2D KBr/Graphene Heterostructures—Influence on Work Function and Friction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Atomic Force Microscopy
2.3. Computational Method
3. Results
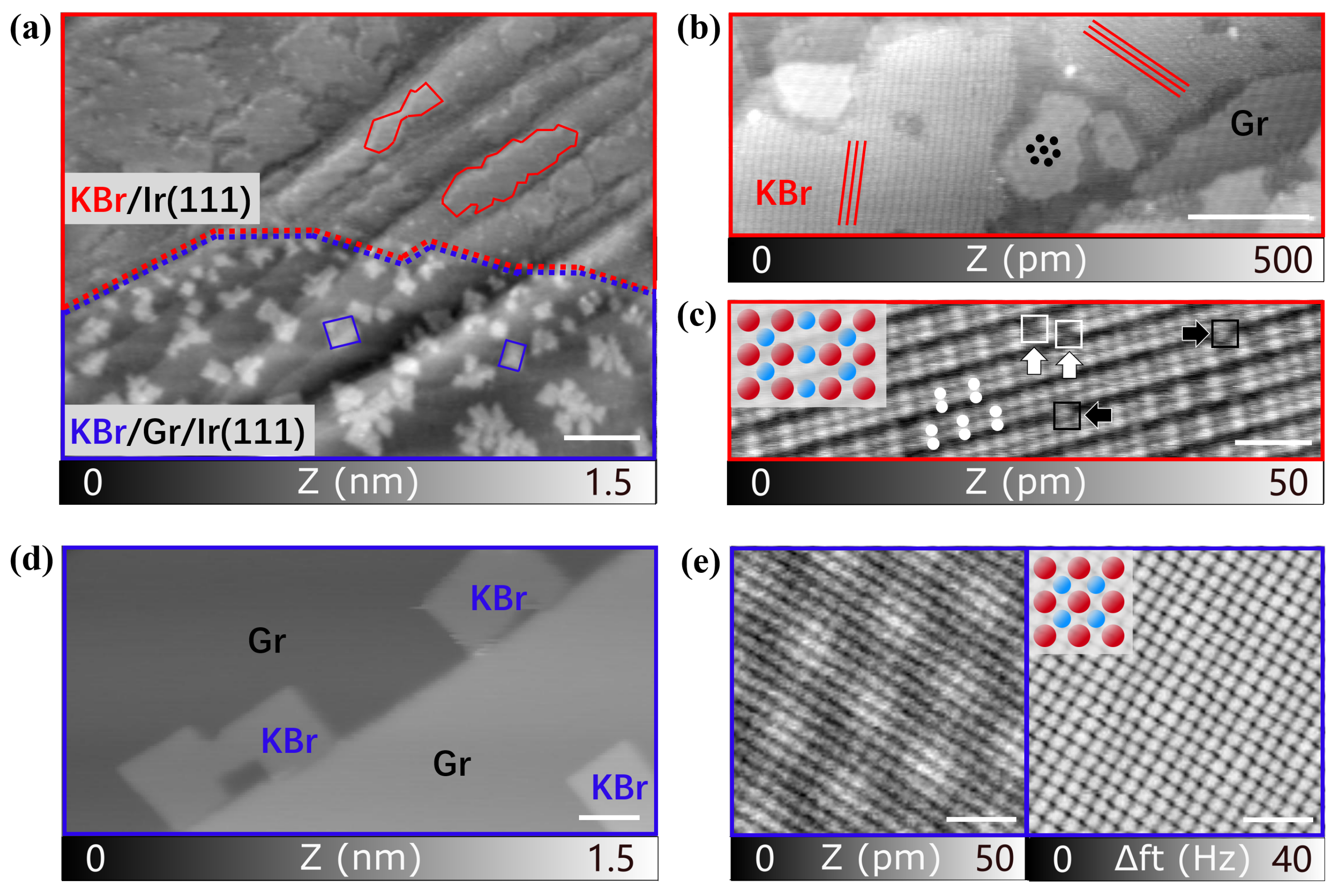
3.1. Structural Characterization
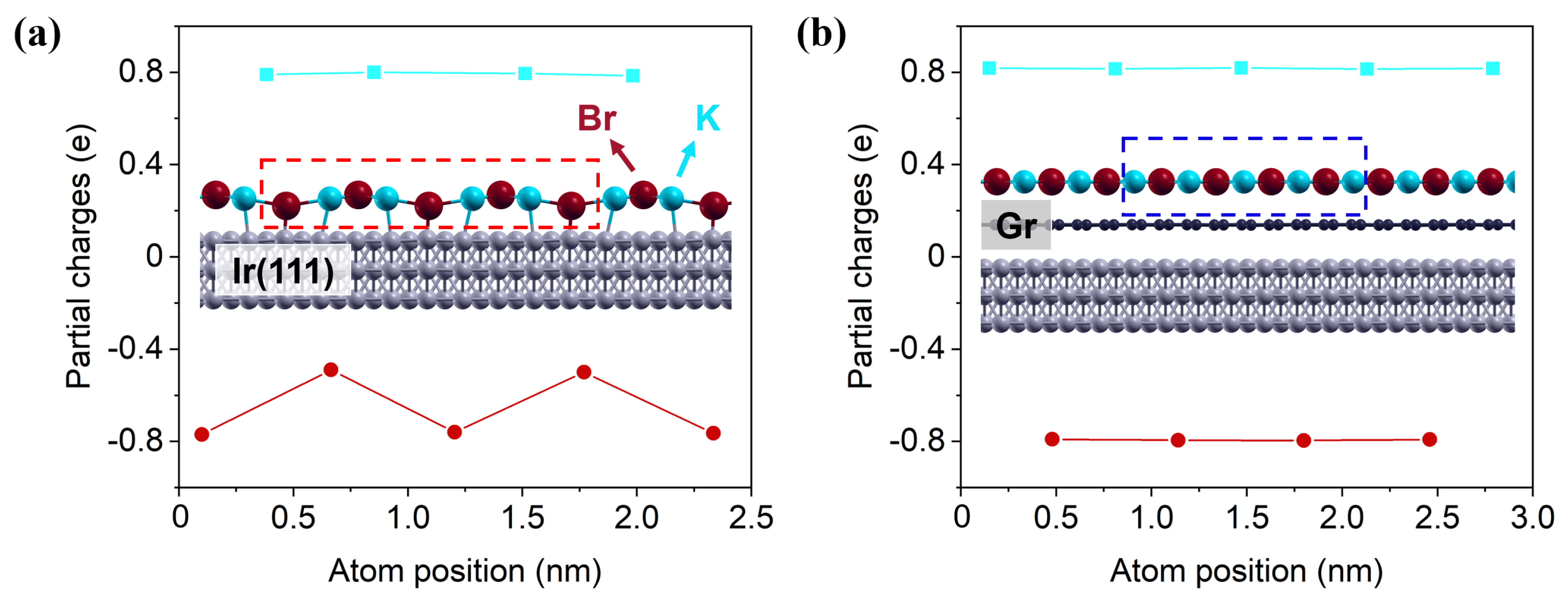
3.2. Work Function & Formation of Dipoles
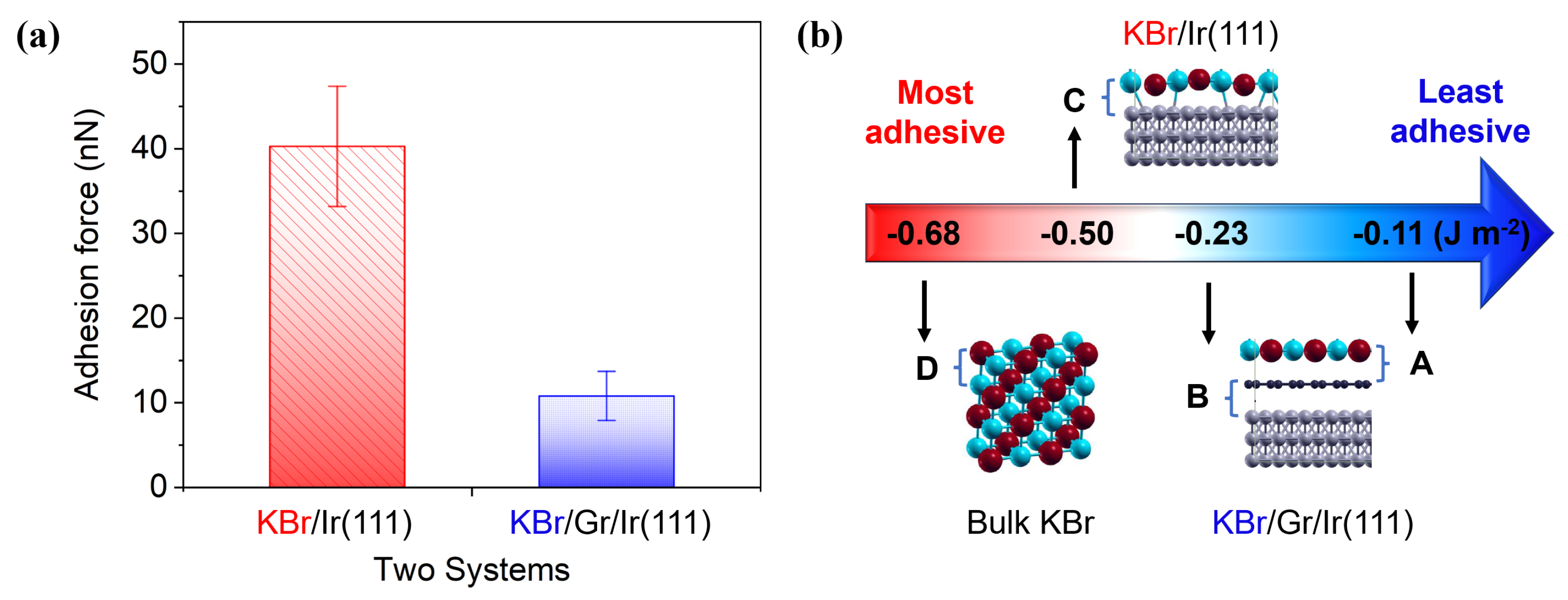
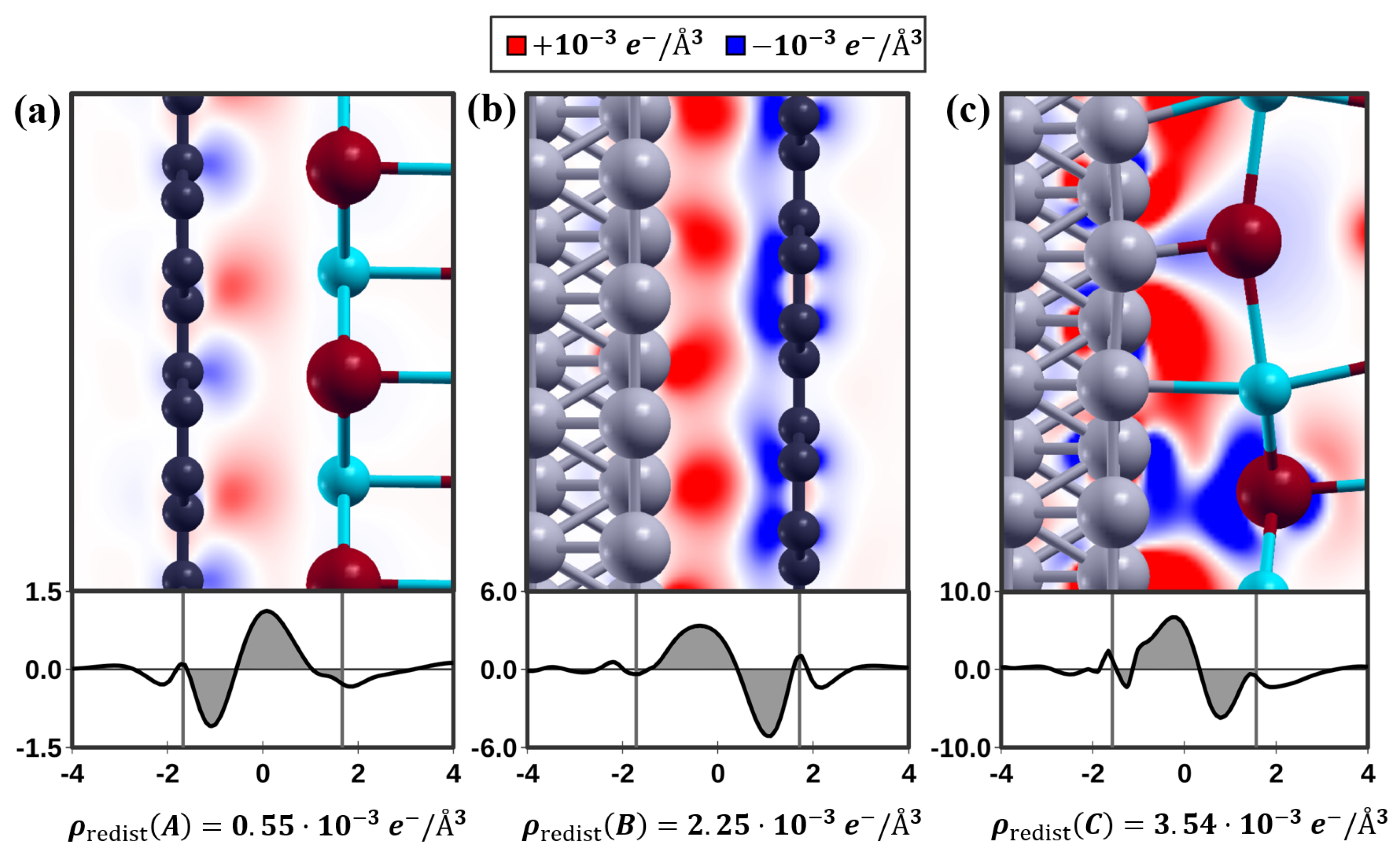
3.3. Friction and Adhesion Forces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, H.Y.; Li, Y.J.; Liu, H.B.; Yin, X.D.; Li, Y.L. Construction of heterostructure materials toward functionality. Chem. Soc. Rev. 2011, 40, 4506–4524. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2011, 5, 449–493. [Google Scholar] [CrossRef]
- Androulidakis, C. Zhang, K.H.; Robertson, M.; Tawfick, S. Tailoring the mechanical properties of 2D materials and heterostructures. 2D Mater. 2018, 5, 032005. [Google Scholar] [CrossRef]
- Frisenda, R.; Molina-Mendoza, A.J.; Mueller, T.; Castellanos-Gomez, A.; Van Der Zant, H.S.J. Atomically thin p–n junctions based on two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3339–3358. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.C.; Li, X.D.; Hu, T.T. Structural and Electronic Properties of Heterostructures Composed of Antimonene and Monolayer MoS2. Nanomaterials 2020, 10, 2358. [Google Scholar] [CrossRef]
