Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Statistical Analysis
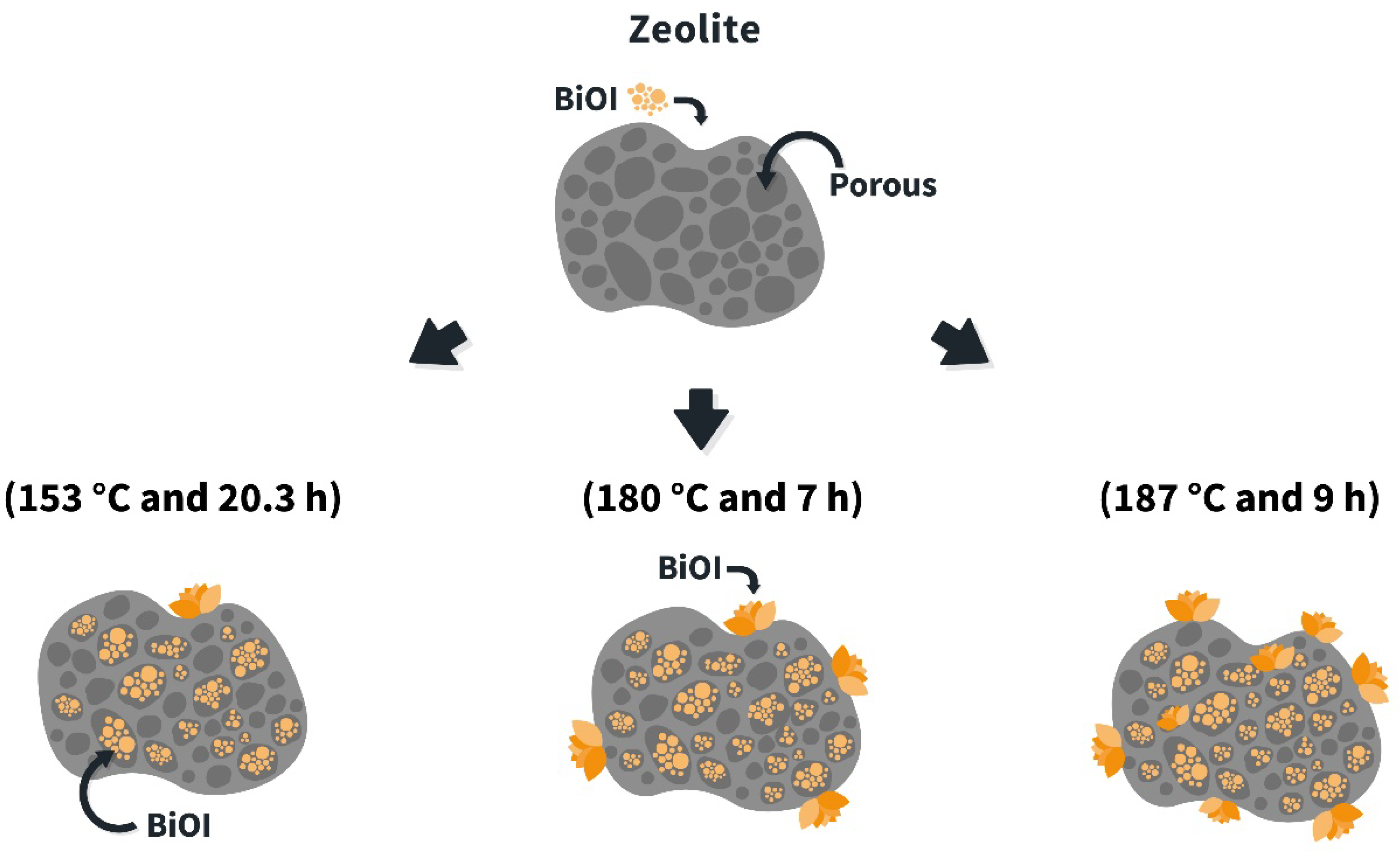
2.3. Synthesis of BiOI/Mordenite Composites
2.4. Photocatalytic Activity Assessment of Synthesized BiOI/Mordenite Composites
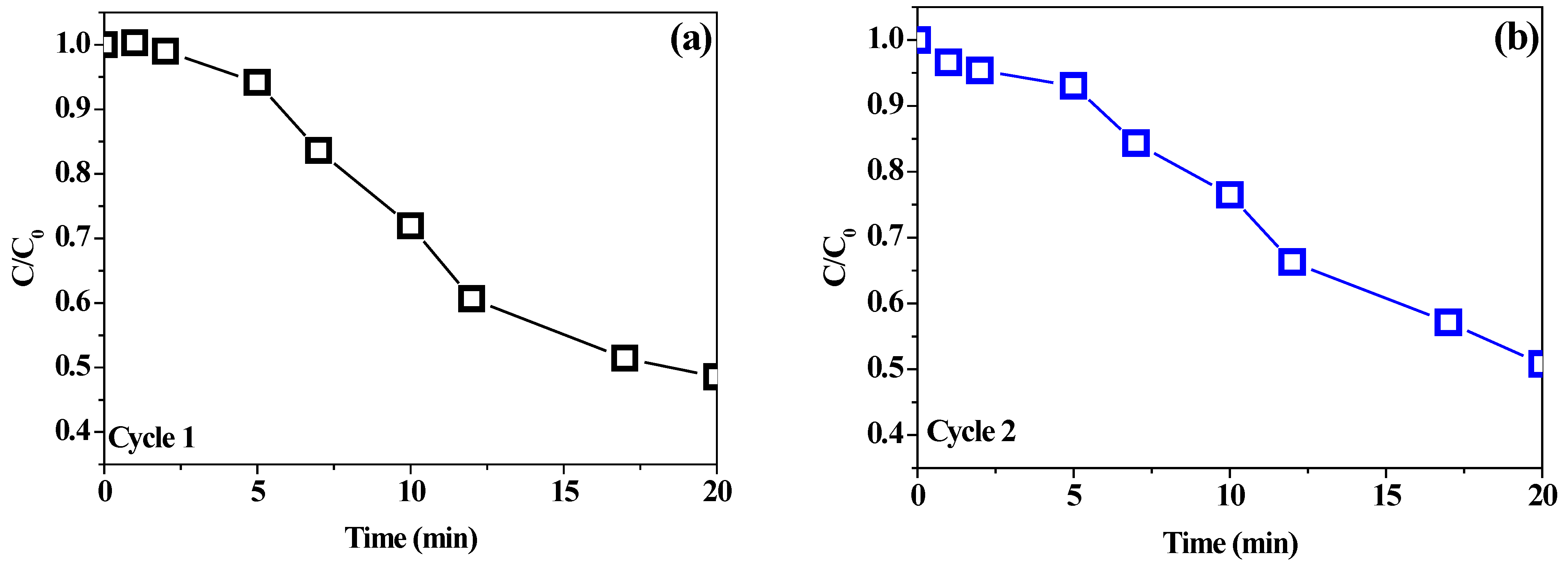
2.5. Stability Tests
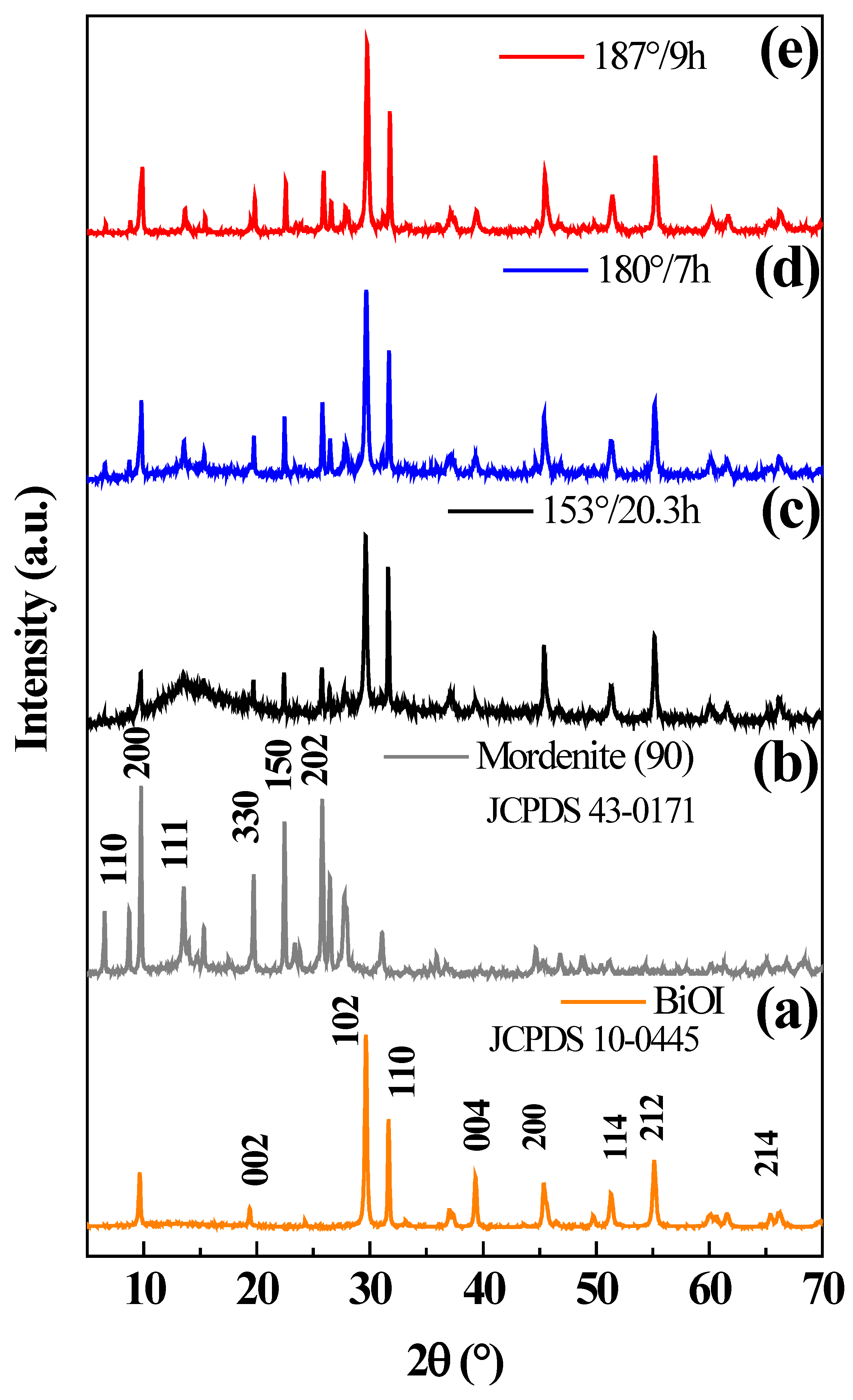
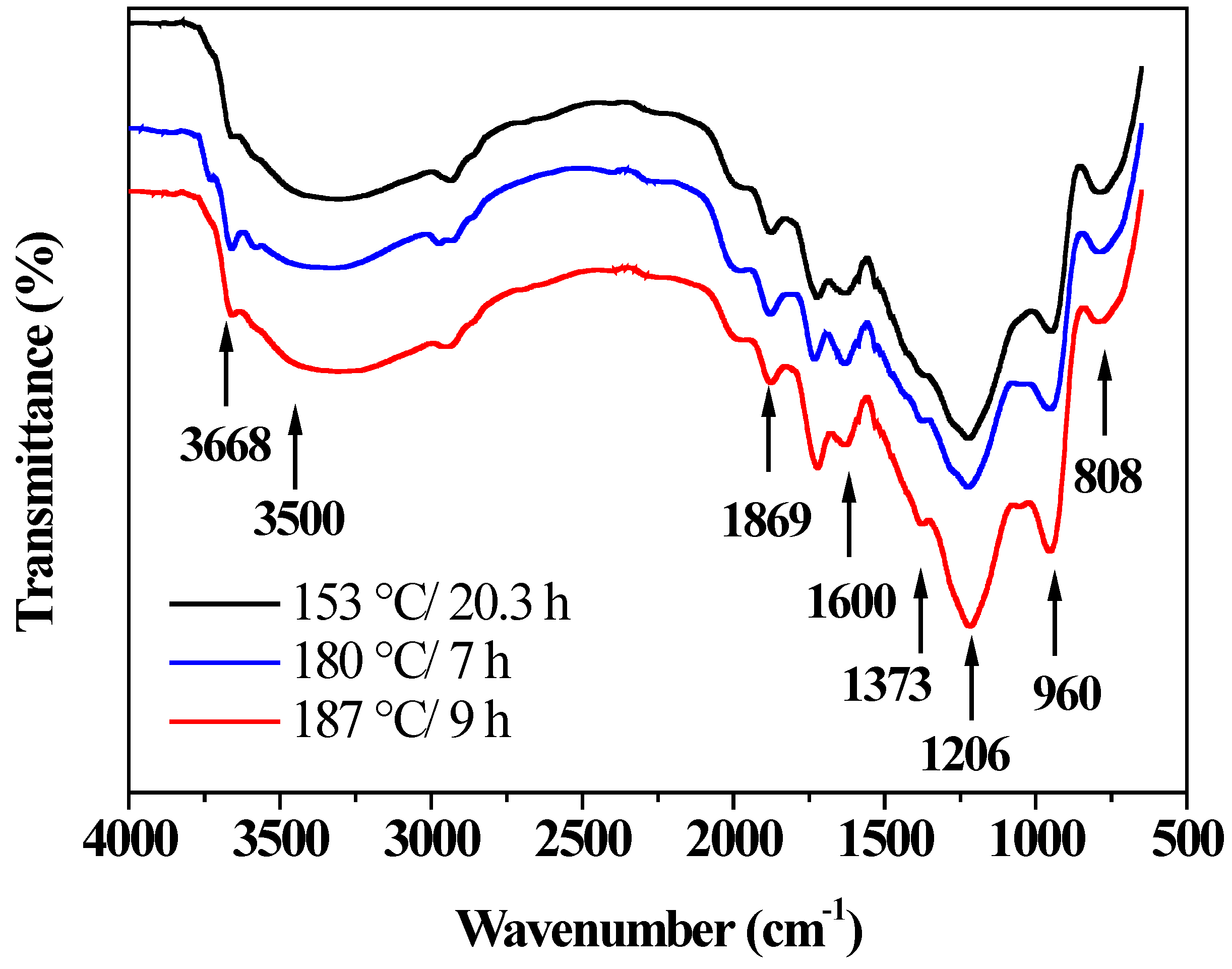
2.6. Physical-Chemical Characterization of Prepared Materials
3. Results and Discussion
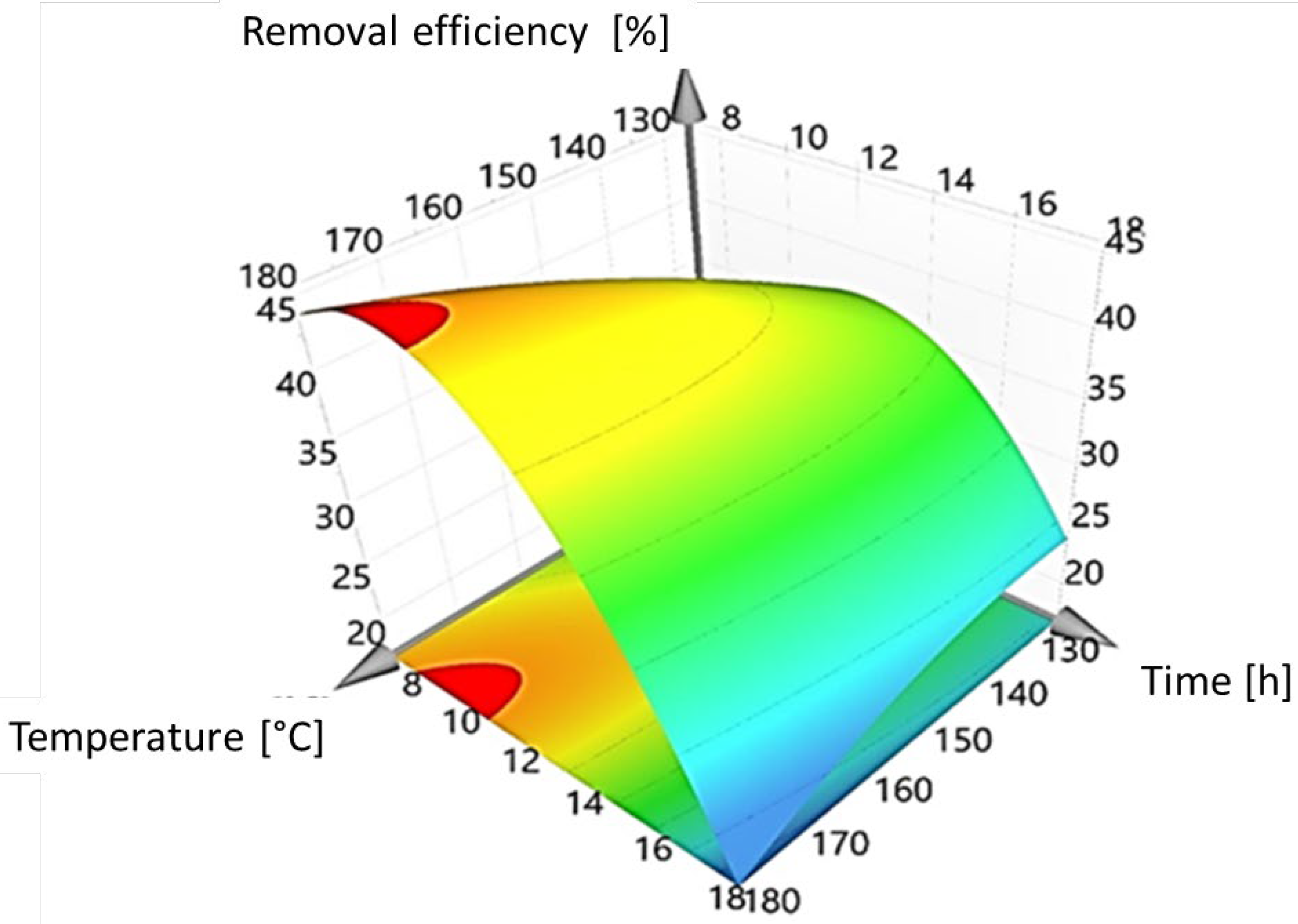
3.1. Optimization of BiOI/Mordenite Composite Synthesis
3.2. Physical and Optical Properties, and Chemical Surface of the Synthesized Materials
3.3. Mechanistic Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genethliou, C.; Kornaros, M.; Dailianis, S. Biodegradation of olive mill wastewater phenolic compounds in a thermophilic anaerobic upflow packed bed reactor and assessment of their toxicity in digester effluents. J. Environ. Manag. 2020, 255, 109882. [Google Scholar] [CrossRef] [PubMed]
- Tri, N.L.M.; Thang, P.Q.; Van Tan, L.; Huong, P.T.; Kim, J.; Viet, N.M.; Al Tahtamouni, T. Removal of phenolic compounds from wastewaters by using synthesized Fe-nano zeolite. J. Water Process Eng. 2020, 33, 101070. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Micellar enhanced ultrafiltration for the valorization of phenolic compounds and polysaccharides from winery wastewaters. J. Water Process Eng. 2020, 38, 101565. [Google Scholar] [CrossRef]
- Pham, T.-H.; Lee, B.-K.; Kim, J. Improved adsorption properties of a nano zeolite adsorbent toward toxic nitrophenols. Process Saf. Environ. Prot. 2016, 104, 314–322. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Yáñez, E.; Santander, P.; Contreras, D.; Yañez, J.; Cornejo, L.; Mansilla, H.D. Homogeneous and heterogeneous degradation of caffeic acid using photocatalysis driven by UVA and solar light. J. Environ. Sci. Health Part A 2016, 51, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, N.V.; Anupama, S.; Navya, K.; Shalini, H.N.; Idris, M.; Hampannavar, U.S. Biological removal of phenol from wastewaters: A mini review. Appl. Water Sci. 2015, 5, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, B.; Li, J.; Lai, B. Decontamination of heavy metal complexes by advanced oxidation processes: A review. Chin. Chem. Lett. 2020, 31, 2575–2582. [Google Scholar] [CrossRef]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Nadeem, N.; Zahid, M. UV-Accelerated Photocatalytic Degradation of Pesticide over Magnetite and Cobalt Ferrite Decorated Graphene Oxide Composite. Plants 2020, 10, 6. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Ikhlaq, A.; Zahid, M.; Alazmi, A. Subcritical and supercritical water oxidation for dye decomposition. J. Environ. Manag. 2021, 290, 112605. [Google Scholar] [CrossRef] [PubMed]
- Viet, N.M.; Trung, D.Q.; Giang, B.L.; Tri, N.L.M.; Thao, P.; Pham, T.; Kamand, F.Z.; Al Tahtamouni, T. Noble metal -doped graphitic carbon nitride photocatalyst for enhancement photocatalytic decomposition of antibiotic pollutant in wastewater under visible light. J. Water Process Eng. 2019, 32, 100954. [Google Scholar] [CrossRef]
- Mera, A.C.; Rodríguez, C.A.; Melendrez, M.; Valdés, H. Synthesis and characterization of BiOI microspheres under standardized conditions. J. Mater. Sci. 2017, 52, 944–954. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.R.; Karthikeyan, S.; Ohtani, B.; Sasaki, K. Fabrication and characterization of ternary sepiolite/g-C3N4/Pd composites for improvement of photocatalytic degradation of ciprofloxacin under visible light irradiation. J. Colloid Interface Sci. 2020, 577, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Pourshirband, N.; Nezamzadeh-Ejhieh, A.; Mirsattari, S.N. The coupled AgI/BiOI catalyst: Synthesis, brief characterization, and study of the kinetic of the EBT photodegradation. Chem. Phys. Lett. 2020, 761, 138090. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Lourenço, V.S.; Carvalho, W.A. Removal of phenol in seawater by heterogeneous photocatalysis using activated carbon materials modified with TiO2. Catal. Today 2020, 388–389, 247–258. [Google Scholar] [CrossRef]
- Mera, A.C.; Moreno, Y.; Pivan, J.-Y.; Peña, O.; Mansilla, H.D. Solvothermal synthesis of BiOI microspheres: Effect of the reaction time on the morphology and photocatalytic activity. J. Photochem. Photobiol. A Chem. 2014, 289, 7–13. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Qian, D.; Zhong, S.; Wang, S.; Lai, Y.; Yang, N.; Jiang, W. Promotion of phenol photodegradation based on novel self-assembled magnetic bismuth oxyiodide core–shell microspheres. RSC Adv. 2017, 7, 36653–36661. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Liu, Z.; Zhang, X.; Cui, T.; Han, J.; Guo, K.; Wang, B.; Li, Y.; Hong, T.; Liu, J.; et al. Three-dimensional flower-like hybrid BiOI–zeolite composites with highly efficient adsorption and visible light photocatalytic activity. RSC Adv. 2014, 4, 45540–45547. [Google Scholar] [CrossRef]
- Yildiz, T.; Yatmaz, H.C.; Öztürk, K. Anatase TiO2 powder immobilized on reticulated Al2O3 ceramics as a photocatalyst for degradation of RO16 azo dye. Ceram. Int. 2020, 46, 8651–8657. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Awasthi, G.P.; Kim, H.J.; Park, C.H.; Kim, C.S. Electrospinning Directly Synthesized Porous TiO2 Nanofibers Modified by Graphitic Carbon Nitride Sheets for Enhanced Photocatalytic Degradation Activity under Solar Light Irradiation. Langmuir 2016, 32, 6163–6175. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Matarangolo, M. ZnO supported on zeolite pellets as efficient catalytic system for the removal of caffeine by adsorption and photocatalysis. Sep. Purif. Technol. 2018, 193, 303–310. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Li, M.; Liu, W.; Tong, M. Facile synthesis of magnetic Fe3O4@BiOI@AgI for water decontamination with visible light irradiation: Different mechanisms for different organic pollutants degradation and bacterial disinfection. Water Res. 2018, 137, 120–129. [Google Scholar] [CrossRef]
- Chang, C.-T.; Wang, J.-J.; Ouyang, T.; Zhang, Q.; Jing, Y.-H. Photocatalytic degradation of acetaminophen in aqueous solutions by TiO2/ZSM-5 zeolite with low energy irradiation. Mater. Sci. Eng. B 2015, 196, 53–60. [Google Scholar] [CrossRef]
- Imam, S.S.; Adnan, R.; Kaus, N.H.M. Immobilization of BiOBr into cellulose acetate matrix as hybrid film photocatalyst for facile and multicycle degradation of ciprofloxacin. J. Alloys Compd. 2020, 843, 155990. [Google Scholar] [CrossRef]
- Yadav, M.; Garg, S.; Chandra, A.; Hernadi, K. Immobilization of green BiOX (X = Cl, Br and I) photocatalysts on ceramic fibers for enhanced photocatalytic degradation of recalcitrant organic pollutants and efficient regeneration process. Ceram. Int. 2019, 45, 17715–17722. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Narayan, R.B.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Amdeha, E.; El-Salamony, R.A. Enhanced removal of p-nitrophenol by ꞵ-Ga2O3-TiO2 photocatalyst immobilized onto rice straw-based SiO2 via factorial optimization of the synergy between adsorption and photocatalysis. J. Environ. Chem. Eng. 2021, 9, 104619. [Google Scholar] [CrossRef]
