Electrochemical Sensor Nanoarchitectonics for Sensitive Detection of Uric Acid in Human Whole Blood Based on Screen-Printed Carbon Electrode Equipped with Vertically-Ordered Mesoporous Silica-Nanochannel Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurements and Instrumentations
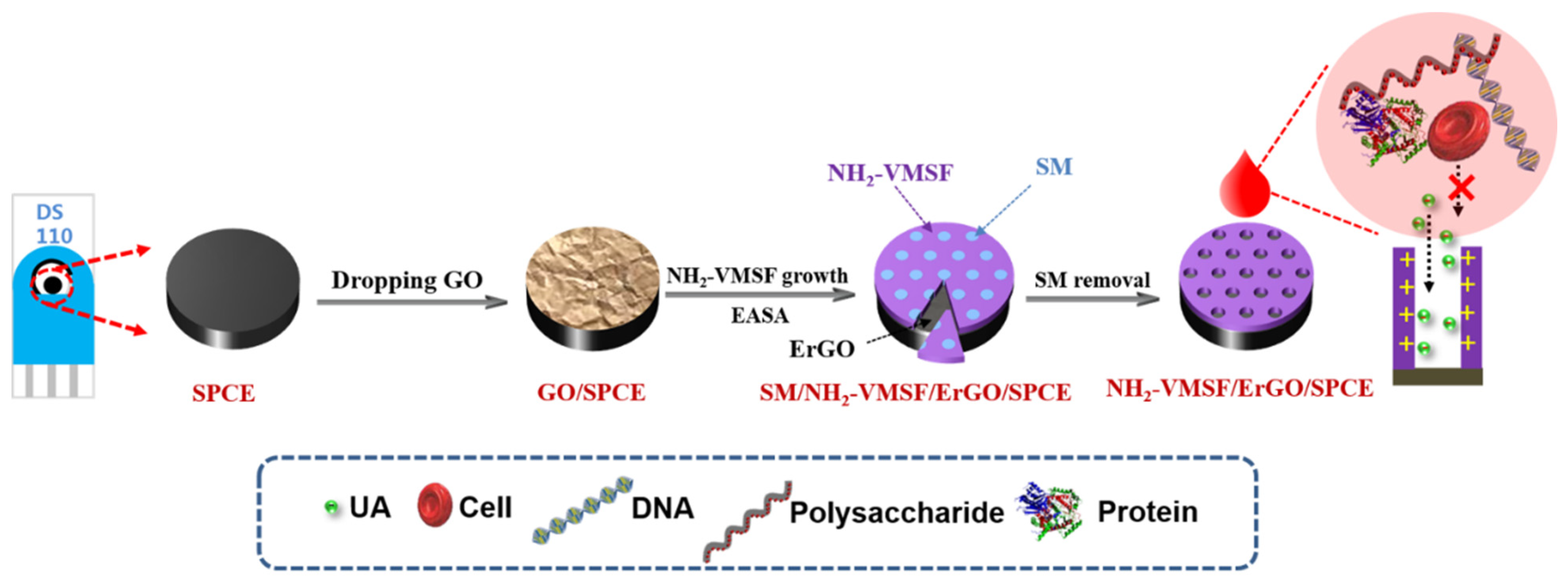
2.3. Preparation of NH2-VMSF-Modified SPCE Using ErGO as Adhersive Layer
2.4. Electrochemical Detection of UA
3. Results and Discussion
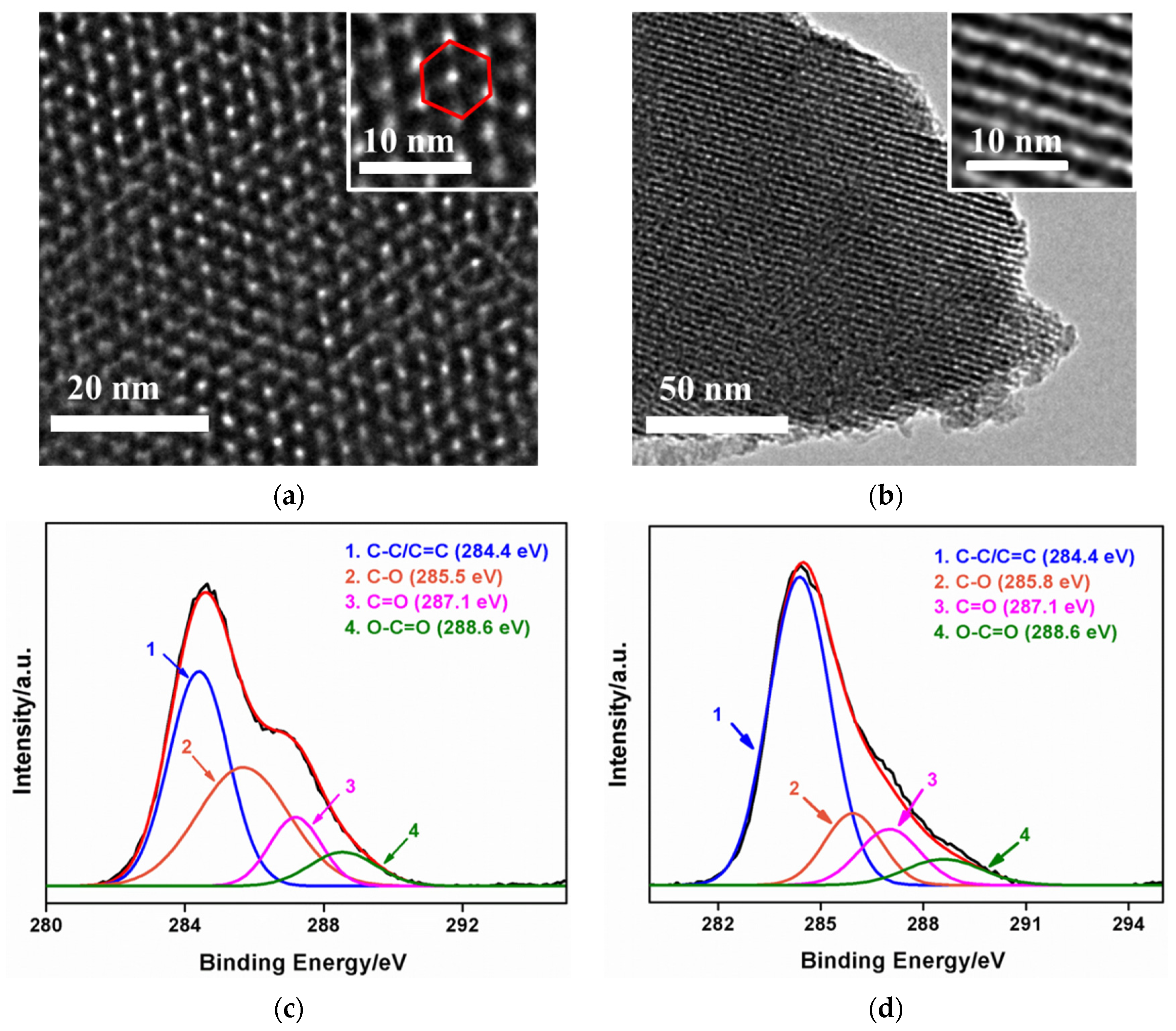
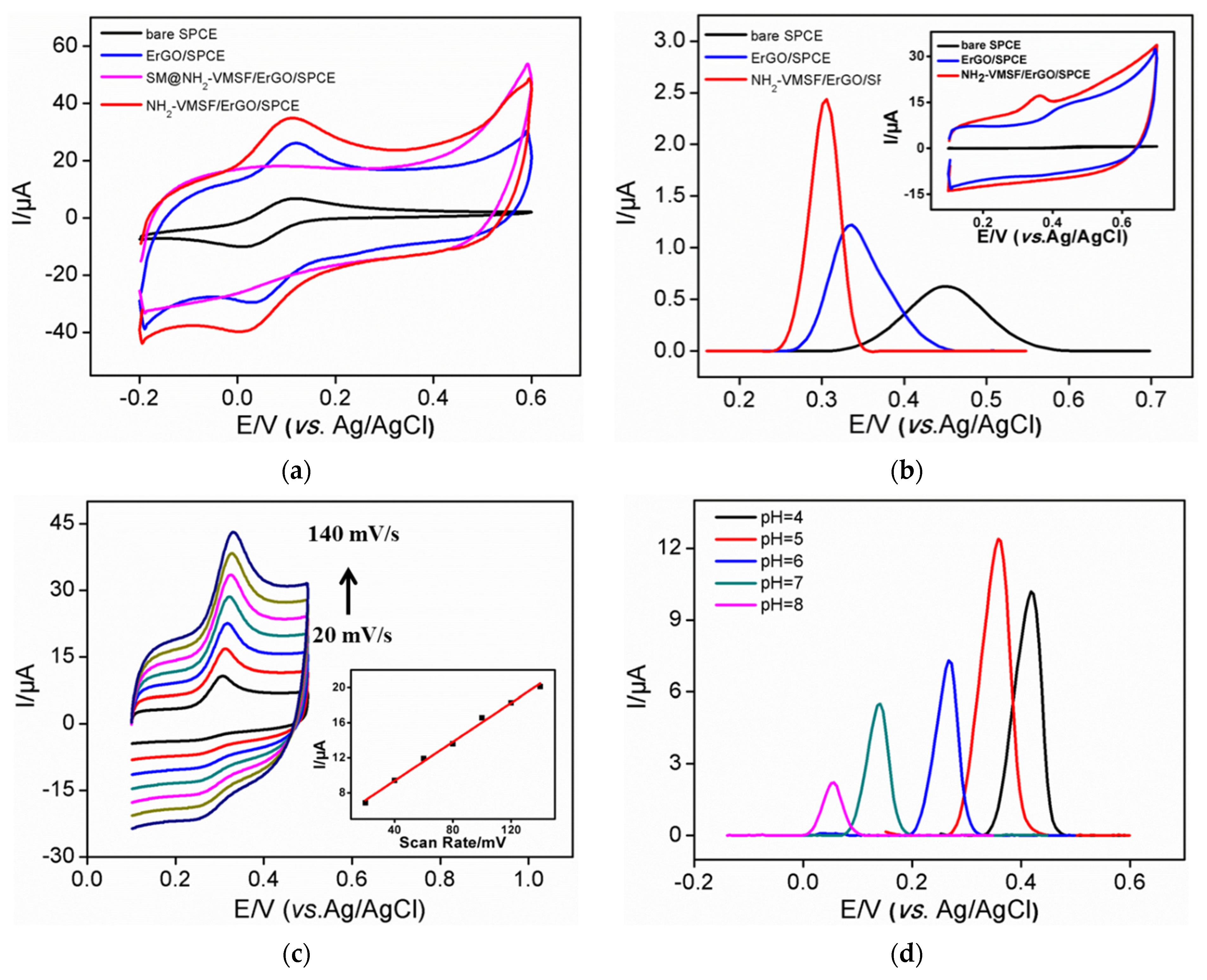
3.1. Stable Coupling of NH2-VMSF with SPCE Using ErGO as Adhersive Layer
3.2. Enrichment Effect and Electrocatalytic Properties of NH2-VMSF/ErGO/SPCE
3.3. Optimization of Conditions for the Detection of Uric Acid
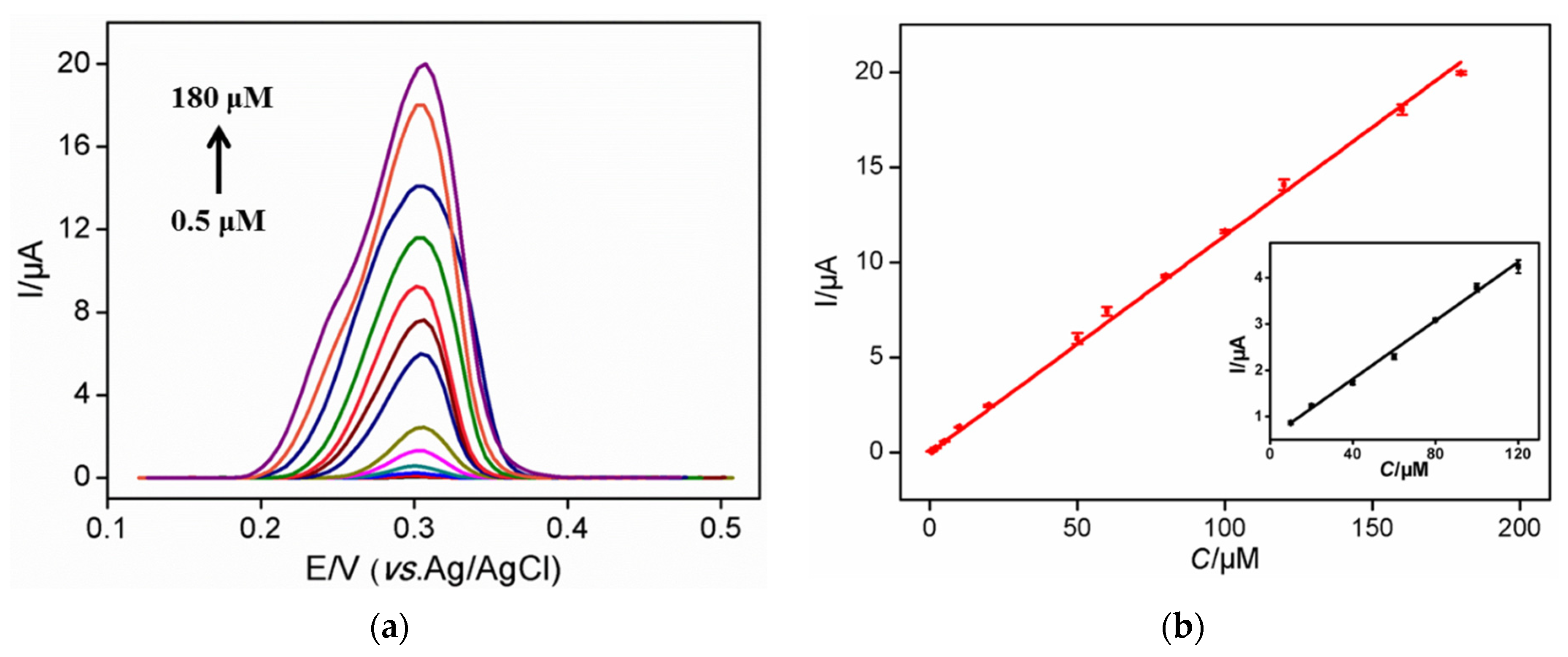
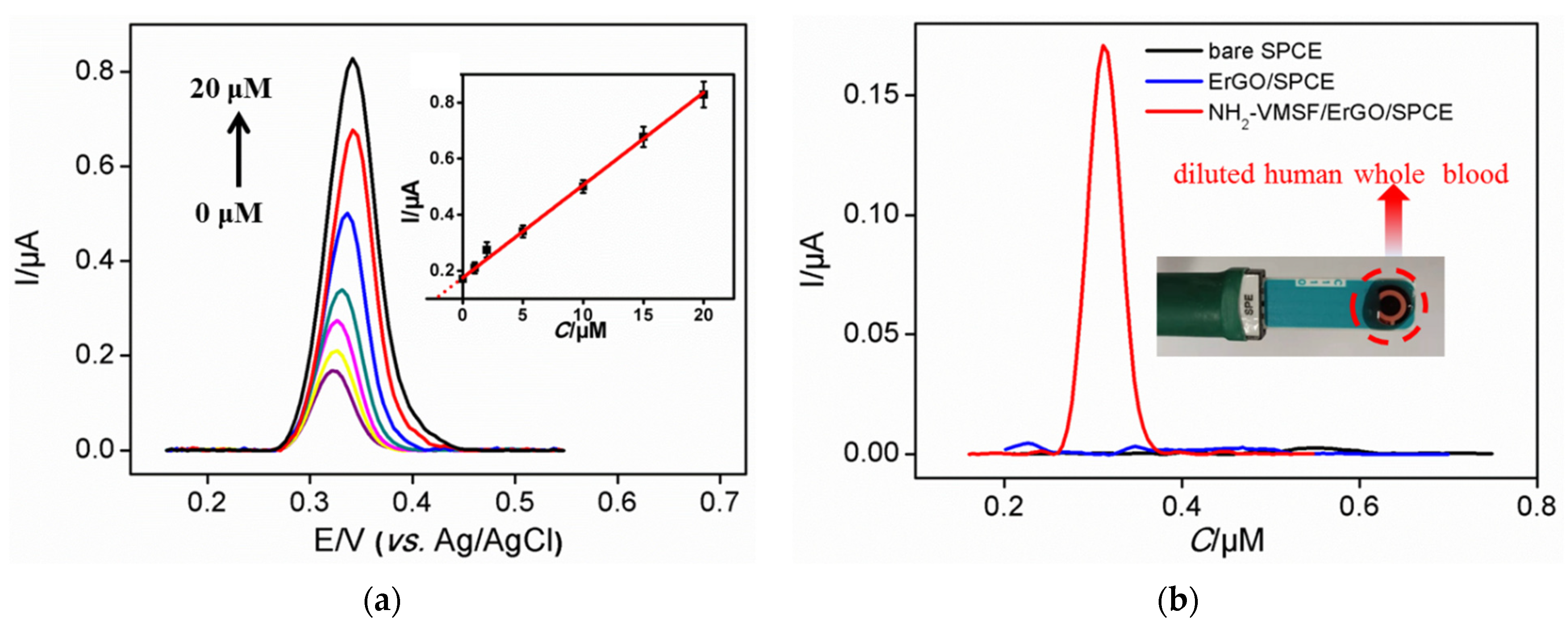
3.4. Sensitive Detection of UA Using NH2-VMSF/ErGO/SPCE
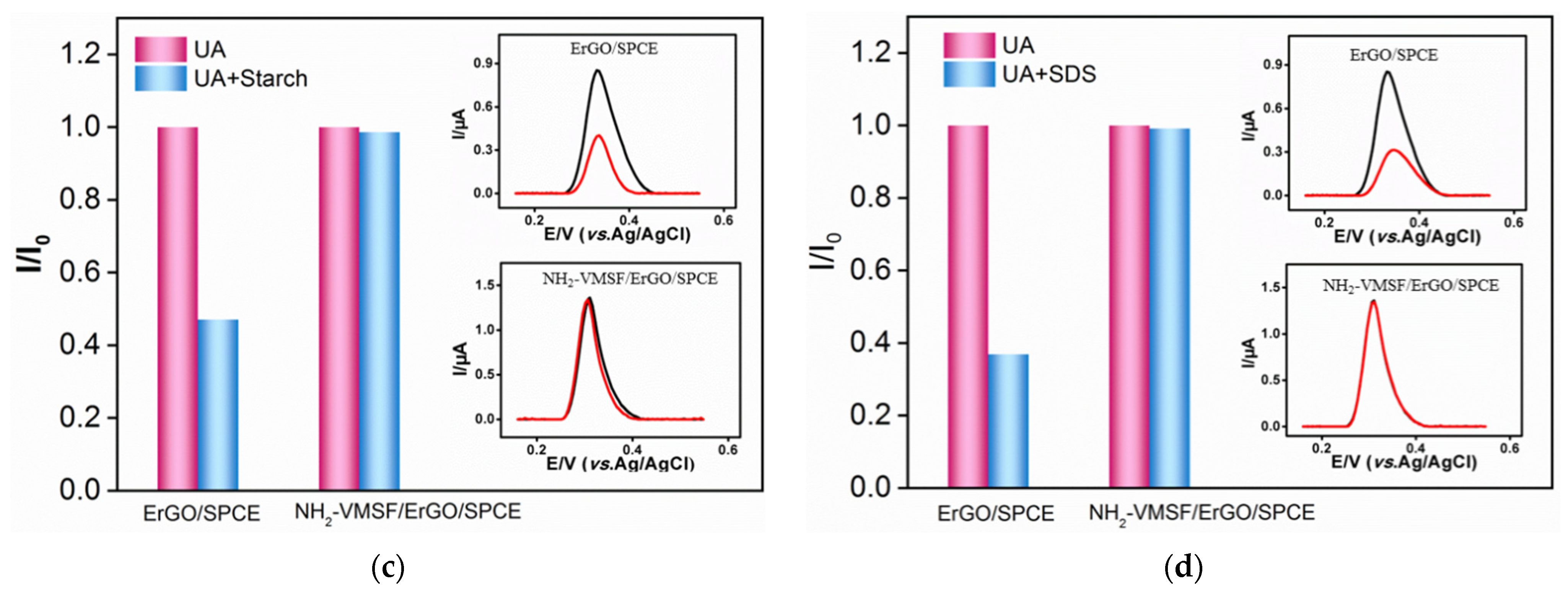
3.5. Anti-Fouling of NH2-VMSF/ErGO/SPCE Sensor
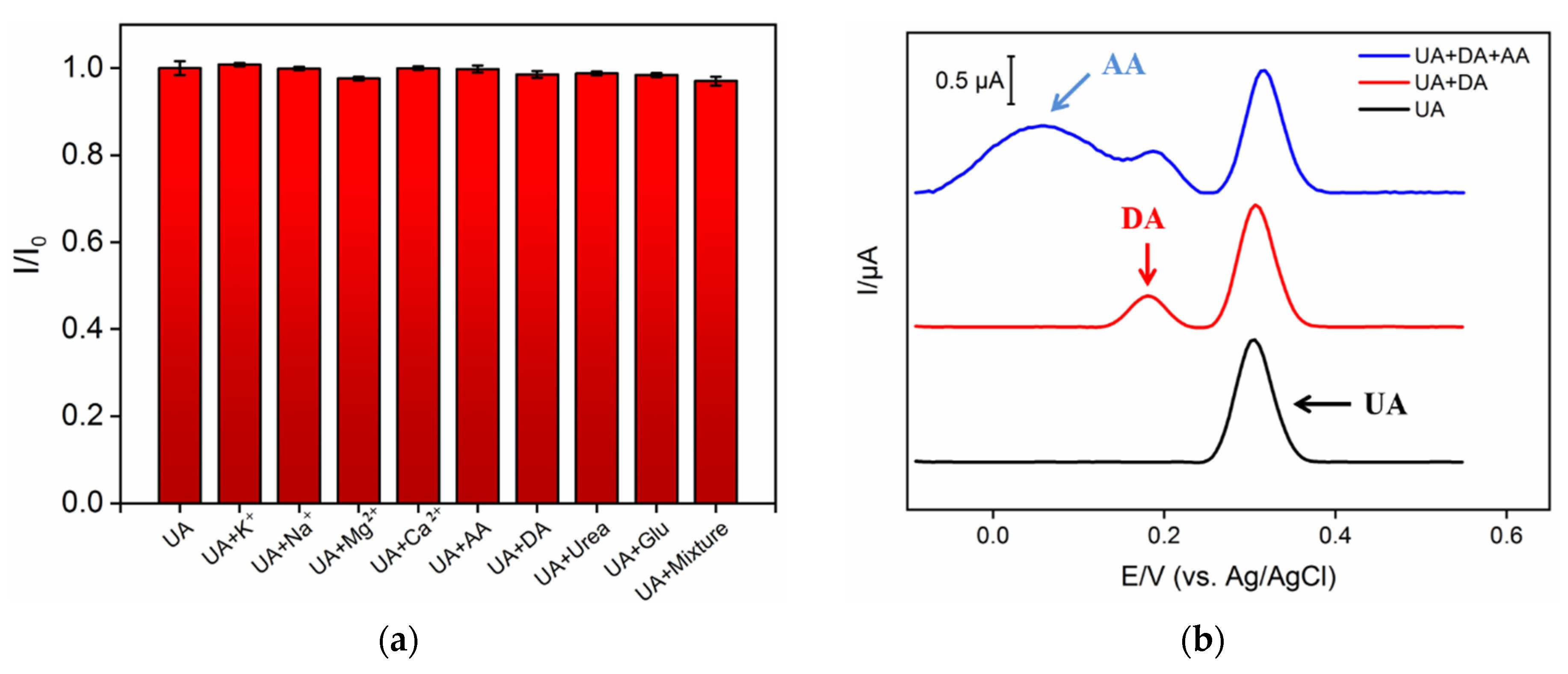
3.6. Stability, Reproducibility, and Selectivity of NH2-VMSF/ErGO/SPCE Sensor
3.7. Detection of UA in Human Whole Blood with Low Sample Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Y.X.; Duan, W.; Jin, Y.; Wo, F.J.; Xi, F.N.; Wu, J.M. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.F.; Slaughter, G. Flexible electrochemical uric acid and glucose biosensor. Bioelectrochemistry 2021, 141, 107870. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Zhong, H.G.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.N.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef]
- Choukairi, M.; Bouchta, D.; Bounab, L.; Elkhamlichi, R.; Chaouket, F.; Raissouni, I.; Rodriguez, I.N. Electrochemical detection of uric acid and ascorbic acid: Application in serum. J. Electroanal. Chem. 2015, 758, 117–124. [Google Scholar] [CrossRef]
- Wan, Y.J.; Zhao, J.W.; Deng, X.C.; Chen, J.; Xi, F.N.; Wang, X.B. Colorimetric and fluorescent dual-modality sensing platform based on fluorescent nanozyme. Front. Chem. 2021, 9, 774486. [Google Scholar] [CrossRef] [PubMed]
- Guo, J. Uric acid monitoring with a smartphone as the electrochemical analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.Q.; Xuan, L.L.; Gong, J.W.; Liu, J.J.; Wang, X.B.; Xi, F.N.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Yan, F.; Lin, X.Y.; Su, B. Vertically ordered silica mesochannel films: Electrochemistry and analytical applications. Analyst 2016, 141, 3482–3495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Ding, Y.; Lu, D.M.; Su, R.B.; Tang, H.L.; Xi, F.N. Silica nanochannel array