Green Synthesis of Laser-Induced Graphene with Copper Oxide Nanoparticles for Deicing Based on Photo-Electrothermal Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser Delivery System
2.2. Preparation of LIG and Cu Oxide Nanoparticles on LIG
2.3. Characterization
3. Results
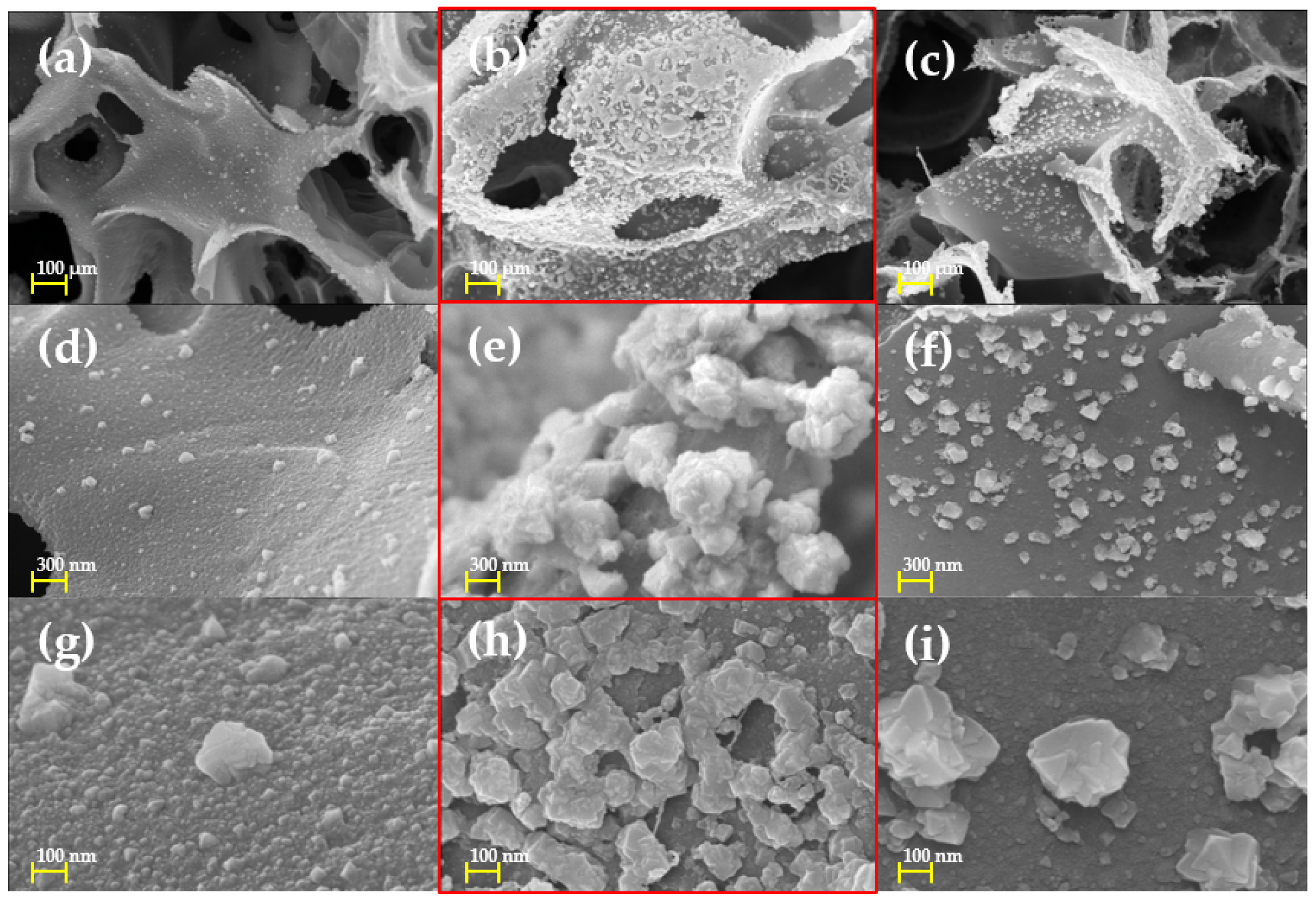
3.1. Morphological Characteristics of Cu/LIG
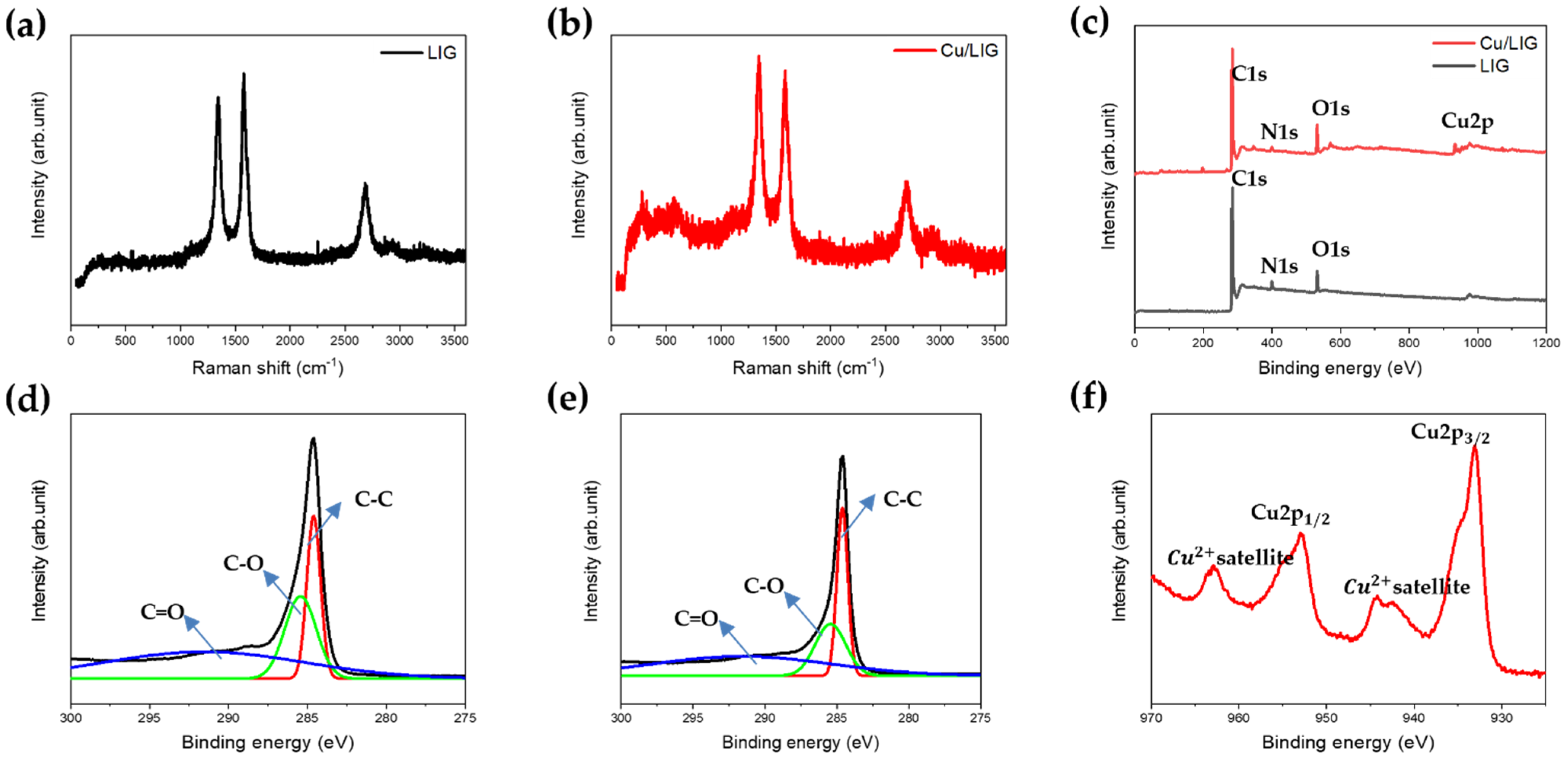
3.2. Chemical Characteristics of Cu/LIG
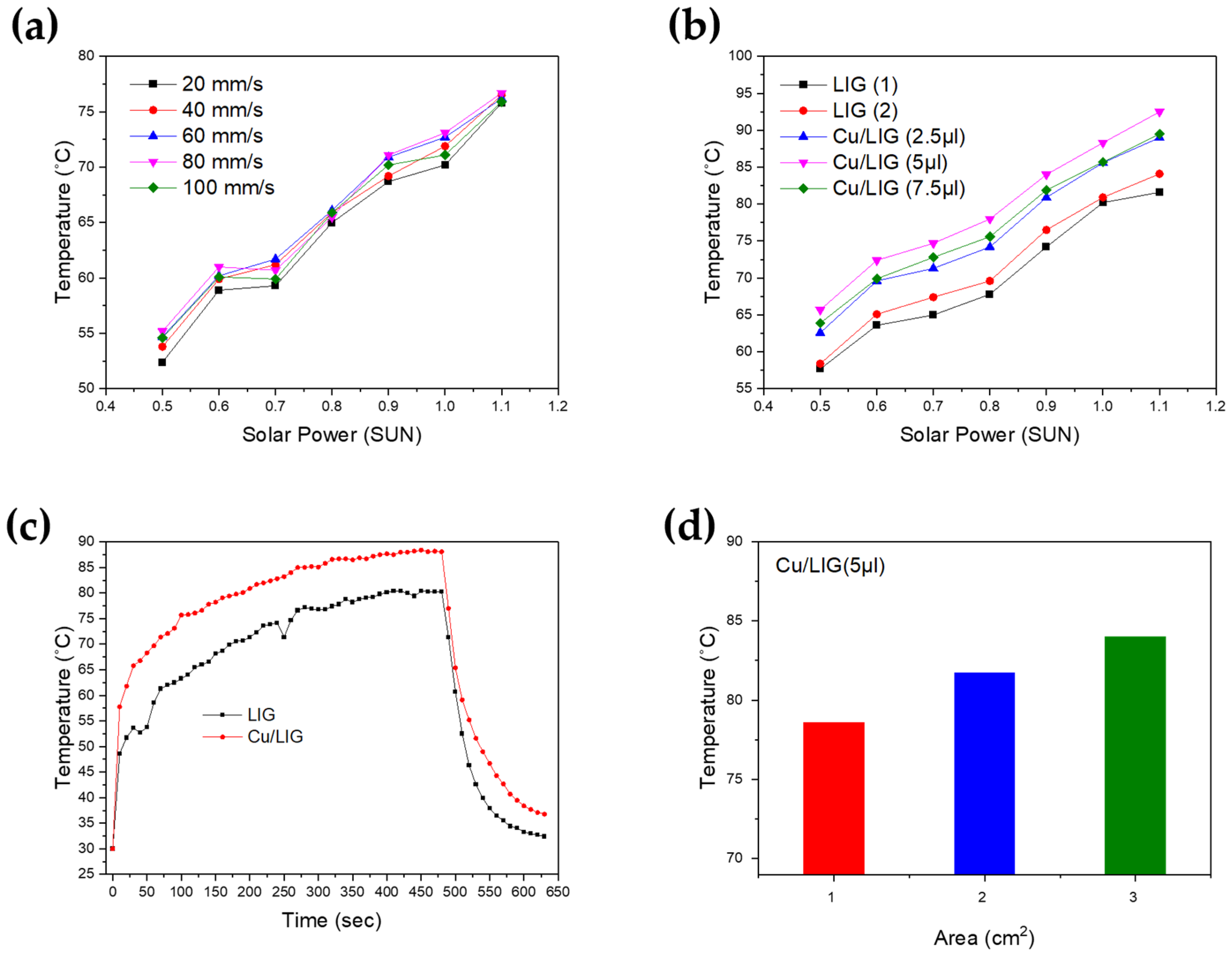
3.3. Photothermal Properties of Cu/LIG
3.4. Electrothermal Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Liu, X.; Liu, H.; Zhang, D. Development of high-efficient synthetic electric heating coating for anti-icing/de-icing. Surf. Coat. Technol. 2018, 349, 340–346. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Sullivan, C.R.; Kozlyuk, V. Variable-resistance conductors (VRC) for power-line de-icing. Cold Reg. Sci. Technol. 2011, 65, 23–28. [Google Scholar] [CrossRef]
- Huneault, M.; Langheit, C.; Caron, J. Combined models for glaze ice accretion and de-icing of current-carrying electrical conductors. IEEE Trans. Power Deliv. 2005, 20, 1611–1616. [Google Scholar] [CrossRef]
- Karim, N.; Zhang, M.; Afroj, S.; Koncherry, V.; Potluri, P.; Nososelov, K.S. Graphene-based surface heater for de-icing applications. RSC Adv. 2018, 8, 16815–16823. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, R.; Luo, N.; Liu, Y.; Wu, Y.; Yu, B.; Liu, S.; Zhou, F. All-Day Anti-Icing/De-Icing Coating by Solar-Thermal and Electric-Thermal Effects. Adv. Mater. Technol. 2021, 6, 2100371. [Google Scholar] [CrossRef]
