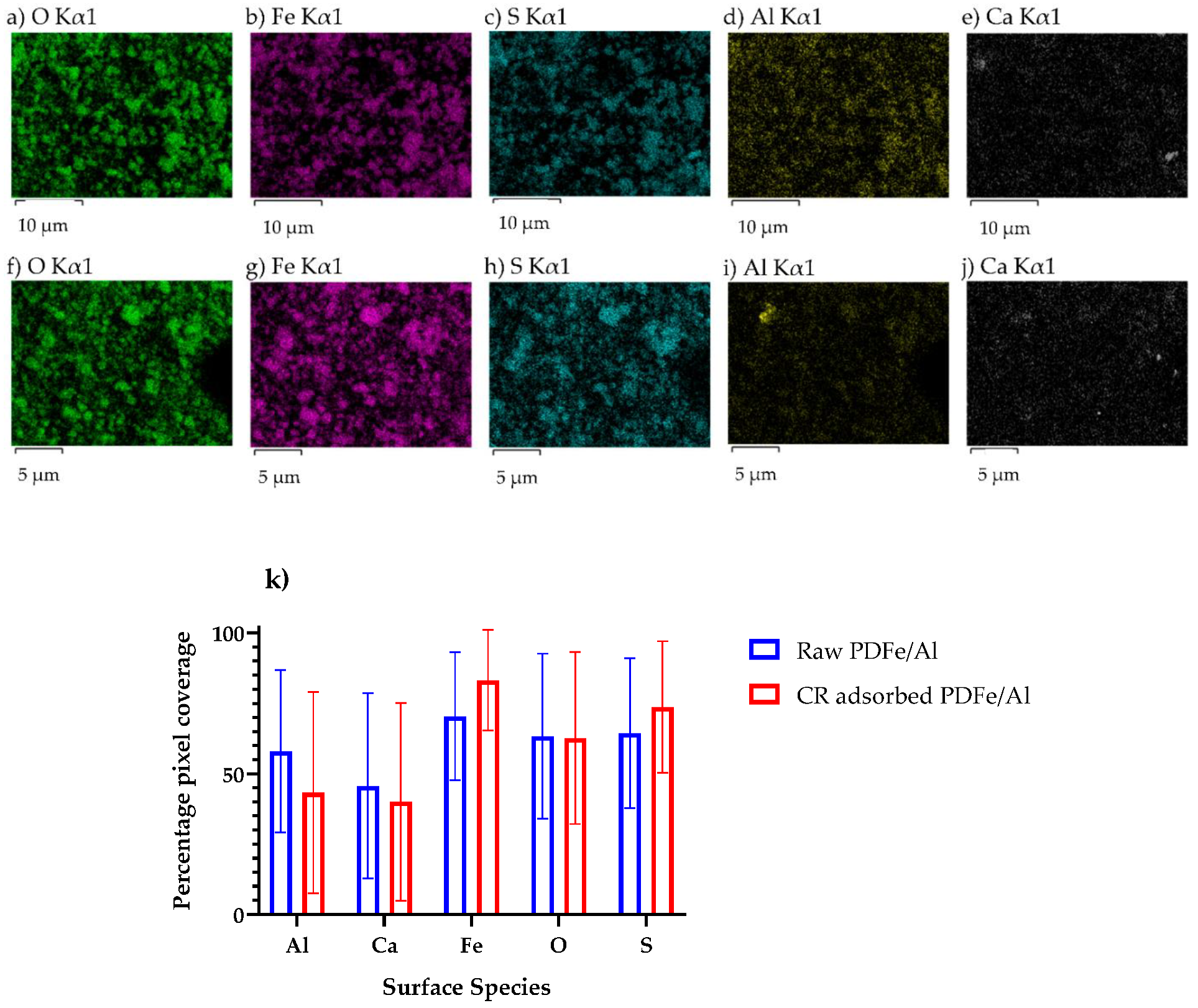
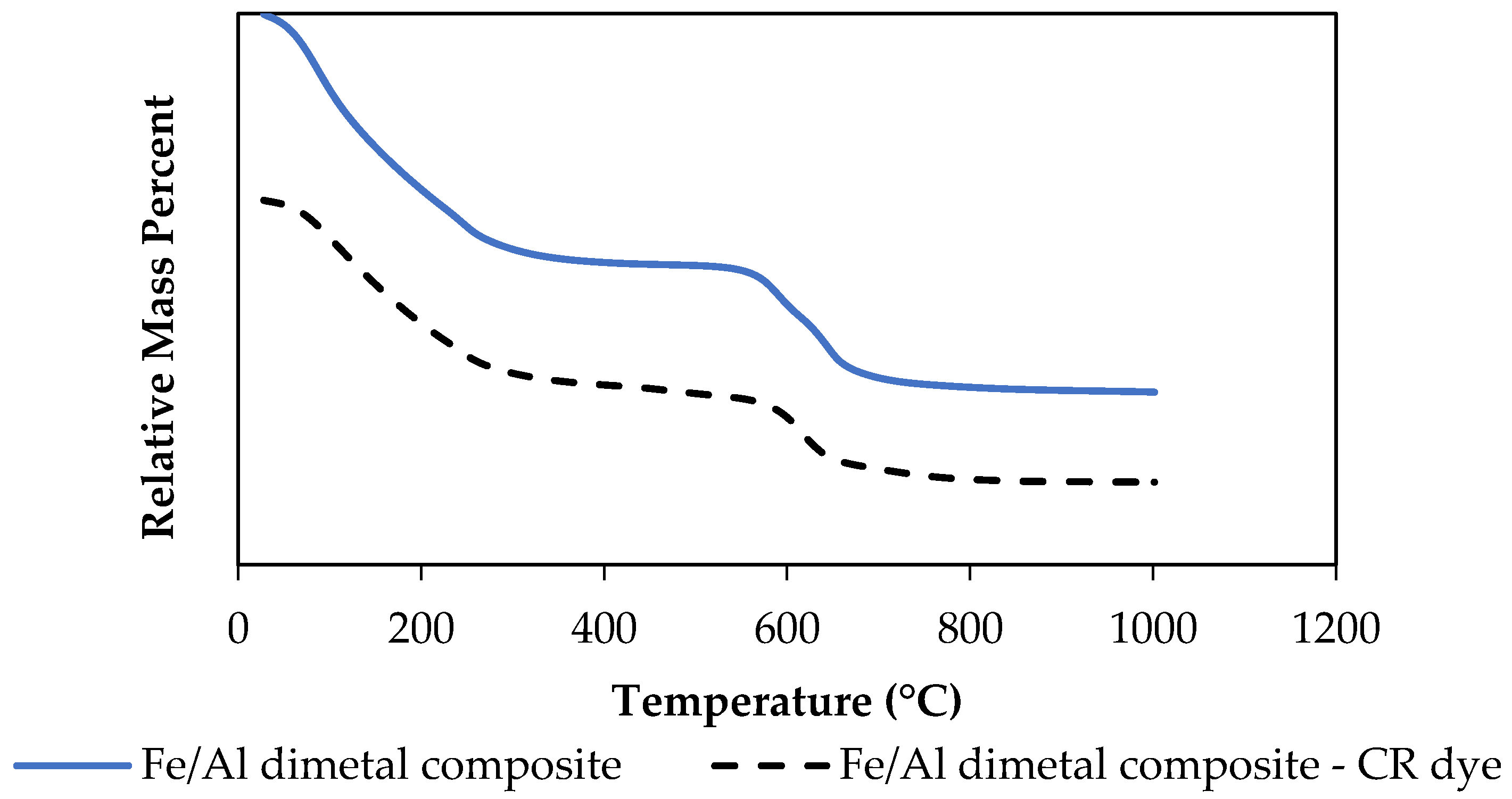
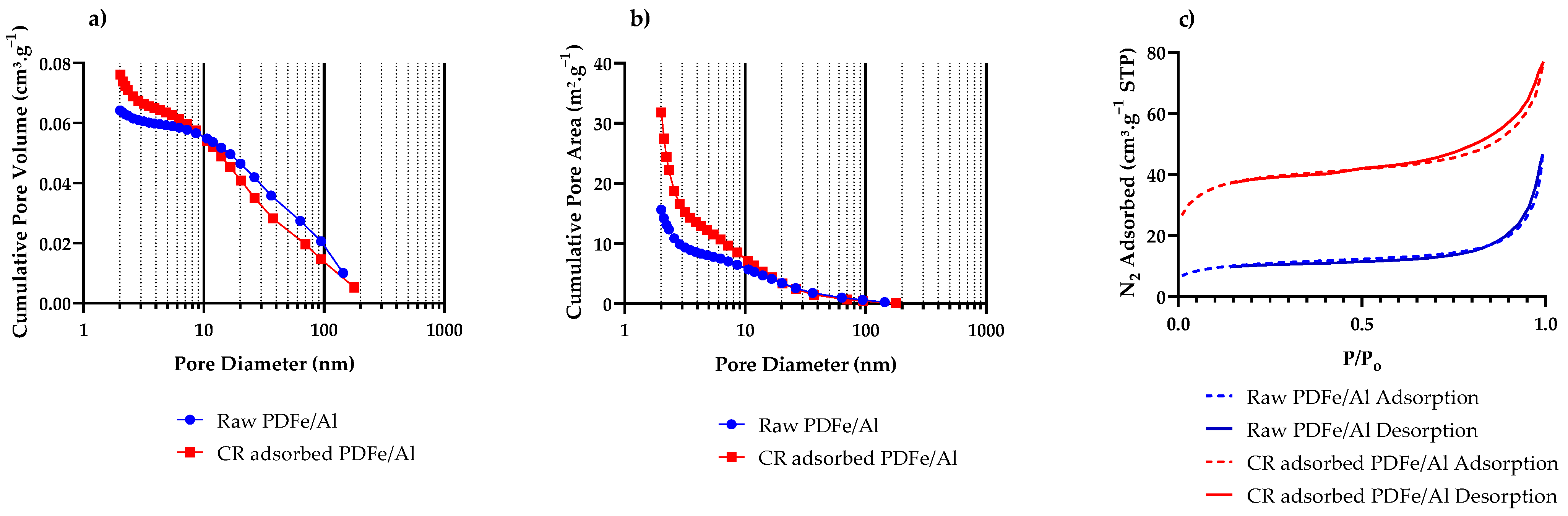
| Experiment No | Initial Concentration (mg·L−1) | Initial pH | Adsorbent Dosage * (g) | Agitation Time (min) | Temperature (°C) |
|---|---|---|---|---|---|
| 1 | 1; 5; 10; 20; 30; 40; 50; 100; 150; 200 | 6–7 | 1 | 30 | 25 |
| 2 | 10 | 2, 3, 4, 5, 6, 7, 8, 9, 10 (±0.2) | 1 | 30 | 25 |
| 3 | 10 | 6–7 | 0.1; 0.5; 1; 2; 3; 4; 5 (±0.0005) | 30 | 25 |
| 4 | 10 | 6–7 | 1 | 1; 5; 10; 15; 20; 25; 30; 40; 50; 60 | 25 |
| 5 | 10 | 6–7 | 1 | 30 | 25; 35; 45; 55; 65 |
| Parameter | Raw PDFe/Al | CR-PDFe/Al |
|---|---|---|
| BET Surface Area (m2·g−1) | 37.58 ± 0.37 | 134.46 ± 2.06 |
| Micropore Area (from t-Plot) (m2·g−1) | 13.67 | 51.85 |
| Pore volume (<179 nm) (cm3·g−1) | 0.0621 | 0.1137 |
| Micropore Volume (cm3·g−1) | 0.0031 | 0.0155 |
| BJH average pore diameter (nm) | 16.47 | 9.58 |