- Yang, W.; Chen, G.R.; Shi, Z.W.; Liu, C.C.; Zhang, L.C.; Xie, G.B.; Cheng, M.; Wang, D.; Yang, R.; Shi, D.; et al. Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat. Mater. 2013, 12, 792–797. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Li, Y.P.; Li, D.Y. Experimental studies on relationships between the electron work function, adhesion, and friction for 3d transition metals. J. Appl. Phys. 2004, 95, 7961–7965. [Google Scholar] [CrossRef]
- Liu, S.Y.; Lu, H.; Li, D.Y. The relationship between the electron work function and friction behavior of passive alloys under different conditions. Appl. Surf. Sci. 2015, 351, 7961–7965. [Google Scholar] [CrossRef]
- Filleter, T.; Emtsev, K.V.; Seyller, T.; Bennewitz, R. Local work function measurements of epitaxial graphene. Appl. Phys. Lett. 2008, 93, 133117. [Google Scholar] [CrossRef]
- Filleter, T.; McChesney, J.L.; Bostwick, A.; Rotenberg, E.; Emtsev, K.V.; Seyller, T.; Horn, K.; Bennewitz, R. Friction and dissipation in epitaxial graphene films. Phys. Rev. Lett. 2009, 102, 086102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavini, F.; Caló, A.; Gao, Y.; Albisetti, E.; Cao, T.F.; Li, G.Q.; Cao, L.Y.; Aruta, C.; Riedo, E. Friction and work function oscillatory behavior for an even and odd number of layers in polycrystalline MoS2. Phys. Rev. Lett. 2018, 10, 8304–8312. [Google Scholar] [CrossRef] [PubMed]
- Sadewasser, S.; Glatzel, T. Kelvin Probe Force Microscopy Measuring and Compensating Electrostatic Forces; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Sadewasser, S.; Glatzel, T. Kelvin Probe Force Microscopy—From Single Charge Detection to Device