Surface Transformation of Spin-on-Carbon Film via Forming Carbon Iron Complex for Remarkably Enhanced Polishing Rate
Abstract
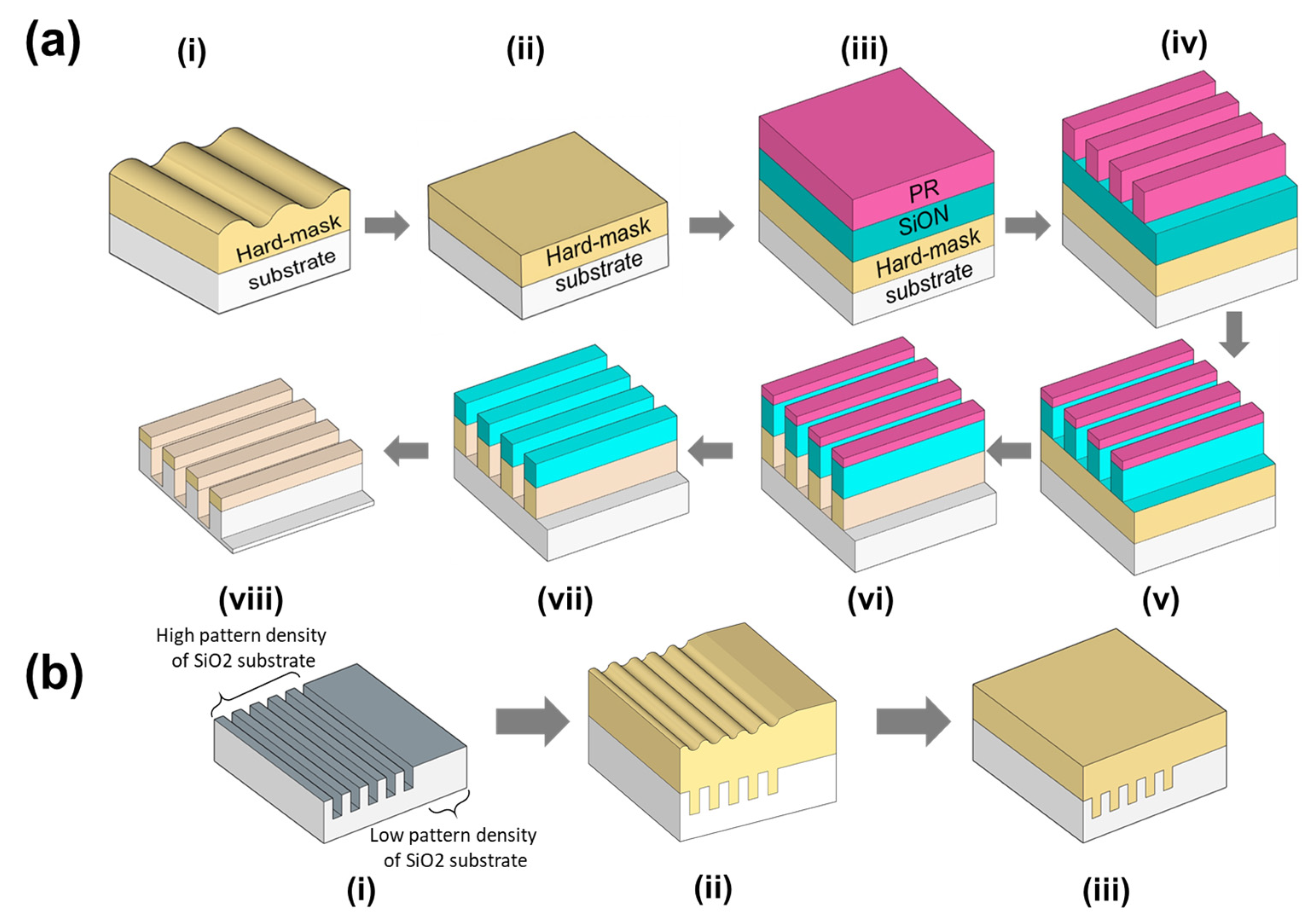
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CMP Conditions
2.3. Characterization
3. Results and Discussion
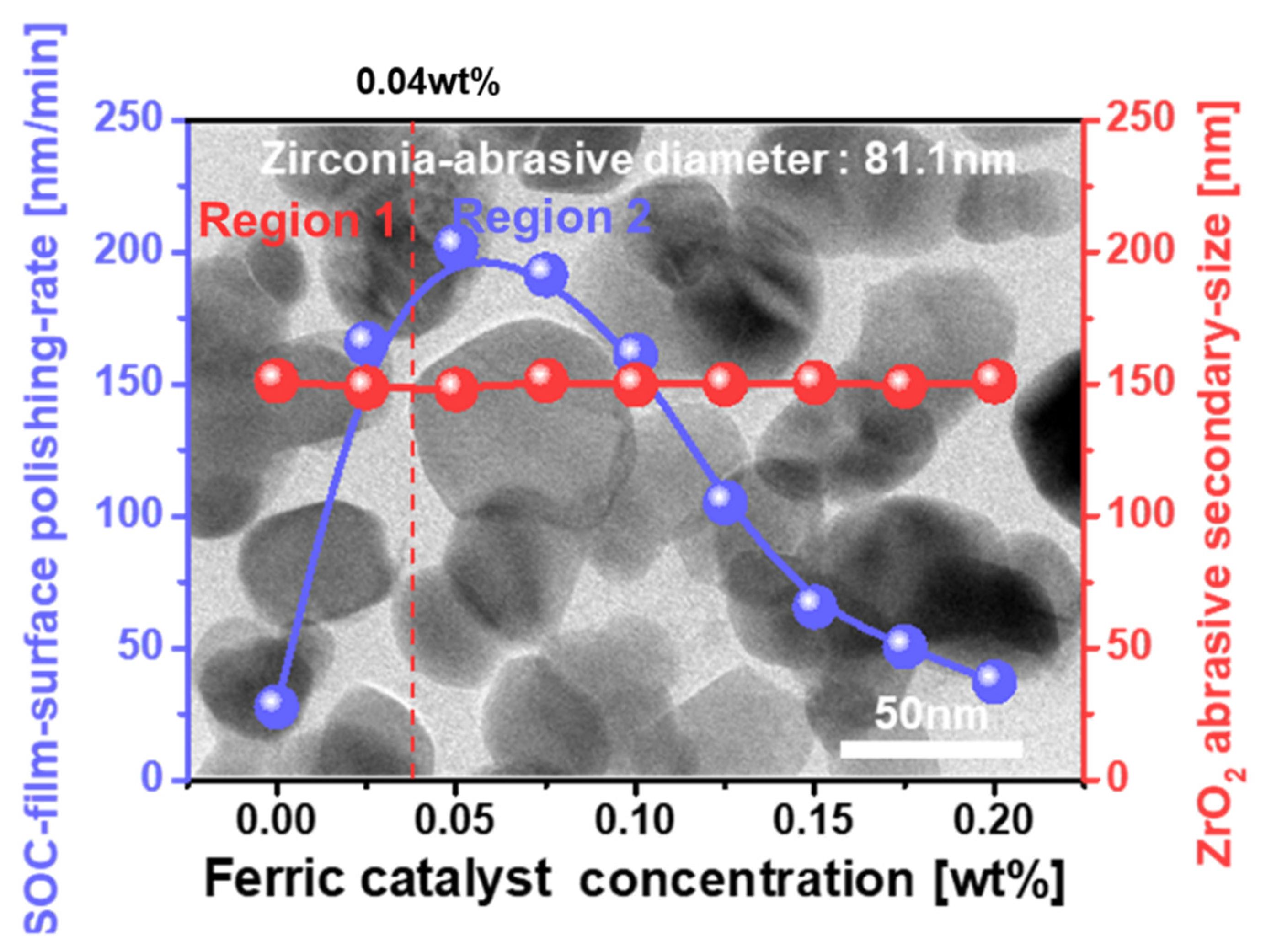
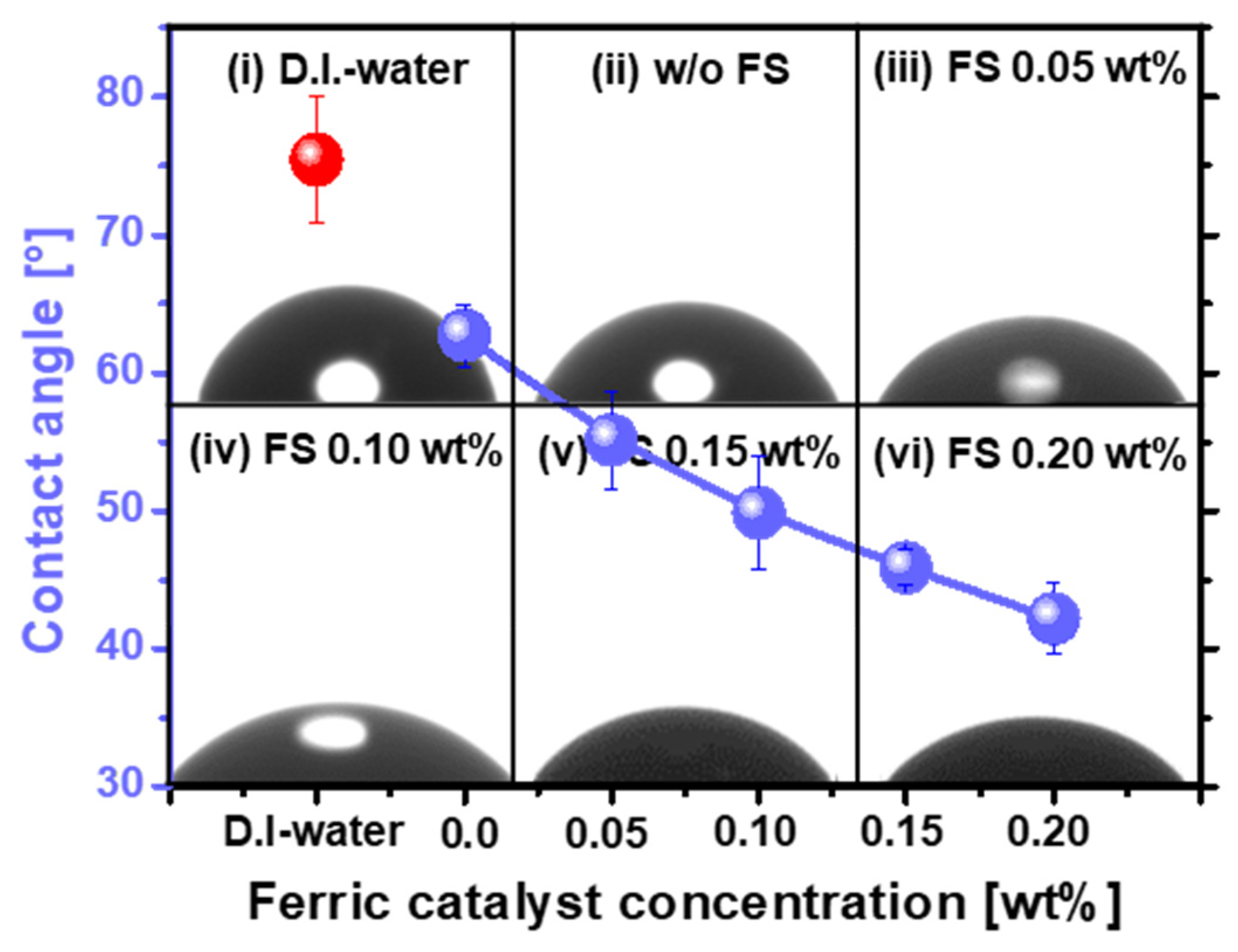
3.1. Dependency of SOC Film Polishing Rate on Ferric Catalyst (Fe2(SO4)3H2O) Concentration
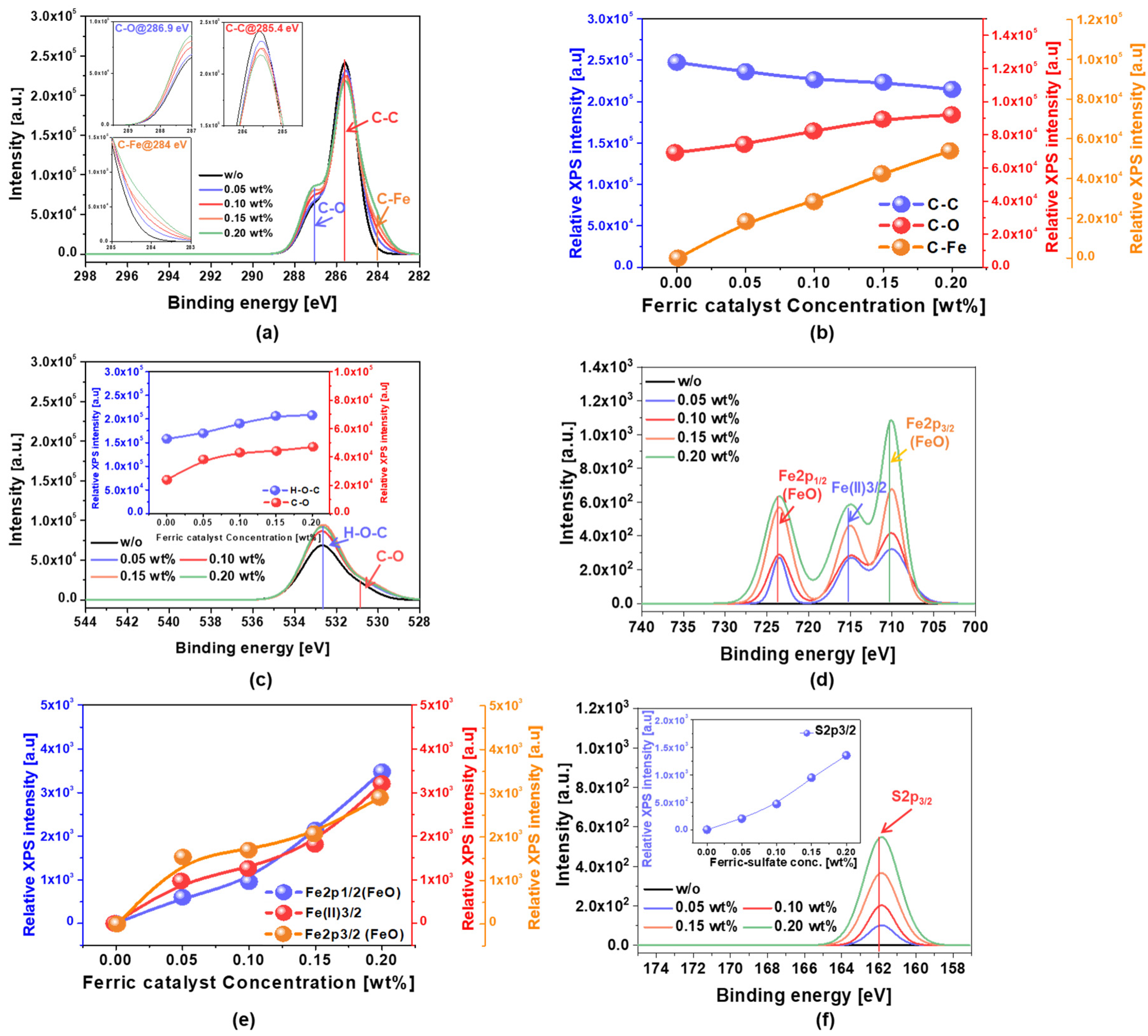
3.2. Dependency of the C-C Bonds Breakage on the Ferric Catalyst (Fe2(SO4)3H2O) Concentration in the SOC Film CMP Slurry
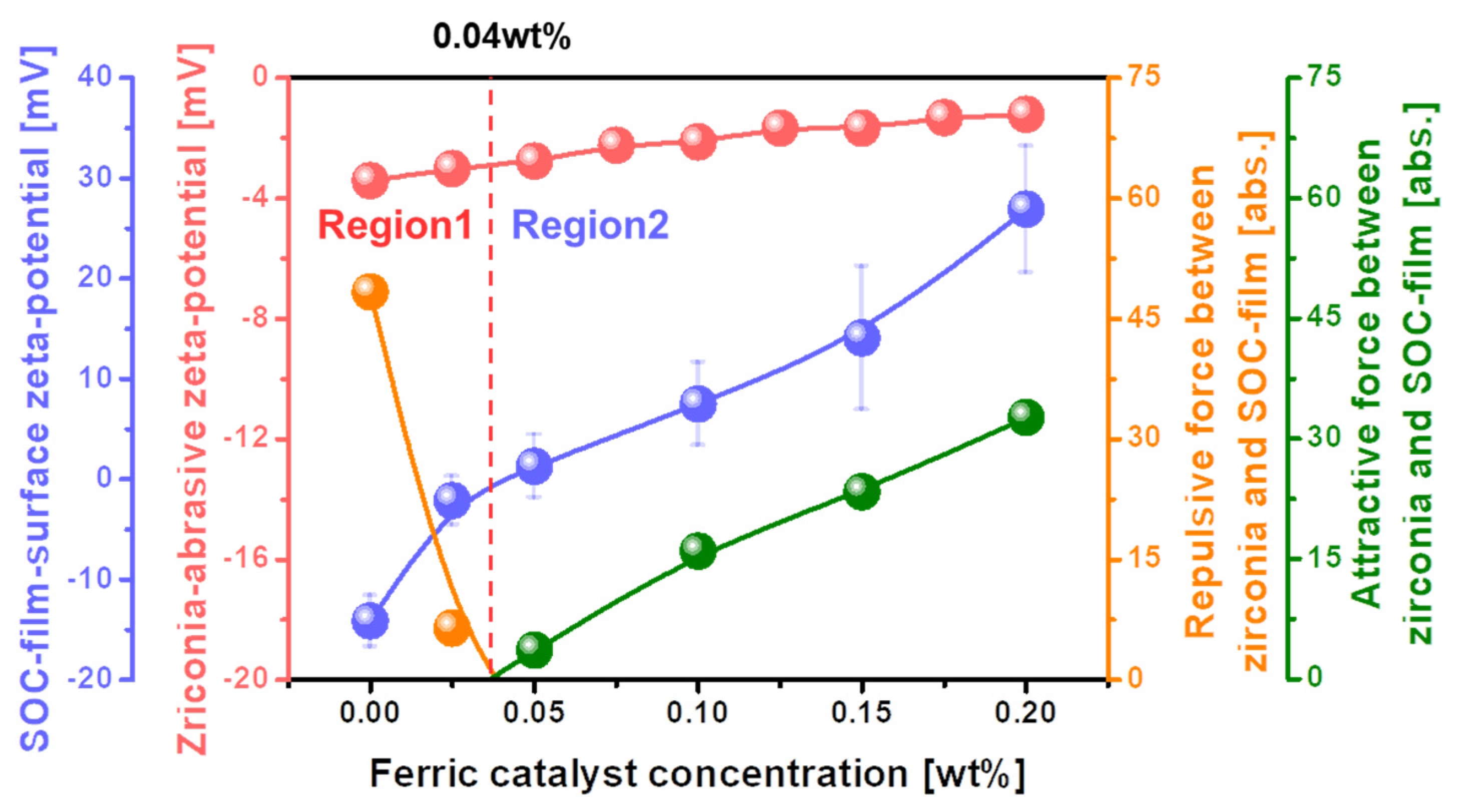
3.3. Dependency of the Mechanical Properties (i.e., Electrostatic Force between Abrasive and SOC Film Surface and Absorption Degree of CMP Slurry) on the Ferric Catalyst (i.e., Fe2(SO4)3) Concentration
3.4. C-C Bond Breakage Mechanism of the SOC Film Surface during CMP Using Ferric Catalyst (i.e., Fe2(SO4)3) Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, G.; Bae, D.I.; Kang, M.; Hwang, S.M.; Kim, S.S.; Seo, B.; Kwon, T.Y.; Lee, T.J.; Moon, C.; Choi, Y.M.; et al. 3nm GAA Technology featuring Multi-Bridge-Channel FET for Low Power and High Performance Applications. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018; pp. 28.7.1–28.7.4. [Google Scholar]