| Kinetic Law | Differential Form * | Analytical Form * | Fitted Parameters * | R2/RMSE |
|---|---|---|---|---|
| Pseudo-first-order [55] | k1 = 1.85·min−1 | 0.991/ 0.138 mg·g−1 | ||
| Pseudo-second-order [55] | k2 = 1.00 g·mg−1·min−1 | 0.998/ 0.0734 mg·g−1 | ||
| Two-phase adsorption [56,57,58] | kfast = 3.18 min−1 kslow = 0.128 min−1 ϕ = 0.863 | 1.000/ 0.0263 mg·g−1 | ||
| Crank internal mass transfer model [55] | De = 3.49 × 10−11 m2·s−1 | 0.991/0.0277 mg·g−1 | ||
| Weber and Morris [55,59] | De1 = 3.49 × 10−11 m2·s−1 De2 = 4.19 × 10−14 m2·s−1 De3 = 0 m2·s−1 | 0.999/ 0.0421 mg·g−1 |
| Adsorbent | Qmax,L * | nF ** | Reference |
|---|---|---|---|
| BTCS functionalised Fe3O4 nanoparticles | 630 mg·g−1 | 1.9596 | [73] |
| γ-Fe2O3–γ-Al2O3 | 416.7 mg·g−1 | 1.13 | [54] |
| PDFe/Al | 411 mg·g−1 | 1.99 | This study |
| Iron doped PVA-chitosan | 315 mg·g−1 | 6.34 | [74] |
