Microporous N-Doped Carbon Obtained from Salt Melt Pyrolysis of Chitosan toward Supercapacitor and Oxygen Reduction Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Chitosan, medium molecular weight (M, 210–300 kDa) and deacetylation degree 84% (Sigma-Aldrich, Darmstadt, Germany)
- -
- Anhydrous Potassium chloride (Sigma-Aldrich, Darmstadt, Germany)
- -
- Anhydrous Zinc chloride (Sigma-Aldrich, Darmstadt, Germany).
2.2. Methods
2.3. Analysis and Characterization Techniques
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernardo, M.; Lapa, N.; Fonseca, I.; Esteves, I.A.A.C. Biomass Valorization to Produce Porous Carbons: Applications in CO2 Capture and Biogas Upgrading to Biomethane—A Mini-Review. Front. Energy Res. 2021, 9, 625188. [Google Scholar] [CrossRef]
- Hammi, N.; Chen, S.; Dumeignil, F.; Royer, S.; El Kadib, A. Chitosan as a sustainable precursor for nitrogen-containing carbon nanomaterials: Synthesis and uses. Mater. Today Sustain. 2020, 10, 100053. [Google Scholar] [CrossRef]
- Primo, A.; Atienzar, P.; Sanchez, E.; Delgado, J.M.; García, H. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-X.; Deng, X.-Q.; Zhang, S.; Shen, Y.-F.; Shan, Y.-Q.; Zhang, Z.-M.; Luque, R.; Duan, P.-G.; Hu, X. Benign-by-design N-doped carbonaceous materials obtained from the hydrothermal carbonization of sewage sludge for supercapacitor applications. Green Chem. 2020, 22, 3885–3895. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Gontarek-Castro, E.; Ollik, K.; Lieder, M. Biomass-Derived Nitrogen Functionalized Carbon Nanodots and Their Anti-Biofouling Properties. Processes 2021, 9, 61. [Google Scholar] [CrossRef]
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787. [Google Scholar] [CrossRef] [Green Version]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Ilnicka, A.; Lukaszewicz, J.P. Synthesis of N-rich microporous carbon materials from chitosan by alkali activation using Na2CO3. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2015, 201, 66–71. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Khan, A.; Goepel, M.; Colmenares, J.C.; Gläser, R. Chitosan-Based N-Doped Carbon Materials for Electrocatalytic and Photocatalytic Applications. ACS Sustain. Chem. Eng. 2020, 8, 4708–4727. [Google Scholar] [CrossRef] [Green Version]
- Rybarczyk, M.K.; Lieder, M.; Jablonska, M. N-doped mesoporous carbon nanosheets obtained by pyrolysis of a chitosan-melamine mixture for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 44969–44977. [Google Scholar] [CrossRef]
- Kucinska, A.; Golembiewski, R.; Lukaszewicz, J.P. Synthesis of N-Rich Activated Carbons from Chitosan by Chemical Activation. Sci. Adv. Mater. 2014, 6, 290–297. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. Heteroatom functionalized activated porous biocarbons and their excellent performance for CO2 capture at high pressure. J. Mater. Chem. A 2017, 5, 21196–21204. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, K.; Subburaj, T.; Jo, Y.N.; Santhoshkumar, P.; Karthikeyan, S.K.S.S.; Vediappan, K.; Gnanamuthu, R.M.; Lee, C.W. Chitosan complements entrapment of silicon inside nitrogen doped carbon to improve and stabilize the capacity of Li-ion batteries. Sci. Rep. 2019, 9, 3318. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Wang, G.; Zhang, M.; Fan, X.; Yu, C.; Yang, J.; Xiao, N.; Qiu, J. Boric acid-mediated B,N-codoped chitosan-derived porous carbons with a high surface area and greatly improved supercapacitor performance. Nanoscale 2015, 7, 5120–5125. [Google Scholar] [CrossRef]