Characterization; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gnecco, E.; Meyer, E. Fundamentals of Friction and Wear on the Nanoscale; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Li, B.; Geng, Y.Q.; Yan, Y.D. Nano/microscale thermal field distribution: Conducting thermal decomposition of pyrolytic-type polymer by heated AFM probes. Nanomaterials 2020, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, Y.F.; Glatzel, T.; Hinaut, A.; Zhang, J.Y.; Meyer, E. Low Friction at the nanoscale of hydrogenated fullerene-like carbon films. Coatings 2020, 10, 643. [Google Scholar] [CrossRef]
- Kawai, S.; Glatzel, T.; Koch, S.; Such, B.; Baratoff, A.; Meyer, E. Systematic achievement of improved atomic-scale contrast via bimodal dynamic force microscopy. Phys. Rev. Lett. 2009, 103, 220801. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Glatzel, T.; Koch, S.; Such, B.; Baratoff, A.; Meyer, E. Ultrasensitive detection of lateral atomic-scale interactions on graphite(0001) via bimodal dynamic force measurements. Phys. Rev. B 2010, 81, 085420. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology and nanomechanics. Wear 2005, 259, 1507–1531. [Google Scholar] [CrossRef]
- Liu, Z.; Hinaut, A.; Peeters, S.; Scherb, S.; Meyer, E.; Righi, M.C.; Glatzel, T. Reconstruction of a 2D layer of KBr on Ir(111) and electromechanical alteration by graphene. Beilstein J. Nanotechnol. 2021, 12, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, S.; Restuccia, P.; Loehlé, S.; Thiebaut, B.; Righi, M.C. Tribochemical reactions of MoDTC lubricant additives with iron by quantum mechanics/molecular mechanics simulations. J. Phys. Chem. C 2020, 124, 13688–13694. [Google Scholar] [CrossRef]