film supported by β-cyclodextrinfunctionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2021, 9, 812086. [Google Scholar] [CrossRef] [PubMed]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yánñez-Sedeño, P.; Campuzano, S.; et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F.; et al. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Biosens. Bioelectron. 2020, 163, 112299. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Jampasa, S.; Ngamrojanavanich, N.; Rengpipat, S.; Chailapakul, O.; Kalcher, K.; Chaiyo, S. Ultrasensitive electrochemiluminescence sensor based on nitrogen-decorated carbon dots for Listeria monocytogenes determination using a screen-printed carbon electrode. Biosens. Bioelectron. 2021, 188, 113323. [Google Scholar] [CrossRef]
- Herl, T.; Matysik, F.M. Investigation of the electrooxidation of thymine on screen-printed carbon electrodes by hyphenation of electrochemistry and mass spectrometry. Anal. Chem. 2020, 92, 6374–6381. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.S.K.; Hwa, K.Y. Facile Synthesis of Ag/AgVO3/N-rGO Hybrid Nanocomposites for electrochemical detection of levofloxacin for complex biological samples using screen-printed carbon paste electrodes. Inorg. Chem. 2021, 60, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Q.; Yan, F.; Yao, L.; Su, B. Anti-biofouling isoporous silica-micelle membrane enabling drug detection in human whole blood. Anal. Chem. 2016, 88, 8364–8368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Hou, H.F.; Wei, H.; Yao, L.N.; Sun, L.; Yu, P.; Su, B.; Mao, L.Q. In vivo monitoring of oxygen in rat brain by carbon fiber microelectrode modified with antifouling nanoporous membrane. Anal. Chem. 2019, 91, 3645–3651. [Google Scholar] [CrossRef]
- Nasir, T.; Zhang, L.; Vilà, N.; Herzog, G.; Walcarius, A. Electrografting of 3-aminopropyltriethoxysilane on a glassy carbon electrode for the improved adhesion of vertically oriented mesoporous silica thin films. Langmuir 2016, 32, 4323–4332. [Google Scholar] [CrossRef]
- Nasir, T.; Herzog, G.; Hébrant, M.; Despas, C.; Liu, L.; Walcarius, A. Mesoporous silica thin films for improved electrochemical detection of paraquat. ACS Sens. 2018, 3, 484–493. [Google Scholar] [CrossRef]
- Karman, C.; Vilà, N.; Walcarius, A. Amplified charge transfer for anionic redox probes through oriented mesoporous silica thin films. ChemElectroChem 2016, 3, 2130–2137. [Google Scholar] [CrossRef]
