Plasmonic Superstructure Arrays Fabricated by Laser Near-Field Reduction for Wide-Range SERS Analysis of Fluorescent Materials
Abstract
:1. Introduction
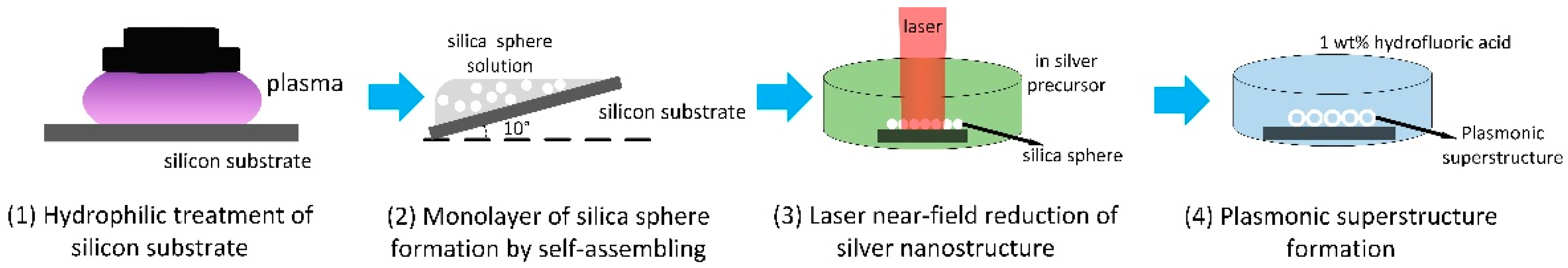
2. Materials and Methods
2.1. Silver Precursor Preparation
2.2. Monolayer Formation of Silica Microspheres
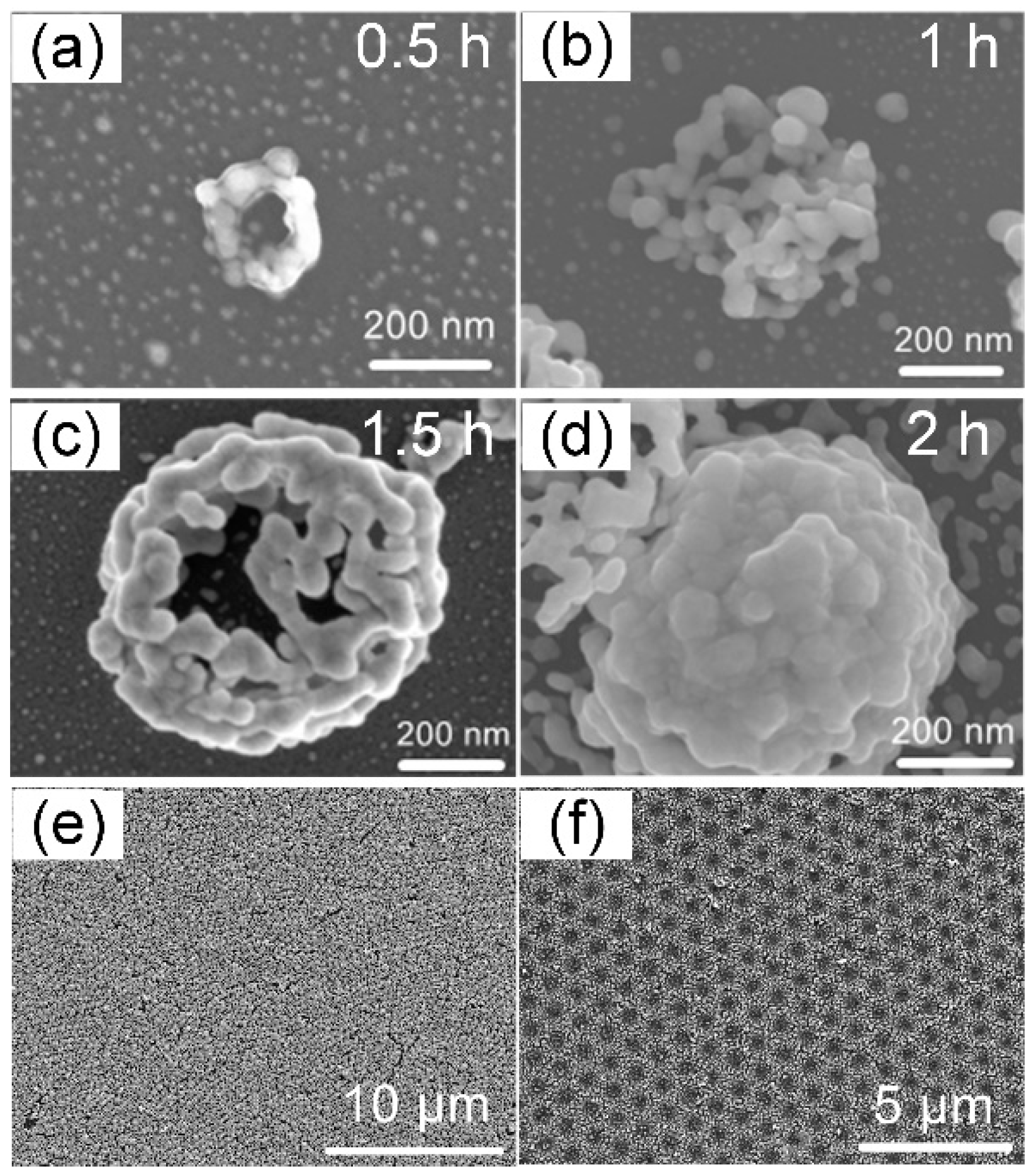
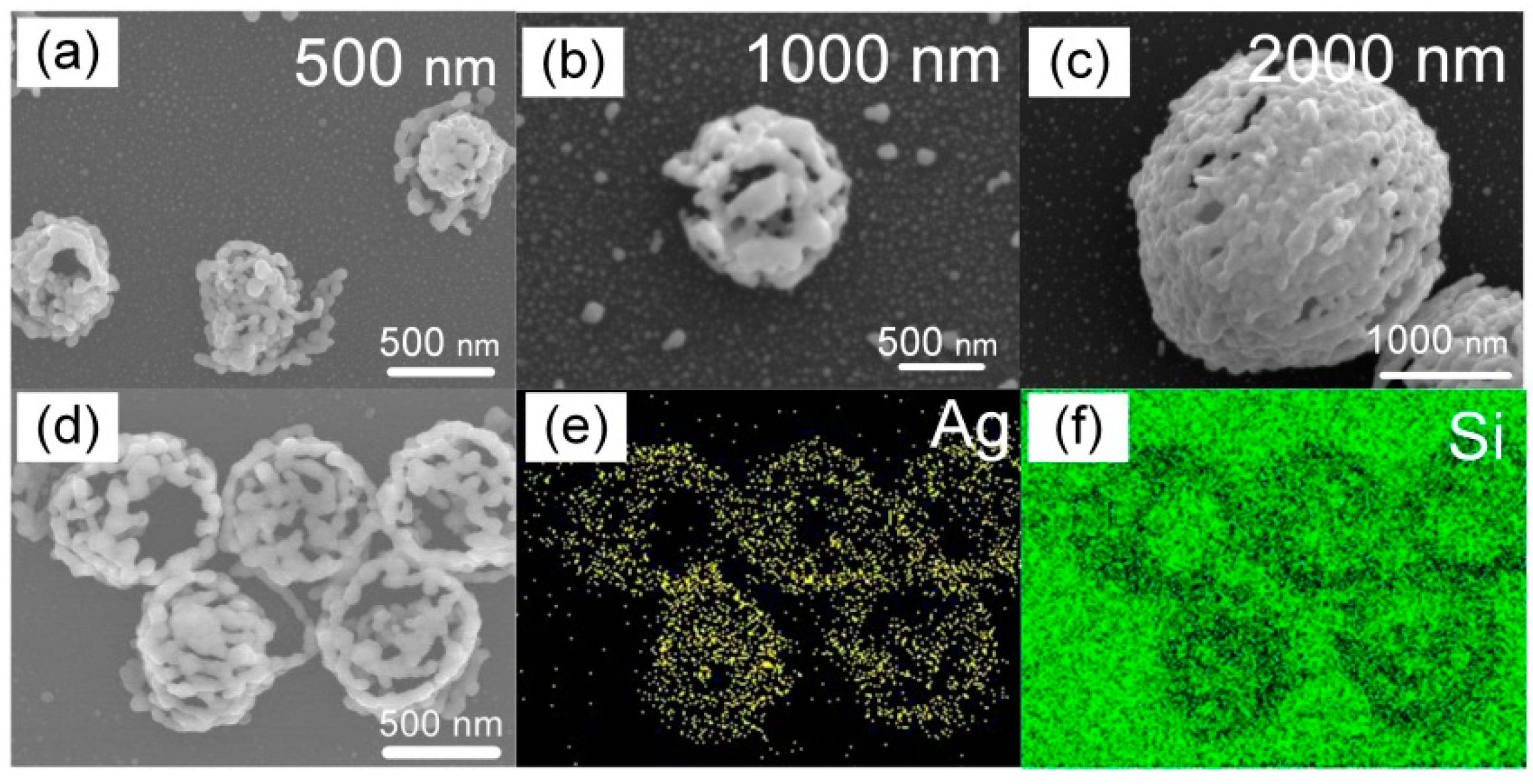
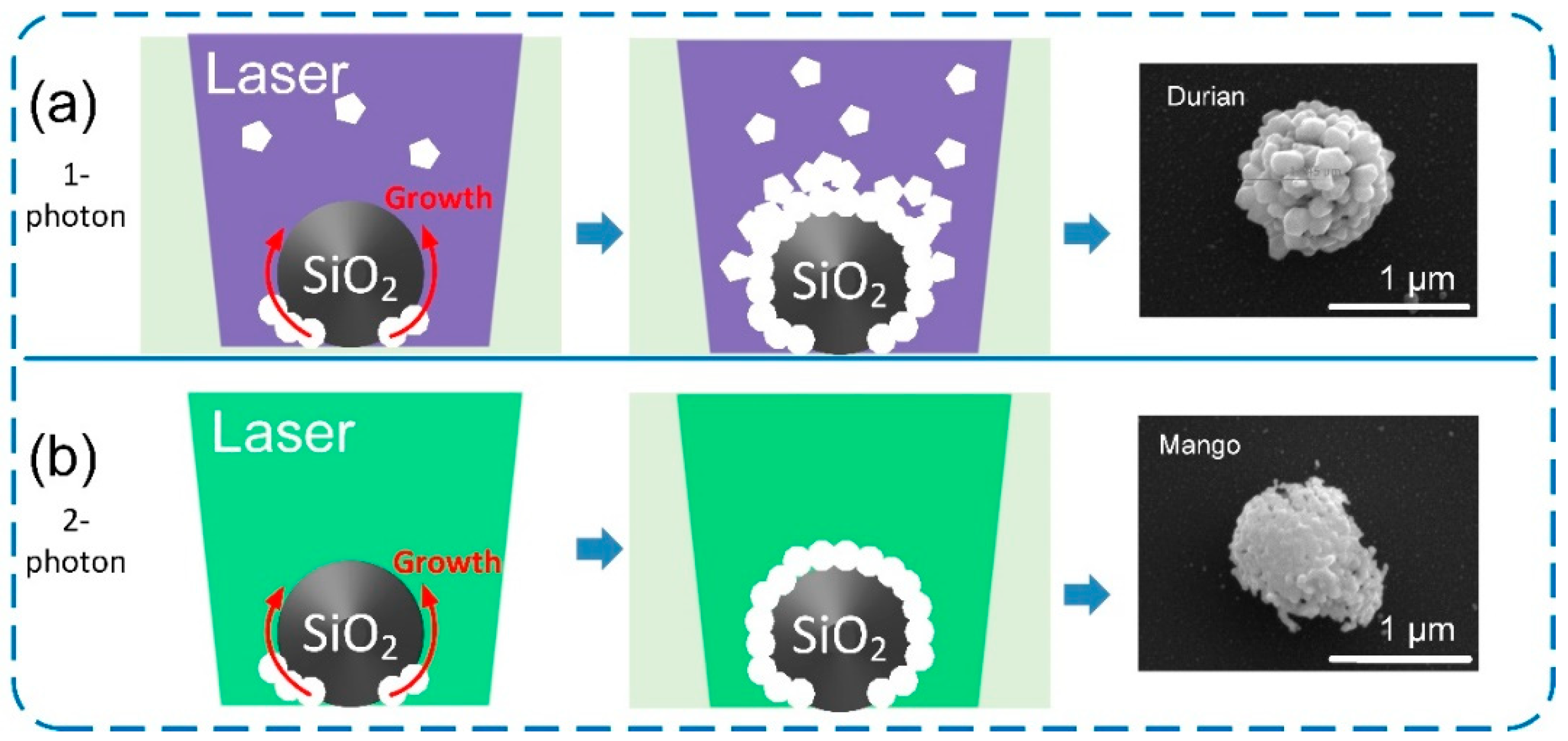
2.3. Laser Near-Field Reduction
2.4. Simulation of Local Electric Field on Plasmonic Superstructure
2.5. Characterization and SERS Measurements
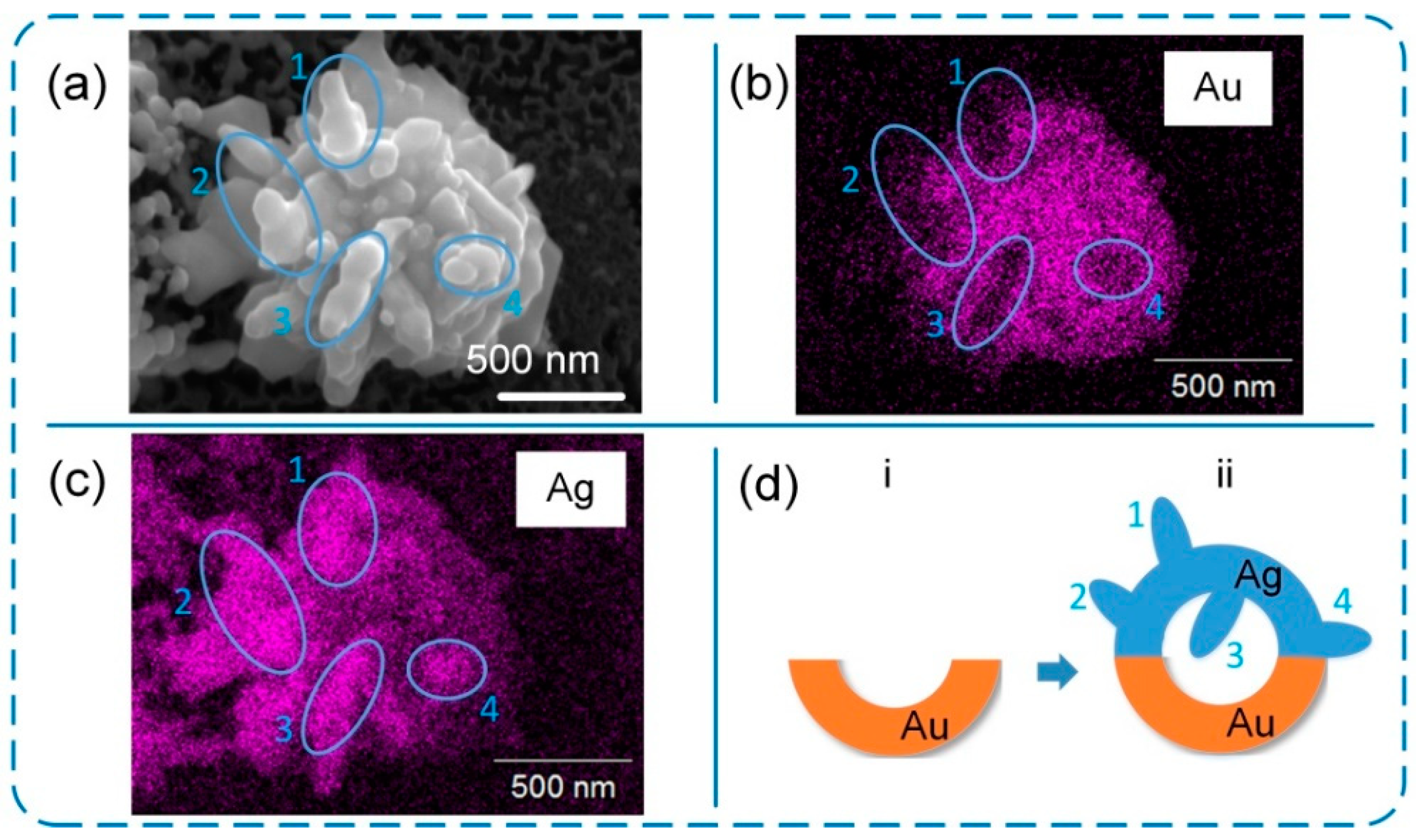
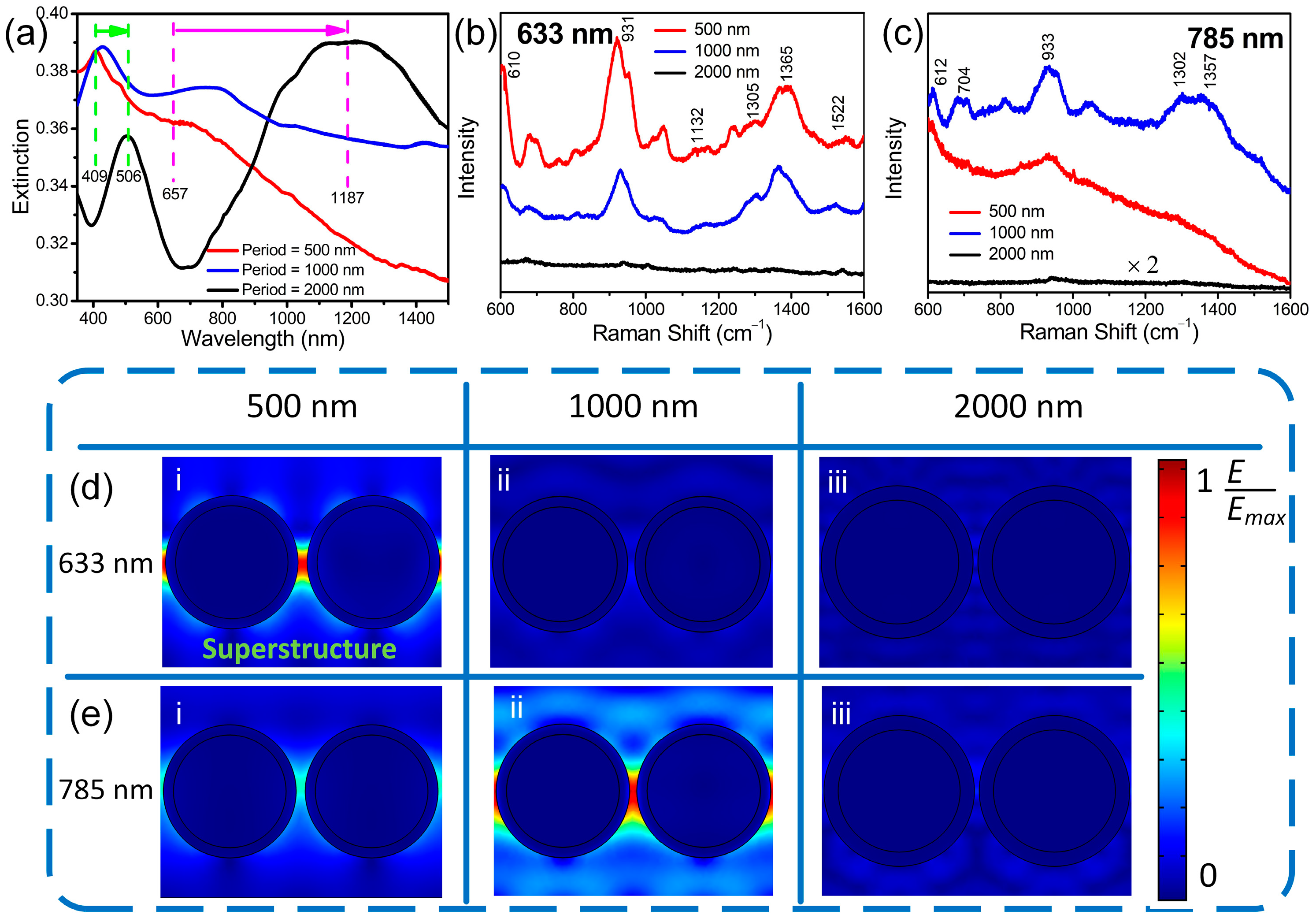
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Cao, G.; Yang, H.; Zhou, X.; Wu, Y. Dynamic Control of Double Plasmon-Induced Transparencies in Aperture-Coupled Waveguide-Cavity System. Plasmonics 2018, 13, 345–352. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Luo, Y.; Yi, Z.; Zhang, J.; Liu, Z.; Yang, W.; Yu, Y.; Wu, X.; Wu, P. A Switchable Terahertz Device Combining Ultra-Wideband Absorption and Ultra-Wideband Complete Reflection. Phys. Chem. Chem. Phys. 2022, 24, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Qin, F.; Yi, Z.; Yao, W.; Liu, Z.; Wu, X.; Wu, P. Ultra-wideband and wide-angle perfect solar energy absorber based on Ti nanorings surface plasmon resonance. Phys. Chem. Chem. Phys. 2021, 23, 17041–17048. [Google Scholar] [CrossRef] [PubMed]