- Li, Y. 3D NAND Memory and Its Application in Solid-State Drives: Architecture, Reliability, Flash Management Techniques, and Current Trends. IEEE Solid-State Circuits Mag. 2020, 12, 56–65. [Google Scholar] [CrossRef]
- Goda, A. 3-D NAND Technology Achievements and Future Scaling Perspectives. IEEE Trans. Electron Devices 2020, 67, 1373–1381. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Ramaswamy, N.; Mouli, C. Memory Technology: Innovations needed for continued technology scaling and enabling advanced computing systems. In Proceedings of the 2020 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 12—18 December 2020; pp. 10.1.1–10.1.8. [Google Scholar] [CrossRef]
- Maejima, H.; Kanda, K.; Fujimura, S.; Takagiwa, T.; Ozawa, S.; Sato, J.; Shindo, Y.; Sato, M.; Kanagawa, N.; Musha, J.; et al. A 512Gb 3b/Cell 3D flash memory on a 96-word-line-layer technology. In Proceedings of the 2018 IEEE International Solid-State Circuits Conference—(ISSCC), San Francisco, CA, USA, 11–15 February 2018; pp. 336–338. [Google Scholar] [CrossRef]
- Wagner, C.; Harned, N. EUV lithography: Lithography gets extreme. Nat. Photon. 2010, 4, 24–26. [Google Scholar] [CrossRef]
- Owa, S.; Nagasaka, H. Advantage and feasibility of immersion lithography. J. Microlithogr. Microfabr. Microsyst. 2004, 3, 97–103. [Google Scholar] [CrossRef]
- Mack, C.A. Pattern collapse. Microlithogr. World 2006, 15, 16–17. [Google Scholar]