- Falzon, B.G.; Robinson, P.; Frenz, S.; Gilbert, B. Development and evaluation of a novel integrated anti-icing/de-icing technology for carbon fibre composite aerostructures using an electro-conductive textile. Compos. Part A Appl. Sci. Manuf. 2005, 68, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Cortés, A.; Jiménez-Suárez, A.; Campo, M.; Ureña, A.; Prolongo, S.G. 3D printed epoxy-CNTs/GNPs conductive inks with application in anti-icing and de-icing systems. Eur. Polym. J. 2020, 141, 110090. [Google Scholar] [CrossRef]
- Yao, X.; Hawkins, S.C.; Falzon, B.G. An advanced anti-icing/de-icing system utilizing highly aligned carbon nanotube webs. Carbon 2018, 136, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Idris, M.K.; Qiu, J.; Melenka, G.W.; Grau, G. Printing electronics directly onto carbon fiber composites: Unmanned aerial vehicle (UAV) wings with integrated heater for de-icing. Eng. Res. Express 2020, 2, 025022. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Xie, Z.; Duo, Y.; Lin, Z.; Fan, T.; Xing, C.; Yu, L.; Wang, R.; Qiu, M.; Zhang, H. The rise of 2D photothermal materials beyond graphene for clean water production. Adv. Sci. Lett. 2020, 7, 1902236. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lei, H.; Kan, A.; Xie, H.; Yu, W. Photothermal applications based on graphene and its derivatives: A state-of-the-art review. Energy 2021, 216, 119262. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 2011, 13, 20836–20843. [Google Scholar] [CrossRef]
- Munoz, R.; Gómez-Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Eletskii, A.V.; Iskandarova, I.M.; Knizhnik, A.A.; Krasikov, D.N. Graphene: Fabrication methods and thermophysical properties. Phys.-Uspekhi 2011, 54, 227. [Google Scholar] [CrossRef]
