Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals, Viral Strain, and Cell Lines
2.2. Characterization of the AgNPs
2.3. Cell-Viability Assay
2.4. Crystal-Violet Staining
2.5. Hoechst Staining
2.6. Annexin-V Analysis
2.7. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Measurement of Viral Titers
2.9. Statistical Analyses
3. Results
3.1. Characteristics of the AgNPs
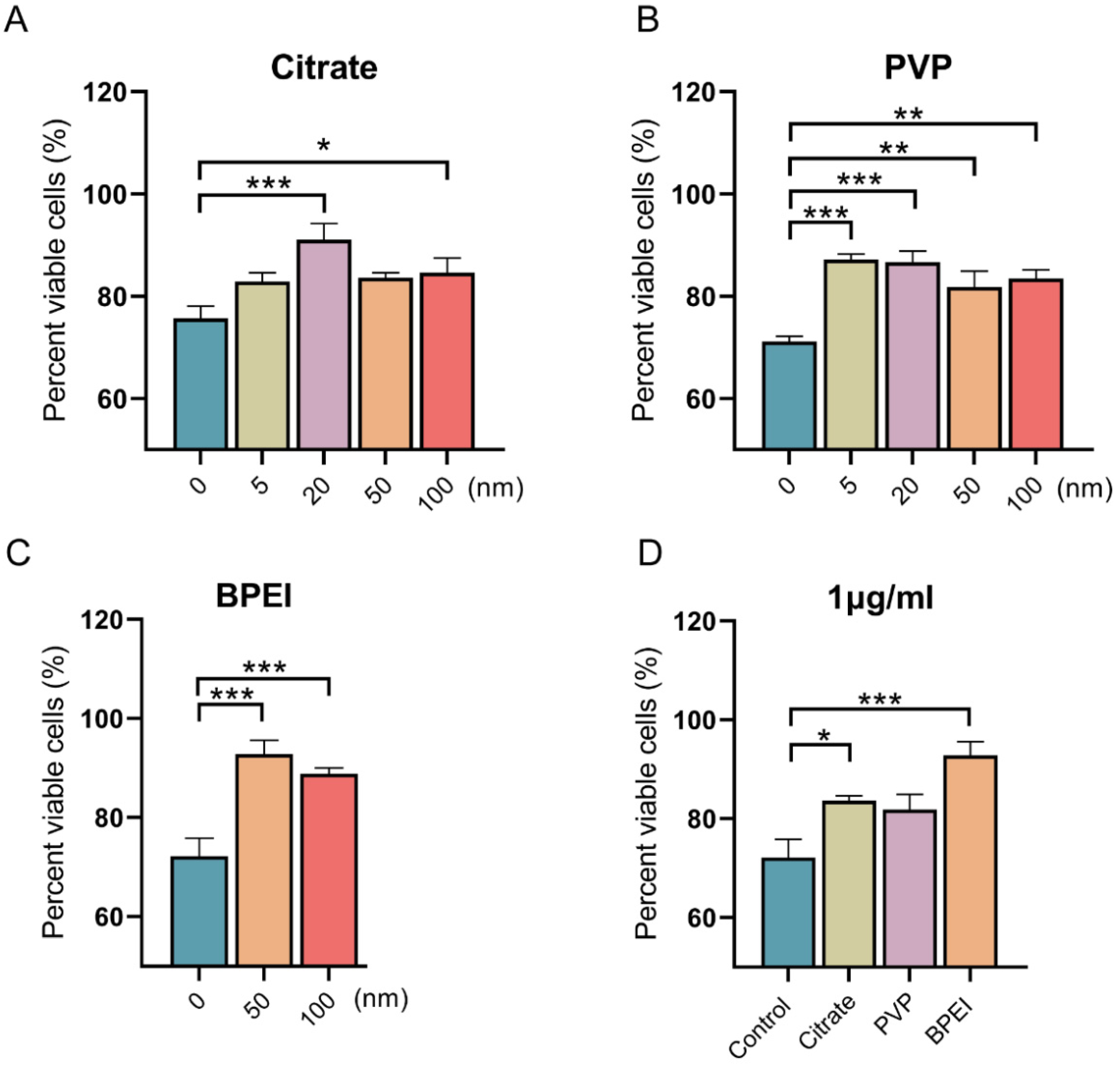
3.2. Effects of AgNPs on the Viability of Vero E6 Cells Infected with SARS-CoV-2
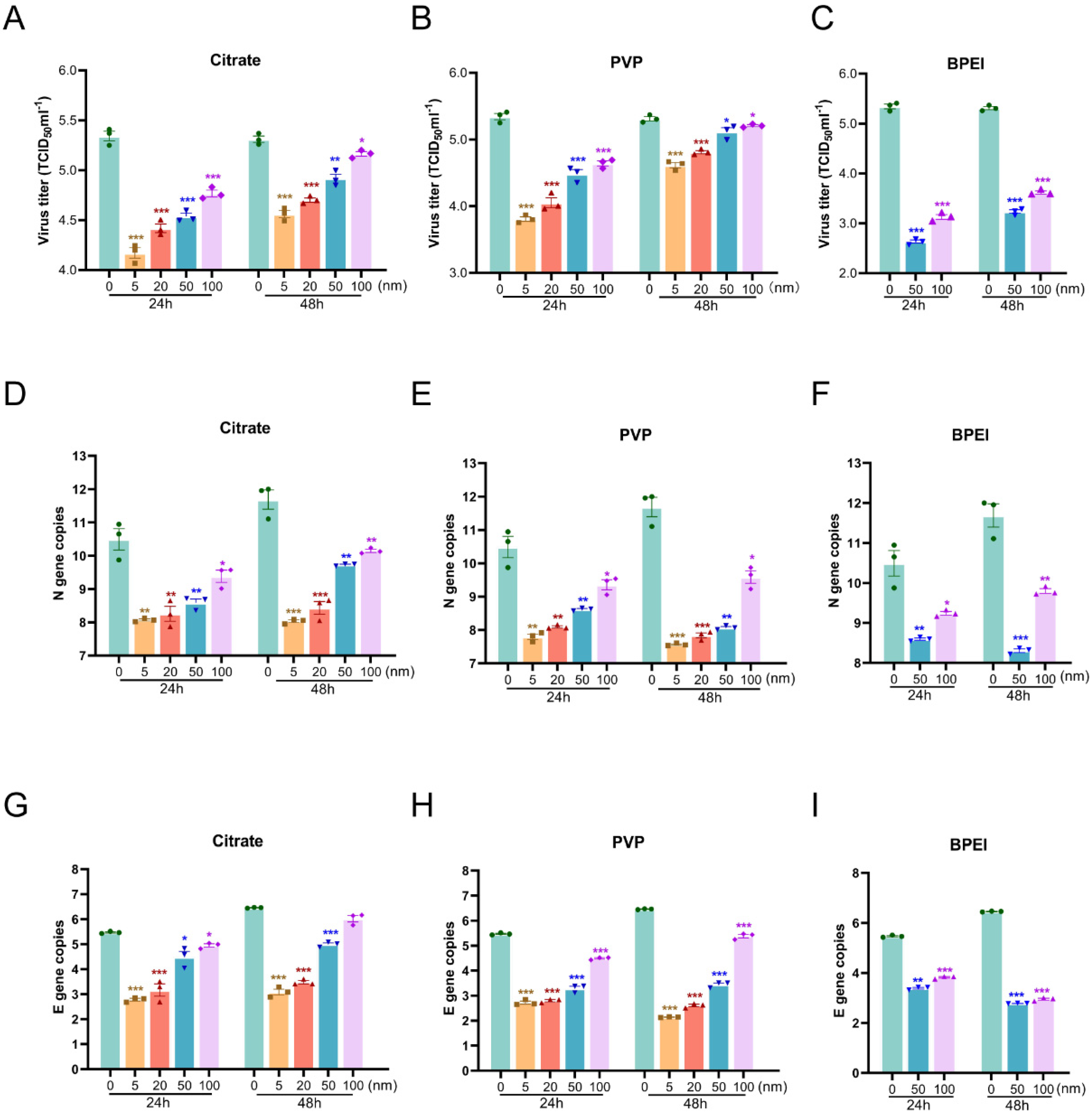
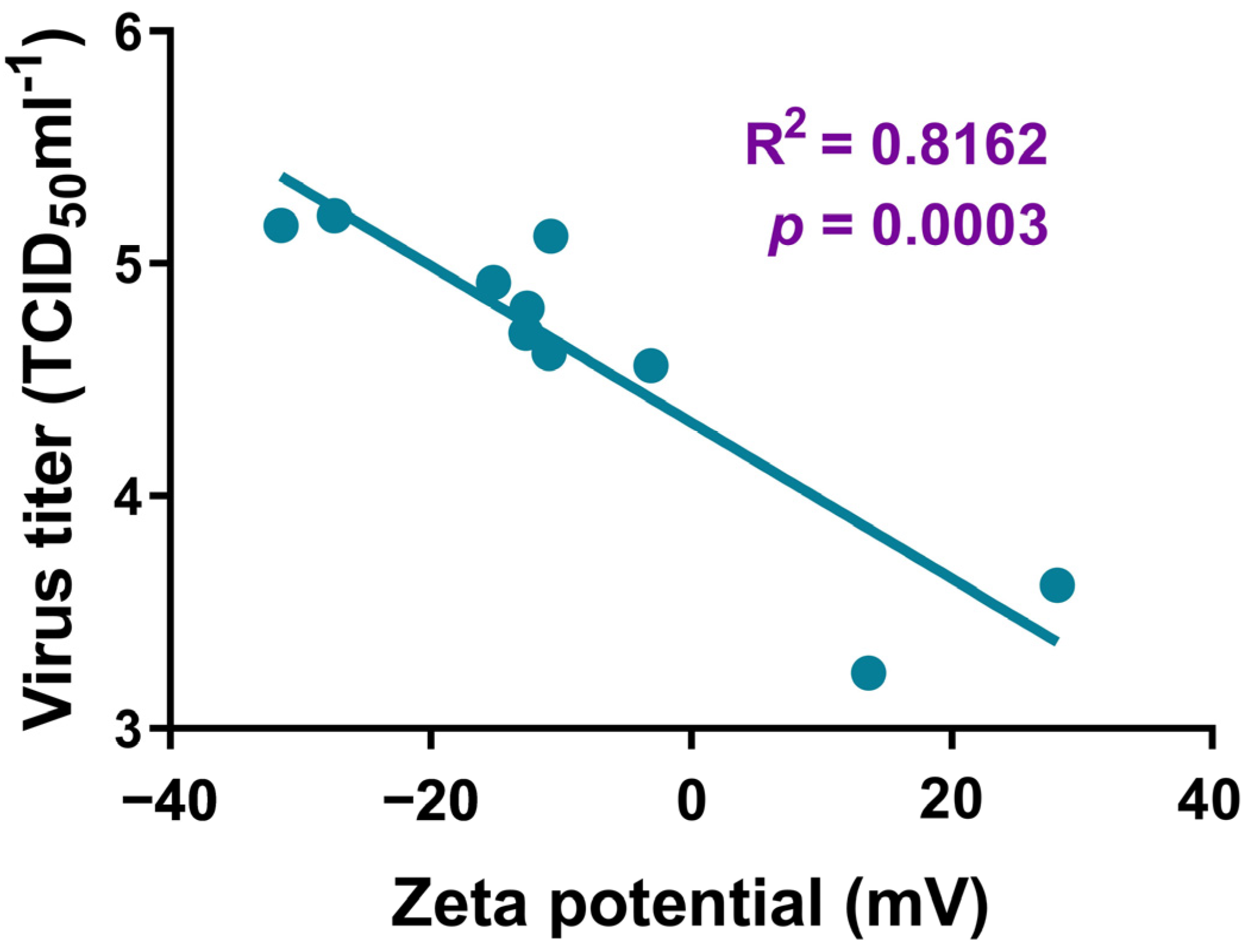
3.3. Effects of AgNPs on the Replication of SARS-CoV-2 in Vero E6 Cells
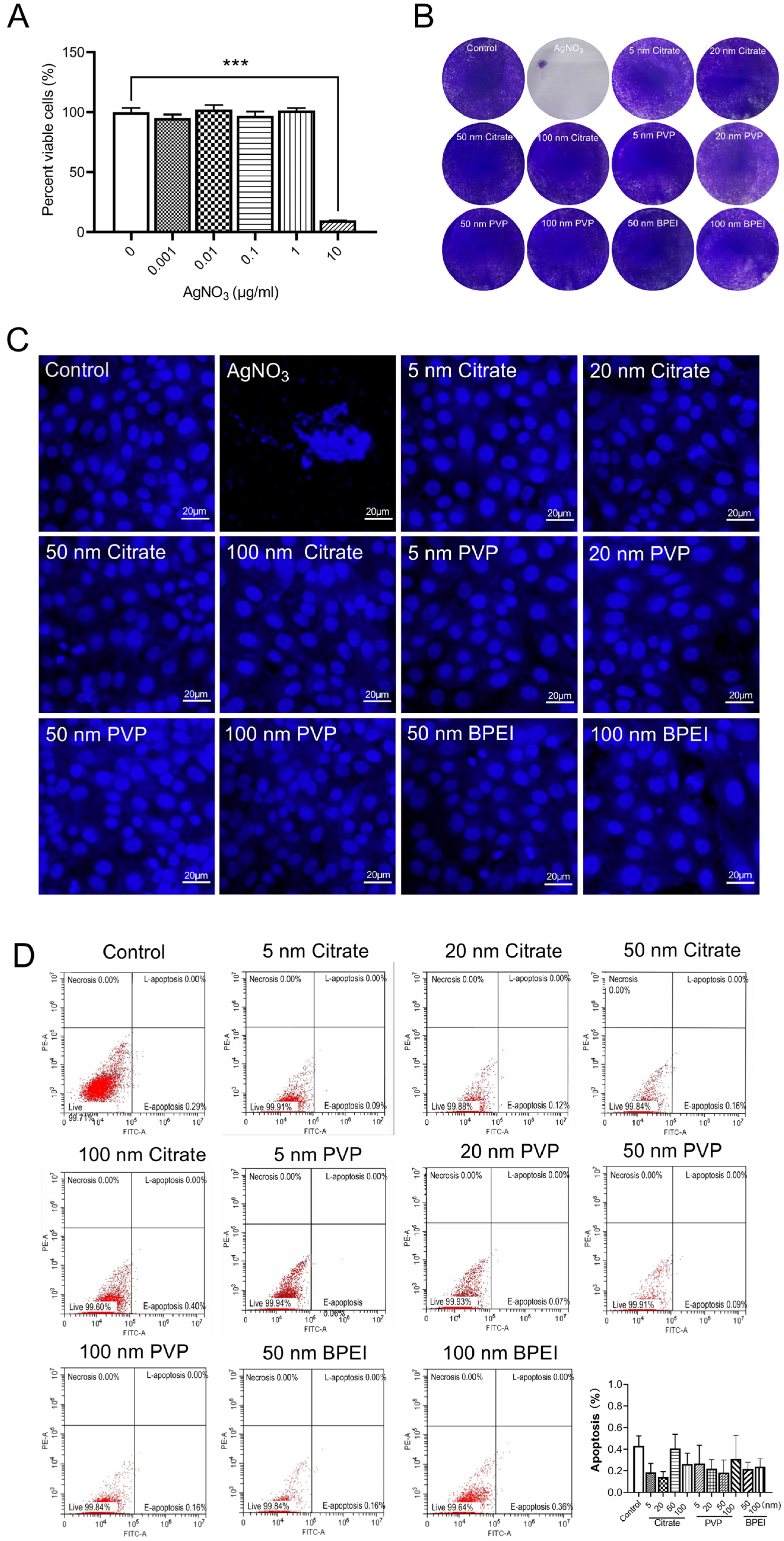