- Domke, W.-D.; Graffenberg, V.L.; Patel, S.; Rich, G.K.; Cao, H.B.; Nealey, P.F. Pattern collapse in high-aspect-ratio DUV and 193-nm resists. In Advances in Resist Technology and Processing XVII, Proceedings of the Microlithography 2000, Santa Clara, CA, USA, 27 February–3 March 2000; SPIE: Bellingham, WA, USA; Volume 3999, pp. 313–321. [CrossRef]
- Noya, G.; Hama, Y.; Ishii, M.; Nakasugi, S.; Kudo, T.; Padmanaban, M. Planarization of topography with spin-on carbon hard mask. In Advances in Patterning Materials and Processes XXXIII, Proceedings of the SPIE Advanced Lithography, San Jose, CA, USA, 21–25 February 2016; SPIE: Bellingham, WA, USA; Volume 9779, p. 97791I. [CrossRef]
- Weigand, M.; Krishnamurthy, V.; Wang, Y.; Lin, Q.; Guerrero, D.; Simmons, S.; Carr, B. Evaluating spin-on carbon materials at low temperatures for high wiggling resistance. In Advanced Etch Technology for Nanopatterning II, Proceedings of the SPIE Advanced Lithography, San Jose, CA, USA, 24–28 February 2013; SPIE: Bellingham, WA, USA; Volume 8685, p. 86850R. [CrossRef]
- Lai, C.C.; Hsing Chang, Y.; Ju Chien, H.; Chen Lu, M. Amorphous carbon process optimization to increase hard mask and lithographic capabilities by its step coverage improvement. In Proceedings of the 2017 6th International Symposium on Next Generation Electronics (ISNE), Keelung, Taiwan, 23–25 May 2017; pp. 6–7. [Google Scholar] [CrossRef]