- Corsini, C.; Peeters, S.; Righi, M.C. Adsorption and dissociation of Ni(acac)2 on iron by ab initio calculations. J. Phys. Chem. A 2020, 124, 8005–8010. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.; Charrin, C.; Duron, I.; Loehlé, S.; Thiebaut, B.; Righi, M.C. Importance of the catalytic effect of the substrate in the functionality of lubricant additives: The case of molybdenum dithiocarbamates. J. Mater. Today Chem. 2021, 21, 100487. [Google Scholar] [CrossRef]
- Losi, G.; Peeters, S.; Delayens, F.; Vezin, H.; Loehlé, S.; Thiebaut, B.; Righi, M.C. Experimental and ab initio characterization of mononuclear molybdenum dithiocarbamates in lubricant mixtures. Langmuir 2021, 37, 4836–4846. [Google Scholar] [CrossRef]
- Janthon, P.; Viñes, F.; Sirijaraensre, J.; Limtrakul, J.; Illas, F. Adding pieces to the CO/Pt(111) puzzle: The role of dispersion. J. Phys. Chem. C 2017, 121, 3970–3977. [Google Scholar] [CrossRef] [Green Version]
- Cutini, M.; Maschio, L.; Ugliengo, P. Exfoliation energy of layered materials by DFT-D: Beware of dispersion! J. Chem. Theory Comput. 2020, 16, 5244–5252. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comp. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [Green Version]