3.4. Evaluation of the Cytotoxicity of AgNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhou, H.; Huang, W.; Zhou, J.; Qiu, M.; Deng, Z.; Chen, L.; Weng, Y.; Cai, L.; Gu, Y.; et al. Cell morphological analysis of SARS-CoV-2 infection by transmission electron microscopy. J. Thorac. Dis. 2020, 12, 4368–4373. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738.e713. [Google Scholar] [CrossRef]
- Prasad, S.; Potdar, V.; Cherian, S.; Abraham, P.; Basu, A. Transmission electron microscopy imaging of SARS-CoV-2. Indian J. Med. Res. 2020, 151, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Caldas, L.A.; Carneiro, F.A.; Higa, L.M.; Monteiro, F.L.; da Silva, G.P.; da Costa, L.J.; Durigon, E.L.; Tanuri, A.; de Souza, W. Ultrastructural analysis of SARS-CoV-2 interactions with the host cell via high resolution scanning electron microscopy. Sci. Rep. 2020, 10, 16099. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.W.; Pan, Y.; Cheng, S.M.S.; Hui, K.P.Y.; Krishnan, P.; Liu, Y.; Ng, D.Y.M.; Wan, C.K.C.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassenaar, T.M.; Zou, Y. 2019_nCoV/SARS-CoV-2: Rapid classification of betacoronaviruses and identification of Traditional Chinese Medicine as potential origin of zoonotic coronaviruses. Lett. Appl. Microbiol. 2020, 70, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. COVID-19 Weekly Epidemiological Update. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19 (accessed on 22 February 2022).
- FDA. Know Your Treatment Options for COVID-19. Available online: https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19 (accessed on 11 January 2022).