- Abdallah, D.J.; McKenzie, D.; Timko, A.; Dioses, A.; Houlihan, F.; Rahman, D.; Miyazaki, S.; Zhang, R.; Kim, W.; Wu, H.; et al. Spin-on tri-layer approaches to high NA 193-nm lithography. In Advances in Resist Materials and Processing Technology XXIV, Proceedings of the SPIE Advanced Lithography, San Jose, CA, USA, 25 February–2 March 2007; SPIE: Bellingham, WA, USA; Volume 6519, p. 65190M. [CrossRef]
- Komura, K.; Hishiro, Y.; Wakamatsu, G.; Takimoto, Y.; Nagai, T.; Kimura, T.; Yamaguchi, Y.; Shimokawa, T.; Breyta, G.; Arellano, N.; et al. Spin-on organic hardmask for topo-patterned substrate. In Advances in Patterning Materials and Processes XXXI, Proceedings of the SPIE Advanced Lithography, San Jose, CA, USA, 23–27 February 2014; SPIE: Bellingham, WA, USA; Volume 9051, p. 905115. [CrossRef]
- Hah, J.H.; Chae, Y.S.; Jang, Y.-K.; Ryoo, M.; Choi, S.-J.; Woo, S.-G.; Cho, H.-K.; Moon, J.-T. Spin-on hard mask with dual-BARC property for 50-nm devices. In Advances in Resist Technology and Processing XXIII, Proceedings of the SPIE 31st International Symposium on Advanced Lithography, San Jose, CA, USA, 19–24 February 2006; SPIE: Bellingham, WA, USA; Volume 6153, p. 61530L. [CrossRef]
- Choi, S.-J. Novel spin-on hard mask with Si-containing bottom antireflective coating for nanolithography. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2007, 25, 868. [Google Scholar] [CrossRef]