- Wolloch, M.; Levita, G.; Restuccia, P.; Righi, M.C. Interfacial charge density and its connection to adhesion and frictional forces. Phys. Rev. Lett. 2018, 121, 026804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comp. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Glatzel, T.; Zimmerli, L.; Koch, S.; Such, B.; Kawai, S.; Meyer, E. Determination of effective tip geometries in Kelvin probe force microscopy on thin insulating films on metals. Nanotechnology 2009, 20, 264016. [Google Scholar] [CrossRef] [PubMed]
- Filleter, T.; Paul, W.; Bennewitz, R. Atomic structure and friction of ultrathin films of KBr on Cu(100). Phys. Rev. B 2008, 77, 035430. [Google Scholar] [CrossRef]
- Holmberg, N.; Laasonen, K.; Peljo, P. Charge distribution and Fermi level in bimetallic nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 2924–2931. [Google Scholar] [CrossRef] [Green Version]
- Lüthi, R.; Meyer, E.; Howald, L.; Bammerlin, M.; Güntherodt, H.J.; Gyalog, T.; Thomas, H. Friction force microscopy in ultrahigh vacuum: An atomic-scale study on KBr(001). Phys. Chem. Chem. Phys. 1995, 1, 129–138. [Google Scholar]
- Wieferink, C.; Krüger, P.; Pollmann, J. Simulations of friction force microscopy on the KBr(001) surface based on ab initio calculated tip-sample forces. Phys. Rev. B 2011, 83, 235328. [Google Scholar] [CrossRef]
- Coraux, J.; Plasa, T.N.; Busse, C.; Michely, T. Structure of epitaxial graphene on Ir(111). New J. Phys. 2008, 10, 043033. [Google Scholar]