- Herrman, L.O.; Valev, V.K.; Tserkezis, C.T.; Barnard, J.S.; Kasera, S.; Scherman, O.A.; Aizpurua, J.; Baumberg, J.J. Threading Plasmonic Nanoparticle Strings with Light. Nat. Commun. 2014, 5, 4568. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Wang, Y.; Song, C.; Zheng, L.; Ma, N.; Liu, X.; Li, S.; Lin, L.; Li, M.; Xu, Y.; et al. Hybrid Au−Ag Nanostructures for Enhanced Plasmon-Driven Catalytic Selective Hydrogenation through Visible Light Irradiation and Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2018, 140, 864–867. [Google Scholar] [CrossRef]
- Matricardi, C.; Hanske, C.; Garcia-Pomar, J.L.; Langer, J.; Mihi, A.; Liz-Marzán, L.M. Gold Nanoparticle Plasmonic Superlattices as Surface-Enhanced Raman Spectroscopy Substrates. ACS Nano 2019, 12, 8521–8539. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Yang, A.; Wang, W.; Hua, Y.; Schaller, R.D.; Schatz, G.C.; Odom, T.W. Band-Edge Engineering for Controlled Multi-Modal Nanolasing in Plasmonic Superlattices. Nat. Nanotechnol. 2017, 12, 889–895. [Google Scholar] [CrossRef]
- Hanke, C.; Tebbe, M.; Kuttner, C.; Bieber, V.; Tsukruk, V.V.; Chanana, M.; König, T.A.F.; Fery, A. Strongly Coupled Plasmonic Modes on Macroscopic Areas via Template-Assisted Colloidal Self-Assembly. Nano Lett. 2014, 14, 6863–6871. [Google Scholar] [CrossRef] [Green Version]
- Plou, J.; García, I.; Charconnet, M.; Astobiza, I.; García-Astrain, C.; Mihi, A.; Carracedo, A.; Liz-Marzán, L.M. Multiplex SERS Detection of Metabolic Alterations in Tumor Extracellular Media. Adv. Funct. Mater. 2020, 30, 1910335. [Google Scholar] [CrossRef]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, L.V.; Shen, J.T. Ultralong Photonic Nanojet Formed by a Two-Layer Dielectric Microsphere. Opt. Lett. 2014, 39, 4120–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maillard, M.; Huang, P.; Brus, L. Silver Nanodisk Growth by Surface Plasmon Enhanced Photoreduction of Adsorbed [Ag+]. Nano Lett. 2003, 3, 1611–1615. [Google Scholar] [CrossRef]