| FexCo3−xO4 | 160.3 mg·g−1 | 1.44 | [75] |
| Calcium Alginate Beads—nano-goethite | 181.1 ± 2.32 mg·g−1 | 2.431 ± 0.343 | [76] |
| Fe2O3–Al2O3 | 126.58 mg·g−1 | 1.85 | [54] |
| Graphine Oxide-CuFe2O4 | 114.21 mg·g−1 | 1.223 | [77] |
| Alumina-Zirconia | 41.07 mg·g−1 | 2.177 | [78] |
| Nano bio-clay composite (Kaolinite/Ulva Lactuca) | 23.7529 mg·g−1 | 1.68 | [79] |
| α-Fe2O3–α-Al2O3 | 1.422 mg·g−1 | −0.665 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muedi, K.L.; Masindi, V.; Maree, J.P.; Haneklaus, N.; Brink, H.G. Effective Adsorption of Congo Red from Aqueous Solution Using Fe/Al Di-Metal Nanostructured Composite Synthesised from Fe(III) and Al(III) Recovered from Real Acid Mine Drainage. Nanomaterials 2022, 12, 776. https://doi.org/10.3390/nano12050776
Muedi KL, Masindi V, Maree JP, Haneklaus N, Brink HG. Effective Adsorption of Congo Red from Aqueous Solution Using Fe/Al Di-Metal Nanostructured Composite Synthesised from Fe(III) and Al(III) Recovered from Real Acid Mine Drainage. Nanomaterials. 2022; 12(5):776. https://doi.org/10.3390/nano12050776
Chicago/Turabian StyleMuedi, Khathutshelo Lilith, Vhahangwele Masindi, Johannes Philippus Maree, Nils Haneklaus, and Hendrik Gideon Brink. 2022. "Effective Adsorption of Congo Red from Aqueous Solution Using Fe/Al Di-Metal Nanostructured Composite Synthesised from Fe(III) and Al(III) Recovered from Real Acid Mine Drainage" Nanomaterials 12, no. 5: 776. https://doi.org/10.3390/nano12050776
APA StyleMuedi, K. L., Masindi, V., Maree, J. P., Haneklaus, N., & Brink, H. G. (2022). Effective Adsorption of Congo Red from Aqueous Solution Using Fe/Al Di-Metal Nanostructured Composite Synthesised from Fe(III) and Al(III) Recovered from Real Acid Mine Drainage. Nanomaterials, 12(5), 776. https://doi.org/10.3390/nano12050776