- Kudo, T.; Rahman, M.D.; McKenzie, D.; Anyadiegwu, C.; Doerrenbaecher, S.; Zahn, W.; Padmanaban, M. Development of spin-on-carbon hard mask for advanced node. In Advances in Patterning Materials and Processes XXXI, Proceedings of the SPIE Advanced Lithography, San Jose, CA, USA, 23-27 February 2014; SPIE: Bellingham, WA, USA; Volume 9051, p. 90511X. [CrossRef]
- Padmanaban, M.; Cho, J.Y.; Kudo, T.; Rahman, D.; Yao, H.; McKenzie, D.; Dioses, A.; Mullen, S.; Wolfer, E.; Yamamoto, K.; et al. Progress in Spin-on Hard Mask Materials for Advanced Lithography. J. Photopolym. Sci. Technol. 2014, 27, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.; Kim, J.; Kim, Y.; Jeong, S.; Lim, S.; Yu, J.Y. Study on planarization performance of spin on hardmask. In Proceedings of the 2018 China Semiconductor Technology International Conference (CSTIC), Shanghai, China, 11–12 March 2018; pp. 1–3. [Google Scholar] [CrossRef]
- Dolgov, A.; Lopaev, D.; Lee, C.J.; Zoethout, E.; Medvedev, V.; Yakushev, O.; Bijkerk, F. Characterization of carbon contamination under ion and hot atom bombardment in a tin-plasma extreme ultraviolet light source. Appl. Surf. Sci. 2015, 353, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a potassium promoter on the Fischer–Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon. Catal. Sci. Technol. 2019, 9, 2728–2741. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Y.; Xu, J. Synthesis of Ag–Ho, Ag–Sm, Ag–Zn, Ag–Cu, Ag–Cs, Ag–Zr, Ag–Er, Ag–Y and Ag–Co metal organic nanoparticles for UV-Vis-NIR wide-range bio-tissue imaging. Photochem. Photobiol. Sci. 2019, 18, 1081–1091. [Google Scholar] [CrossRef]
- Sivaranjini, B.; Mangaiyarkarasi, R.; Ganesh, V.; Umadevi, S. Vertical Alignment of Liquid Crystals Over a Functionalized Flexible Substrate. Sci. Rep. 2018, 8, 8891. [Google Scholar] [CrossRef] [PubMed]
- De Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of Monolayer MoS2 Quantum Dots using Temporally Shaped Femtosecond Laser Ablation of Bulk MoS2 Targets in Water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-H.; Jeong, G.-P.; Kim, P.-S.; Han, M.-H.; Hong, S.-W.; Bae, J.-Y.; Kim, S.-I.; Park, J.-H.; Park, J.-G. Super fine cerium hydroxide abrasives for SiO2 film chemical mechanical planarization performing scratch free. Sci. Rep. 2021, 11, 17736. [Google Scholar] [CrossRef]
- Seo, E.-B.; Bae, J.-Y.; Kim, S.-I.; Choi, H.-E.; Kim, P.; Lee, J.-C.; Son, Y.-H.; Yun, S.-S.; Park, J.-H.; Park, J.-G. Influence of Scavenger on Abrasive Stability Enhancement and Chemical and Mechanical Properties for Tungsten-Film Chemical- Mechanical-Planarization. ECS J. Solid State Sci. Technol. 2020, 9, 065001. [Google Scholar] [CrossRef]