- Galpayage Dona, D.G.; Wang, M.; Liu, M.; Motta, N.; Waclawik, E.; Yan, C. Recent advances in fabrication and characterization of graphene-polymer nanocomposites. Graphene 2012, 1, 30–49. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakabson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Peng, Z.; Wang, T.; Xu, Y.; Zhang, J.; Li, Y.; Nilewski, L.G.; Lin, J.; Tour, J.M. In situ formation of metal oxide nanocrystals embedded in laser-induced graphene. ACS Nano 2015, 9, 9244–9251. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible boron-doped laser-induced graphene microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lin, J.; Ye, R.; Samuel, E.L.; Tour, J.M. Flexible and stackable laser-induced graphene supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3414–3419. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.G.; Yang, K.; Chyan, Y.; Kittrell, C.; Tour, J.M. Laser-induced graphene for flexible and embeddable gas sensors. ACS Nano 2019, 13, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Q.; Tian, H.; Liu, Y.; Ju, Z.Y.; Pang, Y.; Chen, Y.Q.; Wang, D.Y.; Tian, X.G.; Yan, J.C.; Deng, N.Q.; et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, Q.; Ma, Y.; Gao, J.; Zhang, P.; Xia, Y.; Tian, Y.; Sun, X.; Zhong, J.; Sun, X. Facile synthesis of graphene/metal nanoparticle composites via self-catalysis reduction at room temperature. Inorg. Chem. 2013, 52, 3141–3147. [Google Scholar] [CrossRef]
- Liu, K.; Liu, L.; Luo, Y.; Jia, D. One-step synthesis of metal nanoparticle decorated graphene by liquid phase exfoliation. J. Mater. Chem. 2012, 22, 20342–20352. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct electrodeposition of graphene enabling the one-step synthesis of graphene–metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Gonzalez, J.P.A.; Aguilar, R.G.; López, J.O. Synthesis and Characterization of Laser Induced Graphene (LIG) and laser Reduced Graphene Oxide (lrGO) by using a pulsed CO2 laser. MRS Adv. 2019, 4, 3327–3335. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kong, W.; Qin, X.; Qu, F.; Lu, L. Self-powered cathodic photoelectrochemical aptasensor based on in situ–synthesized CuO-Cu 2 O nanowire array for detecting prostate-specific antigen. Microchim. Acta 2020, 187, 1–9. [Google Scholar]
- Deka, B.K.; Hazarika, A.; Kong, K.; Kim, D.; Park, Y.; Park, H.W. Interfacial resistive heating and mechanical properties of graphene oxide assisted CuO nanoparticles in woven carbon fiber/polyester composite. Compos. Part. A Appl. Sci. Manuf. 2016, 80, 159–170. [Google Scholar] [CrossRef]
- Guo, H.; Liu, M.; Xie, C.; Zhu, Y.; Sui, X.; Wen, C.; Li, Q.; Zhao, W.; Yang, J.; Zhang, L. A sunlight-responsive and robust anti-icing/deicing coating based on the amphiphilic materials. Chem. Eng. J. 2020, 402, 126161. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater. Horiz. 2018, 5, 323–343. [Google Scholar] [CrossRef]
- Anguita, J.V.; Ahmad, M.; Haq, S.; Allam, J.; Silva, S.R.P. Ultra-broadband light trapping using nanotextured decoupled graphene multilayers. Sci. Adv. 2016, 2, e1501238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, W.; Chiou, K.; Qiao, Y.; Liu, Y.; Song, C.; Deng, T.; Huang, J. Crumpled graphene ball-based broadband solar absorbers. Nanoscale 2018, 10, 6306–6312. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, W.; Ni, F.; Yu, W.; Liu, X. Forest-like Laser-Induced Graphene Film with Ultrahigh Solar Energy Utilization Efficiency. ACS Nano 2021, 15, 19490–19502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Lv, L.; Zhao, Y.; Qu, L. Vertically aligned graphene sheets membrane for highly efficient solar thermal generation of clean water. ACS Nano 2017, 11, 5087–5093. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, H.; Huo, D.; Wu, F.; Shao, L.; Zheng, P.; Jiang, Y.; Zheng, X.; Qiu, X.; Liu, Y. N-doped graphene-based copper nanocomposite with ultralow electrical resistivity and high thermal conductivity. Sci. Rep. 2018, 8, 9248. [Google Scholar] [CrossRef] [Green Version]
- Phetsang, S.; Kidkhunthod, P.; Chanlek, N.; Jakmunee, J.; Mungkornasawakul, P.; Ounnunkad, K. Copper/reduced graphene oxide film modified electrode for non-enzymatic glucose sensing application. Sci. Rep. 2021, 11, 9302. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, L.; Yu, J.; Zheng, K.; Wang, G.; Chen, X. Improved performance of flexible graphene heater based on repeated laser writing. IEEE Electron Device Lett. 2020, 41, 501–504. [Google Scholar] [CrossRef]
- Lee, J.U.; Lee, C.W.; Cho, S.C.; Shin, B.S. Laser-Induced Graphene Heater Pad for De-icing. Nanomaterials 2021, 11, 3093. [Google Scholar] [CrossRef]
- Gent, R.W.; Dart, N.P.; Cansdale, J.T. Aircraft icing. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2000, 358, 2873–2911. [Google Scholar] [CrossRef]
- Cober, S.G.; Isaac, G.A.; Strapp, J.W. Characterizations of aircraft icing environments that include supercooled large drops. J. Appl. Meteorol. 2001, 40, 1984–2002. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Sullivan, C.R.; Kozlyuk, V.; Petrenko, F.V.; Veerasamy, V. Pulse electro-thermal de-icer (PETD). Cold Reg. Sci. Technol. 2011, 65, 70–78. [Google Scholar] [CrossRef]
- Mason, B.J. The growth of ice crystals in a supercooled water cloud. Q. J. R. Meteorol. Soc. 1953, 79, 104–111. [Google Scholar] [CrossRef]
- Karimi, G.; Lau, I.; Fowler, M.; Pope, M. Parametric study of laser-induced graphene conductive traces and their application as flexible heaters. Int. J. Energy Res. 2021, 45, 13712–13725. [Google Scholar] [CrossRef]