- Du, G.; Feng, P.; Cheng, X.; Li, J.; Luo, X. Immobilizing of ZIF-8 derived ZnO with controllable morphologies on zeolite A for efficient photocatalysis. J. Solid State Chem. 2017, 255, 215–218. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. Facile photocatalytic reactor development using nano-TiO2 immobilized mosquito net and energy efficient UVLED for industrial dyes effluent treatment. J. Environ. Chem. Eng. 2016, 4, 319–327. [Google Scholar] [CrossRef]
- Prasad, C.; Liu, Q.; Tang, H.; Yuvaraja, G.; Long, J.; Rammohan, A.; Zyryanov, G.V. An overview of graphene oxide supported semiconductors based photocatalysts: Properties, synthesis and photocatalytic applications. J. Mol. Liq. 2020, 297, 111826. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A.; Aly, A.A.; Mohamed, T.Y. A facile synthesis of mordenite zeolite nanostructures for efficient bleaching of crude soybean oil and removal of methylene blue dye from aqueous media. J. Mol. Liq. 2017, 248, 302–313. [Google Scholar] [CrossRef]
- Salari, H.; Khasevani, S.G.; Setayesh, S.R.; Gholami, M.R. Enhanced visible light photocatalytic activity of nano-BiOCl/BiVO4/Zeolite p-n heterojunction and Ag/BiOCl/BiVO4 hybrid. Mater. Res. Innov. 2018, 22, 137–143. [Google Scholar] [CrossRef]
- Jaime-Acuña, O.E.; Farfán, R.E.S.-J.; Villavicencio, H.; Herrera, M.; Herrera, O.R. Photoluminescence up-conversion and cathodoluminescence in quaternary CdxZnyOγSδ nanoparticles embedded on zeolite. J. Phys. Chem. Solids 2021, 153, 110004. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Šumić, Z.; Vakula, A.; Tepić, A.; Čakarević, J.; Vitas, J.; Pavlić, B. Modeling and optimization of red currants vacuum drying process by response surface methodology (RSM). Food Chem. 2016, 203, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Mera, A.C.; Rodríguez, C.A.; Pizarro-Castillo, L.; Meléndrez, M.F.; Valdés, H. Effect of temperature and reaction time during solvothermal synthesis of BiOCl on microspheres formation: Implications in the photocatalytic oxidation of gallic acid under simulated solar radiation. J. Sol-Gel Sci. Technol. 2020, 95, 146–156. [Google Scholar] [CrossRef]