- Zhao, L.; Rong, X.; Niu, Y.; Xu, R.; Zhang, T.; Li, T.; Yu, Y.; Hou, Y. Ostwald Ripening Tailoring Hierarchically Porous Na3V2(PO4)2O2F Hollow Nanospheres for Superior High-Rate and Ultrastable Sodium Ion Storage. Small 2020, 16, 200492. [Google Scholar]
- Kim, J.K.; Park, T.; Jang, D. Surface-Enhanced Raman Scattering and Photothermal Effect of Hollow Au Nanourchins with Well-Defined Cavities. J. Nanopart. Res. 2020, 22, 305. [Google Scholar] [CrossRef]
- Mao, P.; Liu, C.; Favraud, G.; Chen, Q.; Han, M.; Fratalocchi, A.; Zhang, S. Broadband Single Molecule SERS Detection Designed by Warped Optical Spaces. Nat. Commun. 2018, 9, 5428. [Google Scholar] [CrossRef]
- Ahn, J.; Wang, D.; Ding, Y.; Zhang, J.; Qin, D. Site-Selective Carving and Co-Deposition: Transformation of Ag Nanocubes into Concave Nanocrystals Encased by Au–Ag Alloy Frames. ACS Nano 2018, 12, 298–307. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.Y.; Kim, M.; Kim, J.H. Multifunctional Hollow Porous Au/Pt Nanoshells for Simultaneous Surface-Enhanced Raman Scattering and Catalysis. Appl. Surf. Sci. 2021, 543, 148831. [Google Scholar] [CrossRef]
- Hu, A.; Zhou, R.; Bai, S.; Yu, Y.; Zhou, W.; Bridges, D.; Deng, Y.; Zhang, L. Introduction to Laser Micro-to-Nano Manufacturing. In Laser Micro-Nano-Manufacturing and 3D Microprinting; Hu, A., Ed.; Springer: Cham, Switzerland, 2020; Volume 309, pp. 1–74. [Google Scholar]