- Lee, J.-U.; Ma, Y.-W.; Jeong, S.-Y.; Shin, B.-S. Direct Fabrication of Ultra-Sensitive Humidity Sensor Based on Hair-Like Laser-Induced Graphene Patterns. Micromachines 2020, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Lee, C.W.; Lee, J.U.; Ma, Y.W.; Shin, B.S. Laser-Induced Biochar Formation through 355 nm Pulsed Laser Irradiation of Wood, and Application to Eco-Friendly pH Sensors. Nanomaterials. 2020, 10, 1904. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-U.; Lee, J.-h.; Lee, C.-W.; Cho, S.-C.; Hong, S.-M.; Ma, Y.-w.; Jeong, S.-Y.; Shin, B.-S. Green Synthesis of Laser-Induced Graphene with Copper Oxide Nanoparticles for Deicing Based on Photo-Electrothermal Effect. Nanomaterials 2022, 12, 960. https://doi.org/10.3390/nano12060960
Lee J-U, Lee J-h, Lee C-W, Cho S-C, Hong S-M, Ma Y-w, Jeong S-Y, Shin B-S. Green Synthesis of Laser-Induced Graphene with Copper Oxide Nanoparticles for Deicing Based on Photo-Electrothermal Effect. Nanomaterials. 2022; 12(6):960. https://doi.org/10.3390/nano12060960
Chicago/Turabian StyleLee, Jun-Uk, Jeong-hoon Lee, Chan-Woo Lee, Su-Chan Cho, Sung-Moo Hong, Yong-won Ma, Sung-Yeob Jeong, and Bo-Sung Shin. 2022. "Green Synthesis of Laser-Induced Graphene with Copper Oxide Nanoparticles for Deicing Based on Photo-Electrothermal Effect" Nanomaterials 12, no. 6: 960. https://doi.org/10.3390/nano12060960
APA StyleLee, J.-U., Lee, J.-h., Lee, C.-W., Cho, S.-C., Hong, S.-M., Ma, Y.-w., Jeong, S.-Y., & Shin, B.-S. (2022). Green Synthesis of Laser-Induced Graphene with Copper Oxide Nanoparticles for Deicing Based on Photo-Electrothermal Effect. Nanomaterials, 12(6), 960. https://doi.org/10.3390/nano12060960