- Contreras, D.; Freer, J.; Rodríguez, J. Veratryl alcohol degradation by a catechol-driven Fenton reaction as lignin oxidation by brown-rot fungi model. Int. Biodeterior. Biodegrad. 2006, 57, 63–68. [Google Scholar] [CrossRef]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Montgomery, D. Diseño y Análisis de Experimentos, 2nd ed.; Limusa Wiley: México City, México, 2004; pp. 21–692. [Google Scholar]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Pardue, H.L. Kinetic aspects of analytical chemistry. Anal. Chim. Acta 1989, 216, 69–107. [Google Scholar] [CrossRef]
- Kontos, A.; Arabatzis, I.; Tsoukleris, D.; Bernard, M.; Petrakis, D.; Falaras, P. Efficient photocatalysts by hydrothermal treatment of TiO2. Catal. Today 2005, 101, 275–281. [Google Scholar] [CrossRef]
- Bertinetto, C.; Engel, J.; Jansen, J. ANOVA simultaneous component analysis: A tutorial review. Anal. Chim. Acta X 2020, 6, 100061. [Google Scholar] [CrossRef]
- Lee, G.-J.; Zheng, Y.-C.; Wu, J.J. Fabrication of hierarchical bismuth oxyhalides (BiOX, X = Cl, Br, I) materials and application of photocatalytic hydrogen production from water splitting. Catal. Today 2018, 307, 197–204. [Google Scholar] [CrossRef]
- Sharma, N.; Pap, Z.; Székely, I.; Focsan, M.; Karacs, G.; Nemeth, Z.; Garg, S.; Hernadi, K. Combination of iodine-deficient BiOI phases in the presence of CNT to enhance photocatalytic activity towards phenol decomposition under visible light. Appl. Surf. Sci. 2021, 565, 150605. [Google Scholar] [CrossRef]
- Reyna-Cavazos, K.A.; La Cruz, A.M.-D.; Rodríguez, F.E.L.; Cuellar, E.L. Synthesis of bismuth oxyiodide (BiOI) by means of microwaves in glycerol with high photocatalytic activity for the elimination of NOx and SO2. Res. Chem. Intermed. 2019, 46, 923–941. [Google Scholar] [CrossRef]
- Lee, G.-J.; Chien, Y.-W.; Anandan, S.; Lv, C.; Dong, J.; Wu, J.J. Fabrication of metal-doped BiOI/MOF composite photocatalysts with enhanced photocatalytic performance. Int. J. Hydrog. Energy 2021, 46, 5949–5962. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Zhao, L.; Zhao, S. Core/shell Fe3O4/BiOI nanoparticles with high photocatalytic activity and stability. J. Nanoparticle Res. 2016, 18, 318. [Google Scholar] [CrossRef]