- Cui, Y.X.; Duan, W.; Jin, Y.; Wo, F.J.; Xi, F.N.; Wu, J.M. Graphene quantum dot-decorated luminescent porous silicon dressing for theranostics of diabetic wounds. Acta Biomater. 2021, 272, 120772. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Jin, Y.; Cui, Y.X.; Xi, F.N.; Liu, X.Y.; Wo, F.J.; Wu, J.M. A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 2021, 272, 120772. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, M.; Fernández, I.; Luna, G.; Díez, P.; Campuzano, S.; Raouafi, N.; Sánchez, A.; Pingarrón, J.M.; Villalonga, R. Label-free electrochemical genosensor based on mesoporous silica thin film. Anal. Bioanal. Chem. 2016, 408, 7321–7327. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.D.; Klausen, L.H.; Yan, N.; Liu, J.; Chen, F.H.; Song, W.; Dong, M.D.; Zhang, Y.M. Growing vertical aligned mesoporous silica thin film on nanoporous substrate for enhanced degradation, drug delivery and bioactivity. Bioact. Mater. 2021, 6, 1452–1463. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.B.; Zhan, J.; Chen, Y.Q.; Hu, J.M. Electrodeposited superhydrophobic mesoporous silica films co-embedded with template and corrosion inhibitor for active corrosion protection. Appl. Surf. Sci. 2020, 508, 145242. [Google Scholar] [CrossRef]
- Saadaoui, M.; Fernández, I.; Sánchez, A.; Díez, P.; Campuzano, S.; Raouafi, N.; Pingarrón, J.M.; Villalonga, R. Mesoporous silica thin film mechanized with a DNAzyme-based molecular switch for electrochemical biosensing. Electrochem. Commun. 2015, 58, 57–61. [Google Scholar] [CrossRef]