- Wróbel-Iwaniec, I.; Díez, N.; Gryglewicz, G. Chitosan-based highly activated carbons for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 5788–5796. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Chi, L.; Wen, X.; Chen, X.; Mijowska, E. Nitrogen/Oxygen Enriched Hierarchical Porous Carbons Derived from Waste Peanut Shells Boosting Performance of Supercapacitors. Adv. Electron. Mater. 2020, 6, 2000450. [Google Scholar] [CrossRef]
- Łuczak, J.; Paszkiewicz, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 1: Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar] [CrossRef]
- Yan, F.; Lartey, M.; Damodaran, K.; Albenze, E.; Thompson, R.L.; Kim, J.; Haranczyk, M.; Nulwala, H.B.; Luebke, D.R.; Smit, B. Understanding the effect of side groups in ionic liquids on carbon-capture properties: A combined experimental and theoretical effort. Phys. Chem. Chem. Phys. 2013, 15, 3264–3272. [Google Scholar] [CrossRef]
- Zhang, S.; Dokko, K.; Watanabe, M. Carbon materialization of ionic liquids: From solvents to materials. Mater. Horiz. 2015, 2, 168–197. [Google Scholar] [CrossRef]
- Huang, X.; Yamasaki, K.; Kudo, S.; Sperry, J.; Hayashi, J. Influence of ionic liquid type on porous carbon formation during the ionothermal pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2020, 145, 104728. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.-P.; Antonietti, M. “Salt Templating”: A Simple and Sustainable Pathway toward Highly Porous Functional Carbons from Ionic Liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Preuss, K.; Porwal, H.; Zhao, T.; Guo, Z.X.; Titirici, M.-M. Salt Templating with Pore Padding: Hierarchical Pore Tailoring towards Functionalised Porous Carbons. ChemSusChem 2017, 10, 199–209. [Google Scholar] [CrossRef]
- Fan, Q.; Ma, C.; Wu, L.; Wei, C.; Wang, H.; Song, Y.; Shi, J. Preparation of cellulose acetate derived carbon nanofibers by ZnCl2 activation as a supercapacitor electrode. RSC Adv. 2019, 9, 6419–6428. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.; Vijayan, S.; Prabhakaran, K. Nitrogen doped microporous carbon by ZnCl2 activation of protein. Mater. Res. Express 2017, 4, 095602. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Liu, X.; Giordano, C.; Antonietti, M. A facile molten-salt route to graphene synthesis. Small 2014, 10, 193–200. [Google Scholar] [CrossRef]
- Baccour, M.; Louvain, N.; Alauzun, J.G.; Stievano, L.; Mutin, P.H.; Boury, B.; Monconduit, L.; Brun, N. Carbonization of polysaccharides in FeCl3/BmimCl ionic liquids: Breaking the capacity barrier of carbon negative electrodes in lithium ion batteries. J. Power Sources 2020, 474, 228575. [Google Scholar] [CrossRef]
- Xie, Z.-L.; White, R.J.; Weber, J.; Taubert, A.; Titirici, M.M. Hierarchical porous carbonaceous materials via ionothermal carbonization of carbohydrates. J. Mater. Chem. 2011, 21, 7434–7442. [Google Scholar] [CrossRef]