- Xiao, M.; Zheng, S.; Shen, D.; Duley, W.W.; Zhou, Y.N. Laser-Induced Joining of Nanoscale Materials: Processing, Properties, and Applications. Nano Today 2020, 35, 100959. [Google Scholar] [CrossRef]
- Xu, C.; Zipfel, W.; Shear, J.B.; Willians, R.M.; Webb, W.W. Multiphoton Fluorescence Excitation: New Spectral Windows for Biological Nonlinear Microscopy. Proc. Nat. Acad. Sci. USA 1996, 93, 10763–10768. [Google Scholar] [CrossRef] [Green Version]
- Sugioka, K. Hybrid Femtosecond Laser Three-Dimensional Micro-and Nanoprocessing: A Review. Int. J. Extrem. Manuf. 2019, 1, 012003. [Google Scholar] [CrossRef]
- Zhao, W.; Xiao, S.; Zhang, Y.; Pan, D.; Wen, J.; Qian, X.; Wang, D.; Cao, H.; He, W.; Quan, M.; et al. Binary “Island” Shaped Arrays with High-Density Hot Spots for Surface-Enhanced Raman Scattering Substrates. Nanoscale 2018, 10, 14220–14229. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, Y.; Wang, C.; Bai, S.; Zhang, T.; Chen, T.; Hu, A. Reusable and Long-Life 3D Ag Nanoparticles Coated Si Nanowire Array as Sensitive SERS Substrate. Appl. Surf. Sci. 2019, 494, 583–590. [Google Scholar] [CrossRef]
- Bai, S.; Sugioka, K. Recent Advances in the Fabrication of Highly Sensitive Surface-Enhanced Raman Scattering Substrates: Nanomolar to Attomolar Level Sensing. Light Adv. Manuf. 2021, 2, 13. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, H.; Yan, L.; Zhang, X.; Falak, A.; Guo, Y.; Chen, P.; Dong, F.; Sun, L.; Chu, W. A Generalized Methodology of Designing 3D SERS Probes with Superior Detection Limit and Uniformity by Maximizing Multiple Coupling Effects. Adv. Sci. 2019, 6, 1900177. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Megharaj, M.; Naidu, R. Surface-Enhanced Raman Scattering (SERS) Detection of Fluorosurfactants in Firefighting Foams. RSA Adv. 2016, 6, 11140–11145. [Google Scholar] [CrossRef]