- Vanheusden, G.; Philipsen, H.; Herregods, S.J.; Vereecken, P.M. Aggregate-free micrometer-thick mesoporous silica thin films on planar and three-dimensional structured electrodes by hydrodynamic diffusion layer control during electrochemically Assisted Self-Assembly. Chem. Mater. 2021, 33, 7075–7088. [Google Scholar] [CrossRef]
- Xu, E.S.; Yang, H.T.; Wu, L.N.; Chen, J.; Wei, W.; Liu, Y.; Liu, S.Q. Label-free poly (ADP-ribose) polymerase-1 activity assay based on perpendicular orientated mesoporous silica films. Sens. Actuators B Chem. 2019, 294, 185–191. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Jang, H.; Park, Y.J.; Chen, X.; Das, T.; Kim, M.S.; Ahn, J.H. Graphene-based flexible and stretchable electronics. Adv. Mater. 2016, 28, 4184–4202. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, C.C.; Cheng, H.H.; Zhao, Y.; Shi, G.Q.; Jiang, L.; Qu, L.T. Facile fabrication of light, flexible and multifunctional graphene fibers. Adv. Mater. 2012, 24, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Zheng, Y.Y.; Pang, Y.Y.; Chen, J.; Zhang, Z.Y.; Xi, F.N.; Chen, P. Graphene quantum dots as full-color and stimulus responsive fluorescence ink for information encryption. J. Colloid Interface