- Seo, E.-B.; Bae, J.-Y.; Kim, S.-I.; Choi, H.-E.; Son, Y.-H.; Yun, S.-S.; Park, J.-H.; Park, J.-G. Interfacial Chemical and Mechanical Reactions between Tungsten-Film and Nano-Scale Colloidal Zirconia Abrasives for Chemical-Mechanical-Planarization. ECS J. Solid State Sci. Technol. 2020, 9, 054001. [Google Scholar] [CrossRef]
- Kim, S.-I.; Jeong, G.-P.; Lee, S.-J.; Lee, J.-C.; Lee, J.-M.; Park, J.-H.; Bae, J.-Y.; Park, J.-G. Scavenger with Protonated Phosphite Ions for Incredible Nanoscale ZrO2-Abrasive Dispersant Stability Enhancement and Related Tungsten-Film Surface Chemical–Mechanical Planarization. Nanomaterials 2021, 11, 3296. [Google Scholar] [CrossRef]
- Mason, M.; Weaver, W. The Settling of Small Particles in a Fluid. Phys. Rev. 1924, 23, 412–426. [Google Scholar] [CrossRef]
- Bergman, R.G. Organometallic chemistry C-H activation. Nature 2007, 446, 391–393. [Google Scholar] [CrossRef]
- Goldman, A.S.; Goldberg, K.I. Organometallic C—H Bond Activation: An Introduction. ACS Symp. Ser. 2004, 885, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Lyons, T.W.; Sanford, M.S.; Formation, I.C.N.B. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-Catalyzed C−C Bond Formation via Heteroatom-Directed C−H Bond Activation. Chem. Rev. 2009, 110, 624–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, C. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 2004, 33, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lin, Z. β-Heteroatom versus β-Hydrogen Elimination: A Theoretical Study. Organometallics 2006, 25, 812–819. [Google Scholar] [CrossRef]