- Huang, M.M.; Xu, L.N. Construction of a New Luminescent Cd(II) Compound for the Detection of Fe3+ and Treatment of Hepatitis B. Open Chem. 2021, 19, 953–960. [Google Scholar] [CrossRef]
- Haider, A.; Ahmed, M.; Faisal, M.; Naseer, M.M. Isatin as a Simple, Highly Selective and Sensitive Colorimetric Sensor for Fluoride Anion. Heterocycl. Commun. 2020, 26, 14–19. [Google Scholar] [CrossRef]
- Lao, Z.; Zheng, Y.; Dai, Y.; Hu, Y.; Ni, J.; Ji, S.; Cai, Z.; Smith, Z.J.; Li, J.; Zhang, L.; et al. Nanogap Plasmonic Structures Fabricated by Switchable Capillary-Force Driven Self-Assembly for Localized Sensing of Anticancer Medicines with Microfluidic SERS. Adv. Funct. Mater. 2020, 30, 1909467. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Y.; Chen, G.; Yang, L.; Xu, S.; Xu, W. Aptamer-Based Surface-Enhanced Raman Scattering-Microfluidic Sensor for Sensitive and Selective Polychlorinated Biphenyls Detection. Anal. Chem. 2015, 87, 9555–9558. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Nanda, J.; Wang, B.; Chen, G.; Hallinan, D.T., Jr. Self-Assembly of Large Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 13457–13470. [Google Scholar] [CrossRef]
- Huang, J.; Chen, F.; Zhang, Q.; Zhan, Y.; Ma, D.; Xu, K.; Zhao, Y. 3D Silver Nanoparticles Decorated Zinc Oxide/Silicon Heterostructured Nanomace Arrays as High-Performance Surface-Enhanced Raman Scattering Substrates. ACS Appl. Mater. Interfaces 2015, 7, 5725–5735. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, P.; Lisi, F.; Ahmed, E.; Liang, K.; Babarao, R.; Gooding, J.J.; Donald, W.A. Metal–Organic Framework-Enhanced Solid-Phase Microextraction Mass Spectrometry for the Direct and Rapid Detection of Perfluorooctanoic Acid in Environmental Water Samples. Anal. Chem. 2020, 92, 6900–6908. [Google Scholar] [CrossRef]
- Qi, L.; Gong, J. Facile in-situ Polymerization of Polyaniline-Functionalized Melamine Sponge Preparation for Mass Spectrometric Monitoring of Perfluorooctanoic Acid and Perfluorooctane Sulfonate from Biological Samples. J. Chromatogr. A 2020, 1616, 460777. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Wiczkowski, W.; Cieślik, E.; Zieliński, H. The Perfluoroalkyl Carboxylic Acids (PFCAs) and Perfluoroalkane Sulfonates (PFSAs) Contamination Level in Spices. Microchem. J. 2015, 121, 150–156. [Google Scholar] [CrossRef]
- Dolman, S.; Pelzing, M. An Optimized Method for the Determination of Perfluorooctanoic Acid, Perfluorooctane Sulfonate and Other Perfluorochemicals in Different Matrices Using Liquid Chromatography/Ion-Trap Mass Spectrometry. J. Chromatogr. B 2011, 879, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yu, H.; Geng, W.C.; Hu, X.Y.; Wang, Y.Y.; Li, Z.; Wang, Y.; Guo, D.S. Guanidinocalix[5]arene for Sensitive Fluorescence Detection and Magnetic Removal of Perfluorinated Pollutants. Nat. Commun. 2019, 10, 5762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, Y.; Liu, Y.; Long, S.; Du, L.; Liang, J.; Huang, C.; Swihart, M.T.; Tan, K. Core-Shell Quantum Dots Coated with Molecularly Imprinted Polymer for Selective Photoluminescence Sensing of Perfluorooctanoic Acid. Talanta 2019, 194, 1–6. [Google Scholar] [CrossRef]