- Busse, C.; Lazić, P.; Djemour, R.; Coraux, J.; Gerber, T.; Atodiresei, N.; Caciuc, V.; Brako, R.; Blügel, S.; Zegenhagen, J.; et al. Graphene on Ir(111): Physisorption with chemical modulation. Phys. Rev. Lett. 2011, 107, 036101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrel, S. Chemical Vapor Deposition Growth of Molybdenum Disulfide and Its Nanoscale Tribological Correlation with Raman Spectroscopy; Rutgers The State University of New Jersey-New Brunswick: New Brunswick, NJ, USA, 2017. [Google Scholar]

| System | (eV) | (eV) |
|---|---|---|
| Ir(111) | 5.76 | 5.77 |
| KBr /Gr/Ir(111) | 4.71 | 4.96 |
| Gr/Ir(111) | 4.56 | 4.74 |
| KBr/Ir(111) | 4.06 | 4.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Hinaut, A.; Peeters, S.; Scherb, S.; Meyer, E.; Righi, M.C.; Glatzel, T. 2D KBr/Graphene Heterostructures—Influence on Work Function and Friction. Nanomaterials 2022, 12, 968. https://doi.org/10.3390/nano12060968
Liu Z, Hinaut A, Peeters S, Scherb S, Meyer E, Righi MC, Glatzel T. 2D KBr/Graphene Heterostructures—Influence on Work Function and Friction. Nanomaterials. 2022; 12(6):968. https://doi.org/10.3390/nano12060968
Chicago/Turabian StyleLiu, Zhao, Antoine Hinaut, Stefan Peeters, Sebastian Scherb, Ernst Meyer, Maria Clelia Righi, and Thilo Glatzel. 2022. "2D KBr/Graphene Heterostructures—Influence on Work Function and Friction" Nanomaterials 12, no. 6: 968. https://doi.org/10.3390/nano12060968
APA StyleLiu, Z., Hinaut, A., Peeters, S., Scherb, S., Meyer, E., Righi, M. C., & Glatzel, T. (2022). 2D KBr/Graphene Heterostructures—Influence on Work Function and Friction. Nanomaterials, 12(6), 968. https://doi.org/10.3390/nano12060968