Sci. 2020, 579, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, M.Q.; Zou, H.Q.; Liu, J.S.; Wang, J.; Wang, L.D. Non-enzymatic electrochemical detection of uric acid with electrodeposited Nafion film. J. Electroanal. Chem. 2019, 841, 129–134. [Google Scholar] [CrossRef]
- Baccarin, M.; Rowley-Neale, S.J.; Cavalheiro, É.T.; Smith, G.C.; Banks, C.E. Nanodiamond based surface modified screen-printed electrodes for the simultaneous voltammetric determination of dopamine and uric acid. Microchim. Acta 2019, 186, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirakunakorn, R.; Khumngern, S.; Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Uric acid enzyme biosensor based on a screen-printed electrode coated with Prussian blue and modified with chitosan-graphene composite cryogel. Microchem. J. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Xing, X.G.; Yao, B.B.; Wu, Q.; Zhang, R.; Yao, L.; Xu, J.G.; Gao, G.H.; Chen, W. Continual and accurate home monitoring of uric acid in urine samples with uricase-packaged nanoflowers assisted portable electrochemical uricometer. Biosens. Bioelectron. 2022, 198, 113804. [Google Scholar] [CrossRef]
- Turkkan, G.; Bas, S.Z.; Atacan, K.; Ozmen, M. An electrochemical sensor based on a Co3O4–ERGO nanocomposite modified screen-printed electrode for detection of uric acid in artificial saliva. Anal. Methods 2022, 14, 67–75. [Google Scholar] [CrossRef]