- Walekar, L.S.; Zheng, M.; Zheng, L.; Long, M. Selenium and Nitrogen Co-Doped Carbon Quantum Dots as a Fluorescent Probe for Perfluorooctanoic Acid. Microchim. Acta 2019, 186, 278. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, L.; Zhu, P.; Chen, Q.; Tan, K. An Erythrosin B-Based “Turn On” Fluorescent Sensor for Detecting Perfluorooctane Sulfonate and Perfluorooctanoic Acid in Environmental Water Samples. Spectrochim. Acta A 2018, 201, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wu, J.; Sobhani, Z.; Amin, M.A.; Tang, Y. Aggregated-Fluorescent Detection of PFAS with a Simple Chip. Anal. Methods 2019, 11, 163–170. [Google Scholar] [CrossRef]
- Cao, X.; Wang, C.; Lu, Y.; Zhang, M.; Khan, K.; Song, S.; Wang, P.; Wang, C. Occurrence, Sources and Health Risk of Polyfluoroalkyl Substances (PFASs) in Soil, Water and Sediment from a Drinking Water Source Area. Ecotoxicol. Environ. Saf. 2019, 174, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Bai, F.Y.; Chang, Y.C.; Chen, P.C.; Chen, C.Y. Concentrations of Perfluoroalkyl Substances in Foods and the Dietary Exposure among Taiwan General Population and Pregnant Women. J. Food Drug Anal. 2018, 26, 994–1004. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, Y.; Liang, J.; Long, S.; Chen, X.; Tan, K. A Simple and Highly Sensitive Assay of Perfluorooctanoic Acid Based on Resonance Light Scattering Technique. Spectrochim. Acta A 2016, 159, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]



| Methods | Quantitative Detection Range (ppb) | Detection Limit (ppb) | Ref. |
|---|---|---|---|
| Mass spectrometry | NA | 0.011 | [35] |