- Mera, A.C.; Moreno, Y.; Contreras, D.; Escalona, N.; Melendrez, M.; Mangalaraja, R.V.; Mansilla, H.D. Improvement of the BiOI photocatalytic activity optimizing the solvothermal synthesis. Solid State Sci. 2017, 63, 84–92. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xie, X.; Li, C.; Si, Y.; Zhang, M.; Yan, Q. Synthesis flower-like BiVO4/BiOI core/shell heterostructure photocatalyst for tetracycline degradation under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 9311–9321. [Google Scholar] [CrossRef]
- Wei, Z.; Zheng, N.; Dong, X.; Zhang, X.; Ma, H.; Zhang, X.; Xue, M. Green and controllable synthesis of one-dimensional Bi2O3/BiOI heterojunction for highly efficient visible-light-driven photocatalytic reduction of Cr(VI). Chemosphere 2020, 257, 127210. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-H.; Zhang, X.-L.; Zhang, N.; Zhang, J.-Y.; Zhang, R.; Liu, Y.-F.; Fang, Y.-Z. A visible-light driven Bi2S3@ZIF-8 core–shell heterostructure and synergistic photocatalysis mechanism. Dalton Trans. 2018, 47, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, X.; Fang, J.; Zhu, X.; Chu, J.; Li, B. Microemulsion synthesis, characterization of bismuth oxyiodine/titanium dioxide hybrid nanoparticles with outstanding photocatalytic performance under visible light irradiation. Appl. Surf. Sci. 2012, 258, 3771–3778. [Google Scholar] [CrossRef]
- Wang, S.; Guan, Y.; Wang, L.; Zhao, W.; He, H.; Xiao, J.; Yang, S.; Sun, C. Fabrication of a novel bifunctional material of BiOI/Ag3VO4 with high adsorption–photocatalysis for efficient treatment of dye wastewater. Appl. Catal. B Environ. 2015, 168–169, 448–457. [Google Scholar] [CrossRef]
- Supamathanon, N.; Khabuanchalad, S. Development of K-mordenite catalyst for biodiesel production from soybean oil. Mater. Today Proc. 2019, 17, 1412–1422. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Tamizhdurai, P.; Ramesh, A.; Chiu, T.-W.; Mangesh, V.; Veerarajan, S.; Shanthi, K. Platinum incorporated mordenite zeolite modified glassy carbon electrode used for selective electrochemical detection of mercury ions. Microporous Mesoporous Mater. 2020, 292, 109770. [Google Scholar] [CrossRef]