| Sample a | Spiked (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Human whole blood a | 0 | 5.30 | 1.6 | |
| 1.00 | 6.37 | 2.0 | 107 | |
| 5.00 | 10.3 | 2.2 | 100 | |
| 10.0 | 15.2 | 2.3 | 99.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical Sensor Nanoarchitectonics for Sensitive Detection of Uric Acid in Human Whole Blood Based on Screen-Printed Carbon Electrode Equipped with Vertically-Ordered Mesoporous Silica-Nanochannel Film. Nanomaterials 2022, 12, 1157. https://doi.org/10.3390/nano12071157
Ma K, Yang L, Liu J, Liu J. Electrochemical Sensor Nanoarchitectonics for Sensitive Detection of Uric Acid in Human Whole Blood Based on Screen-Printed Carbon Electrode Equipped with Vertically-Ordered Mesoporous Silica-Nanochannel Film. Nanomaterials. 2022; 12(7):1157. https://doi.org/10.3390/nano12071157
Chicago/Turabian StyleMa, Kai, Luoxing Yang, Jun Liu, and Jiyang Liu. 2022. "Electrochemical Sensor Nanoarchitectonics for Sensitive Detection of Uric Acid in Human Whole Blood Based on Screen-Printed Carbon Electrode Equipped with Vertically-Ordered Mesoporous Silica-Nanochannel Film" Nanomaterials 12, no. 7: 1157. https://doi.org/10.3390/nano12071157