- WHO Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 26 February 2022).
- Merkl, P.; Long, S.; McInerney, G.M.; Sotiriou, G.A. Antiviral Activity of Silver, Copper Oxide and Zinc Oxide Nanoparticle Coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Mosselhy, D.A.; Virtanen, J.; Kant, R.; He, W.; Elbahri, M.; Sironen, T. COVID-19 Pandemic: What about the Safety of Anti-Coronavirus Nanoparticles? Nanomaterials 2021, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef] [Green Version]
- Khoshnevisan, K.; Maleki, H.; Baharifar, H. Nanobiocide Based-Silver Nanomaterials Upon Coronaviruses: Approaches for Preventing Viral Infections. Nanoscale Res. Lett. 2021, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral Potential of Nanoparticles-Can Nanoparticles Fight Against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kontodimas, K.; Bosmann, M. Nanomedicine: A Diagnostic and Therapeutic Approach to COVID-19. Front. Med. 2021, 8, 648005. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, H. The Handbook of Prescriptions for Emergencies; Jin Dynasty (265–420), China; Available online: https://www.uuzuowen.com/gudianbook/gudaiyishu/zhouhoubeijifang/ (accessed on 26 February 2022).
- Sun, R.; Wei, W.; Zhan, P.; Liu, X. An overview of the application of silver-containing medicines in traditional medicine. Asia Pac. Tradit. Med. 2021, 17, 182–184. [Google Scholar]
- Li, S. Compendium of Materia Medica; Ming Dynasty (1368–1644), China; Available online: https://yuedu.163.com/source/1c78ee3280734e1282b6f1d563429654_4 (accessed on 26 February 2022).