- Ibrahim, M.; Jalil, A.; Zakaria, W.; Fatah, N.; Hamid, M.; Izan, S.; Setiabudi, H. n-Hexane hydroisomerization over Zr-modified bicontinuous lamellar silica mordenite supported Pt as highly selective catalyst: Molecular hydrogen generated protonic acid sites and optimization. Int. J. Hydrog. Energy 2021, 46, 4019–4035. [Google Scholar] [CrossRef]
- Ren, L.; Wang, B.; Lu, K.; Peng, R.; Guan, Y.; Jiang, J.-G.; Xu, H.; Wu, P. Selective conversion of methanol to propylene over highly dealuminated mordenite: Al location and crystal morphology effects. Chin. J. Catal. 2021, 42, 1147–1159. [Google Scholar] [CrossRef]
- Zainudin, N.F.; Abdullah, A.Z.; Mohamed, A.R. Characteristics of supported nano-TiO2/ZSM-5/silica gel (SNTZS): Photocatalytic degradation of phenol. J. Hazard. Mater. 2010, 174, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Liu, Q.; Wang, X.; Ren, S.; Yang, J.; Zhao, D.; Xi, W.; Yao, L. Performance impact and poisoning mechanism of arsenic over commercial V2O5–WO3/TiO2 SCR catalyst. Catal. Commun. 2015, 72, 121–126. [Google Scholar] [CrossRef]
- Torkian, N.; Bahrami, A.; Hosseini-Abari, A.; Momeni, M.M.; Abdolkarimi-Mahabadi, M.; Bayat, A.; Hajipour, P.; Rourani, H.A.; Abbasi, M.S.; Torkian, S.; et al. Synthesis and characterization of Ag-ion-exchanged zeolite/TiO2 nanocomposites for antibacterial applications and photocatalytic degradation of antibiotics. Environ. Res. 2021, 207, 112157. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yang, J.; Hu, G.; Yang, C.; Chen, Y.; Liu, Q.; Ren, S.; Li, J. Optimization of TiO2/ZSM-5 photocatalysts: Energy band engineering by solid state diffusion method with calcination. J. Environ. Chem. Eng. 2021, 9, 105563. [Google Scholar] [CrossRef]
- Triana, M.A.; López, A.F.; Camargo, R.J. Síntesis, caracterización y evaluación fotocatalítica de puntos cuánticos de cdse cubiertos con 2 tipos de Tioles. Inf. Tecnológica 2015, 26, 121–134. [Google Scholar] [CrossRef] [Green Version]




| Experimental Runs | Natural Variables | Coded Variables | Response Values | |||
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | X1 | X2 | Y Experimental (%) | Y Predicted (%) | |
| 1 | 153.0 | 20.3 | 0 | 6.1 | 7.6 | |
| 2 | 180.0 | 18.0 | +1 | +1 | 17.8 | 16.6 |
| 3 | 153.0 | 4.7 | 0 | 29.4 | 29.0 | |
| 4 | 153.0 | 12.5 | 0 | 0 | 42.2 | 41.2 |