- Goldman, A.S. Carbon-Carbon bonds get a break. Nature 2010, 463, 435–436. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-M.; Lee, J.-C.; Kim, S.-I.; Lee, S.-J.; Bae, J.-Y.; Park, J.-H.; Park, J.-G. Surface Transformation of Spin-on-Carbon Film via Forming Carbon Iron Complex for Remarkably Enhanced Polishing Rate. Nanomaterials 2022, 12, 969. https://doi.org/10.3390/nano12060969
Lee J-M, Lee J-C, Kim S-I, Lee S-J, Bae J-Y, Park J-H, Park J-G. Surface Transformation of Spin-on-Carbon Film via Forming Carbon Iron Complex for Remarkably Enhanced Polishing Rate. Nanomaterials. 2022; 12(6):969. https://doi.org/10.3390/nano12060969
Chicago/Turabian StyleLee, Jun-Myeong, Jong-Chan Lee, Seong-In Kim, Seung-Jae Lee, Jae-Yung Bae, Jin-Hyung Park, and Jea-Gun Park. 2022. "Surface Transformation of Spin-on-Carbon Film via Forming Carbon Iron Complex for Remarkably Enhanced Polishing Rate" Nanomaterials 12, no. 6: 969. https://doi.org/10.3390/nano12060969
APA StyleLee, J.-M., Lee, J.-C., Kim, S.-I., Lee, S.-J., Bae, J.-Y., Park, J.-H., & Park, J.-G. (2022). Surface Transformation of Spin-on-Carbon Film via Forming Carbon Iron Complex for Remarkably Enhanced Polishing Rate. Nanomaterials, 12(6), 969. https://doi.org/10.3390/nano12060969