| Mass spectrometry | 0.1–50 | NA | [36] |
| Mass spectrometry | 0.103–0.223 | NA | [37] |
| Mass spectrometry | 0.05–1 | 0.025 | [38] |
| Fluorescence | NA | 10 | [39] |
| Fluorescence | 100–6000 | 10 | [40] |
| Fluorescence | 4000–28,000 | 720 | [41] |
| Fluorescence | 20–4000 | 5 | [42] |
| Fluorescence | 41–41,000 | NA | [43] |
| Light scattering | 40–10,000 | 4.4 | [46] |
| SERS | 50–500 | 50 | [21] |
| SERS | 11–100,000 (500 nm period) 11–400,000 (combined use of 500 nm and 1000 nm periods) | 11 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Hu, A.; Hu, Y.; Ma, Y.; Obata, K.; Sugioka, K. Plasmonic Superstructure Arrays Fabricated by Laser Near-Field Reduction for Wide-Range SERS Analysis of Fluorescent Materials. Nanomaterials 2022, 12, 970. https://doi.org/10.3390/nano12060970
Bai S, Hu A, Hu Y, Ma Y, Obata K, Sugioka K. Plasmonic Superstructure Arrays Fabricated by Laser Near-Field Reduction for Wide-Range SERS Analysis of Fluorescent Materials. Nanomaterials. 2022; 12(6):970. https://doi.org/10.3390/nano12060970
Chicago/Turabian StyleBai, Shi, Anming Hu, Youjin Hu, Ying Ma, Kotaro Obata, and Koji Sugioka. 2022. "Plasmonic Superstructure Arrays Fabricated by Laser Near-Field Reduction for Wide-Range SERS Analysis of Fluorescent Materials" Nanomaterials 12, no. 6: 970. https://doi.org/10.3390/nano12060970
APA StyleBai, S., Hu, A., Hu, Y., Ma, Y., Obata, K., & Sugioka, K. (2022). Plasmonic Superstructure Arrays Fabricated by Laser Near-Field Reduction for Wide-Range SERS Analysis of Fluorescent Materials. Nanomaterials, 12(6), 970. https://doi.org/10.3390/nano12060970