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [Green Version]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Nath, K.; Parekh, Y.; Enayathullah, M.G.; Bokara, K.K.; Sinhamahapatra, A. Antimicrobial silver nanoparticle-photodeposited fabrics for SARS-CoV-2 destruction. Colloid Interface Sci. Commun. 2021, 45, 100542. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelwal, N.; Kaur, G.; Kumar, N.; Tiwari, A. Application of silver nanoparticles in viral inhibition: A new hope for antivirals. Dig. J. Nanomater. Biostruct. 2014, 9, 175–186. [Google Scholar]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sunderland, K.; Mao, C. Virus-Derived Peptides for Clinical Applications. Chem. Rev. 2017, 117, 10377–10402. [Google Scholar] [CrossRef] [Green Version]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 2020, 10, 5932–5942. [Google Scholar] [CrossRef] [PubMed]
- Djellabi, R.; Basilico, N.; Delbue, S.; D’Alessandro, S.; Parapini, S.; Cerrato, G.; Laurenti, E.; Falletta, E.; Bianchi, C.L. Oxidative Inactivation of SARS-CoV-2 on Photoactive AgNPs@TiO(2) Ceramic Tiles. Int. J. Mol. Sci. 2021, 22, 8836. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, M.M.; Abelyan, N.; Abdelgawad, M.A.; Musa, A.; Ghoneim, M.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Alnusaire, T.S.; Abdelwahab, S.F.; et al. Strawberry and Ginger Silver Nanoparticles as Potential Inhibitors for SARS-CoV-2 Assisted by In Silico Modeling and Metabolic Profiling. Antibiotics 2021, 10, 824. [Google Scholar] [CrossRef]
- Ahamed, M.; Alsalhi, M.S.; Siddiqui, M.K. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- House, J.S.; Bouzos, E.; Fahy, K.M.; Francisco, V.M.; Lloyd, D.T.; Wright, F.A.; Motsinger-Reif, A.A.; Asuri, P.; Wheeler, K.E. Low-Dose Silver Nanoparticle Surface Chemistry and Temporal Effects on Gene Expression in Human Liver Cells. Small 2020, 16, e2000299. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Zhuang, X.; Zhang, H.; Li, Y.; Zhu, Y.; Lu, J.; Ge, C.; Cong, J.; Li, T.; Li, N.; et al. Inhibition of Autophagy Suppresses SARS-CoV-2 Replication and Ameliorates Pneumonia in hACE2 Transgenic Mice and Xenografted Human Lung Tissues. J. Virol. 2021, 95, e0153721. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allawadhi, P.; Singh, V.; Khurana, A.; Khurana, I.; Allwadhi, S.; Kumar, P.; Banothu, A.K.; Thalugula, S.; Barani, P.J.; Naik, R.R.; et al. Silver nanoparticle based multifunctional approach for combating COVID-19. Sens. Int. 2021, 2, 100101. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-Lopez, I.; Alvarado-Vera, M.; Patron-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef] [PubMed]

| AgNPs | Size by TEM (nm) | Hydrodynamic Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| PVP-5 | 6.0 ± 2.9 | 11.1 ± 1.4 | −10.9 ± 4.3 |
| PVP-20 | 18.9 ± 3.5 | 35.6 ± 6.6 | −12.6 ± 1.4 |
| PVP-50 | 48.8 ± 5.6 | 49.2 ± 1.0 | −10.8 ± 1.2 |
| PVP-100 | 96.2 ± 8.2 | 122.6 ± 1.0 | −27.4 ± 0.6 |
| Citrate-5 | 6.2 ± 1.8 | 7.8 ± 2.2 | −3.1 ± 0.8 |
| Citrate-20 | 22.0 ± 3.7 | 35.0 ± 14.6 | −12.7 ± 1.0 |
| Citrate-50 | 47.5 ± 5.8 | 62.2 ± 1.0 | −15.2 ± 0.8 |
| Citrate-100 | 114.1 ± 11.9 | 114.4 ± 0.5 | −31.5 ± 0.6 |
| BPEI-50 | 47.5 ± 5.3 | 71.4 ± 3.8 | 13.6 ± 1.0 |
| BPEI-100 | 101.5 ± 10.9 | 114.3 ± 0.4 | 28.1 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. https://doi.org/10.3390/nano12060990
He Q, Lu J, Liu N, Lu W, Li Y, Shang C, Li X, Hu L, Jiang G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials. 2022; 12(6):990. https://doi.org/10.3390/nano12060990
Chicago/Turabian StyleHe, Qinghao, Jing Lu, Nian Liu, Wenqing Lu, Yu Li, Chao Shang, Xiao Li, Ligang Hu, and Guibin Jiang. 2022. "Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size" Nanomaterials 12, no. 6: 990. https://doi.org/10.3390/nano12060990
APA StyleHe, Q., Lu, J., Liu, N., Lu, W., Li, Y., Shang, C., Li, X., Hu, L., & Jiang, G. (2022). Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials, 12(6), 990. https://doi.org/10.3390/nano12060990