| 5 | 153.0 | 12.5 | 0 | 0 | 41.2 | 41.2 |
| 6 | 180.0 | 7.0 | +1 | −1 | 43.6 | 43.8 |
| 7 | 153.0 | 12.5 | 0 | 0 | 40.3 | 41.2 |
| 8 | 126.0 | 7.0 | −1 | −1 | 27.1 | 26.9 |
| 9 | 191.2 | 12.5 | 0 | 40.1 | 40.5 | |
| 10 | 114.8 | 12.5 | 0 | 32.6 | 33.6 | |
| 11 | 126.0 | 18.0 | −1 | +1 | 25.4 | 23.8 |
| Photocatalytic Tests | Experimental Removal Efficiency (%) | Predicted Removal Efficiency Ranges (%) |
|---|---|---|
| 1 | 50.3 | 48.5–51.5 |
| 2 | 47.9 | 48.5–51.5 |
| 3 | 51.5 | 48.5–51.5 |
| Average | 49.9 | |
| Individual BiOI | 42.8 |
| Materials | BET (m2 g−1) | Pore Diameter (nm) | Pore Volume (cm3 g−1) | Eg (eV) |
|---|---|---|---|---|
| Pure BiOI | 7 | 16.3 | 0.031 | 1.93 |
| Mordenite | 547 | 2.40 | 0.331 | --- |
| BiOI/mordenite: 153 °C/20.3 h | 318 | 3.18 | 0.255 | 1.93 |
| BiOI/mordenite: 180 °C/7 h | 360 | 2.61 | 0.238 | 1.93 |
| BiOI/mordenite: 187 °C/9 h | 371 | 3.01 | 0.282 | 1.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Alcaíno, A.; Robles-Araya, N.; Avalos, C.; Alfonso-Alvarez, A.; Rodríguez, C.A.; Valdés, H.; Sánchez-Flores, N.A.; Durán-Alvarez, J.C.; Bizarro, M.; Romero-Salguero, F.J.; et al. Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater. Nanomaterials 2022, 12, 1161. https://doi.org/10.3390/nano12071161
Gallegos-Alcaíno A, Robles-Araya N, Avalos C, Alfonso-Alvarez A, Rodríguez CA, Valdés H, Sánchez-Flores NA, Durán-Alvarez JC, Bizarro M, Romero-Salguero FJ, et al. Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater. Nanomaterials. 2022; 12(7):1161. https://doi.org/10.3390/nano12071161
Chicago/Turabian StyleGallegos-Alcaíno, Alejandra, Nathaly Robles-Araya, Camila Avalos, Alexander Alfonso-Alvarez, Carlos A. Rodríguez, Héctor Valdés, Norma A. Sánchez-Flores, Juan C. Durán-Alvarez, Monserrat Bizarro, Francisco J. Romero-Salguero, and et al. 2022. "Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater" Nanomaterials 12, no. 7: 1161. https://doi.org/10.3390/nano12071161
APA StyleGallegos-Alcaíno, A., Robles-Araya, N., Avalos, C., Alfonso-Alvarez, A., Rodríguez, C. A., Valdés, H., Sánchez-Flores, N. A., Durán-Alvarez, J. C., Bizarro, M., Romero-Salguero, F. J., & Mera, A. C. (2022). Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater. Nanomaterials, 12(7), 1161. https://doi.org/10.3390/nano12071161