- Leżańska, M.; Olejniczak, A.; Łukaszewicz, J.P. Hierarchical porous carbon templated with silica spheres of a diameter of 14 nm from pure chitosan or a chitosan/ZnCl2 solution. J. Porous Mater. 2018, 25, 1633–1648. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-H.; Zhang, B. Using thermal analysis technology to assess the thermal stability of 1,3-dimethylimidazolium nitrate. Process Saf. Environ. Prot. 2019, 124, 181–186. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Sørensen, L.H.; Filinchuk, Y.; Reed, D.; Book, D.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. Mixed-Anion and Mixed-Cation Borohydride KZn(BH4)Cl2: Synthesis, Structure and Thermal Decomposition. Eur. J. Inorg. Chem. 2010, 2010, 1608–1612. [Google Scholar] [CrossRef] [Green Version]
- Rybarczyk, M.K.; Gontarek, E.; Lieder, M.; Titirici, M.M. Salt melt synthesis of curved nitrogen-doped carbon nanostructures: ORR kinetics boost. Appl. Surf. Sci. 2018, 435, 543–551. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Yuksel, R.; Kaplan, B.R.; Bicer, E.; Yurum, A.; Gursel, S.A.; Unalan, H.E. All-carbon hybrids for high performance supercapacitors. Int. J. Energy Res. 2018, 42, 3575–3587. [Google Scholar] [CrossRef]
- Yuksel, R.; Buyukcakir, O.; Panda, P.K.; Lee, S.H.; Jiang, Y.; Singh, D.; Hansen, S.; Adelung, R.; Mishra, Y.K.; Ahuja, R.; et al. Necklace-like Nitrogen-Doped Tubular Carbon 3D Frameworks for Electrochemical Energy Storage. Adv. Funct. Mater. 2020, 30, 1909725. [Google Scholar] [CrossRef]
- Papurello, D.; Santarelli, M.; Fiorilli, S. Physical Activation of Waste-Derived Materials for Biogas Cleaning. Energies 2018, 11, 2338. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; He, C.; Qiu, S.; Zhang, J.; Wang, X.; Yang, Y. Molten-salt-templated fabrication of N,S co-doped hierarchically porous carbons for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 13, 10113–10122. [Google Scholar] [CrossRef]
- Pampel, J.; Fellinger, T.-P. Opening of Bottleneck Pores for the Improvement of Nitrogen Doped Carbon Electrocatalysts. Adv. Energy Mater. 2016, 6, 1502389. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Nasir, M.; Bakandritsos, A.; Pykal, M.; Jakubec, P.; Zboril, R.; Otyepka, M.; Pumera, M. Cyanographene and Graphene Acid: The Functional Group of Graphene Derivative Determines the Application in Electrochemical Sensing and Capacitors. ChemElectroChem 2019, 6, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Díez, N.; Fuertes, A.B.; Sevilla, M. Molten salt strategies towards carbon materials for energy storage and conversion. Energy Storage Mater. 2021, 38, 50–69. [Google Scholar] [CrossRef]
- Caturla, F.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Ramkumara, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015, 44, 6158–6168. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Jones, R.T.; Pramanik, N.C.; Jean-Fulcrand, A.; Garnweitner, G. A Hybrid Electrochemical Energy Storage Device Using Sustainable Electrode Materials. ChemistrySelect 2020, 5, 1597–1606. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]


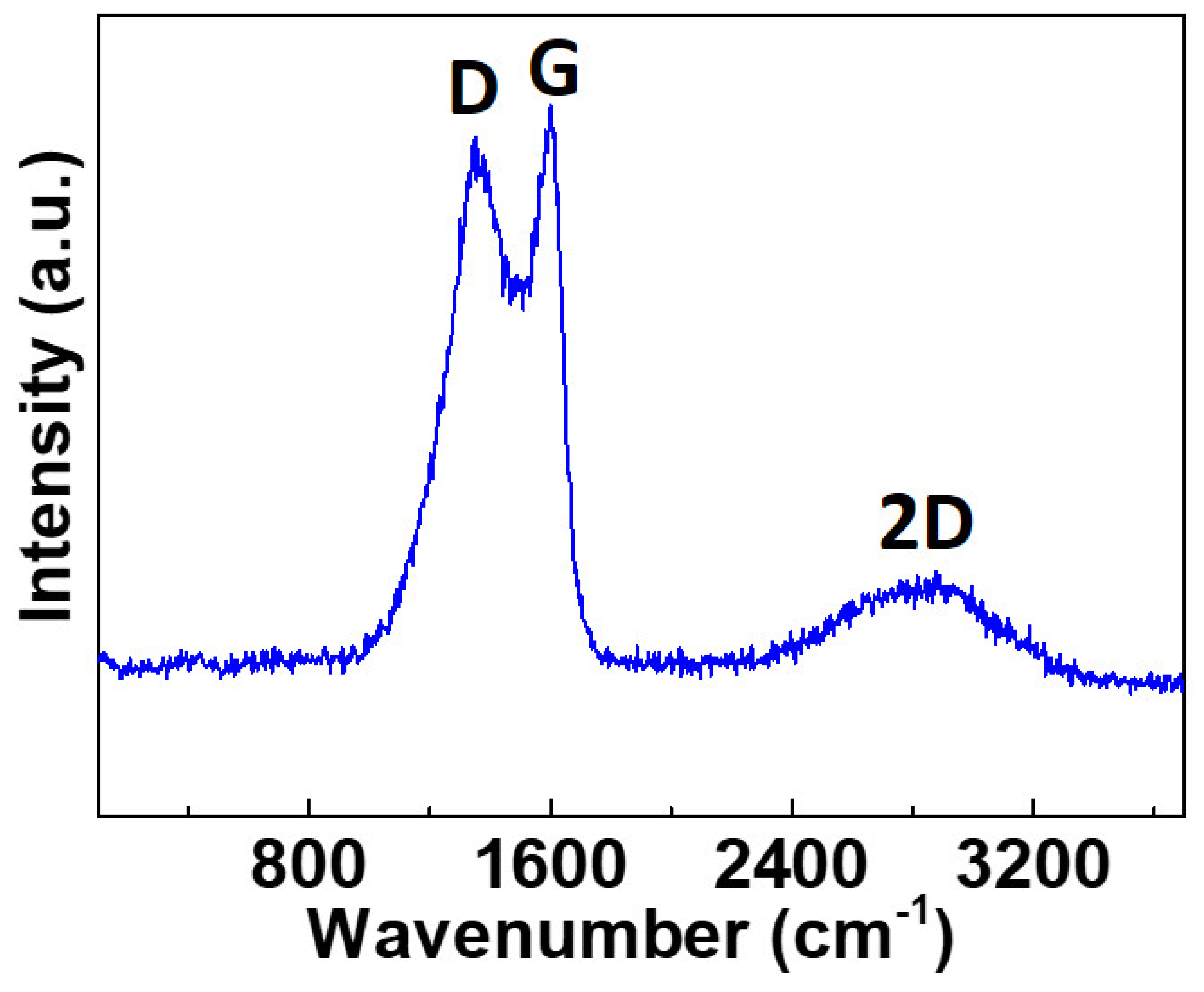
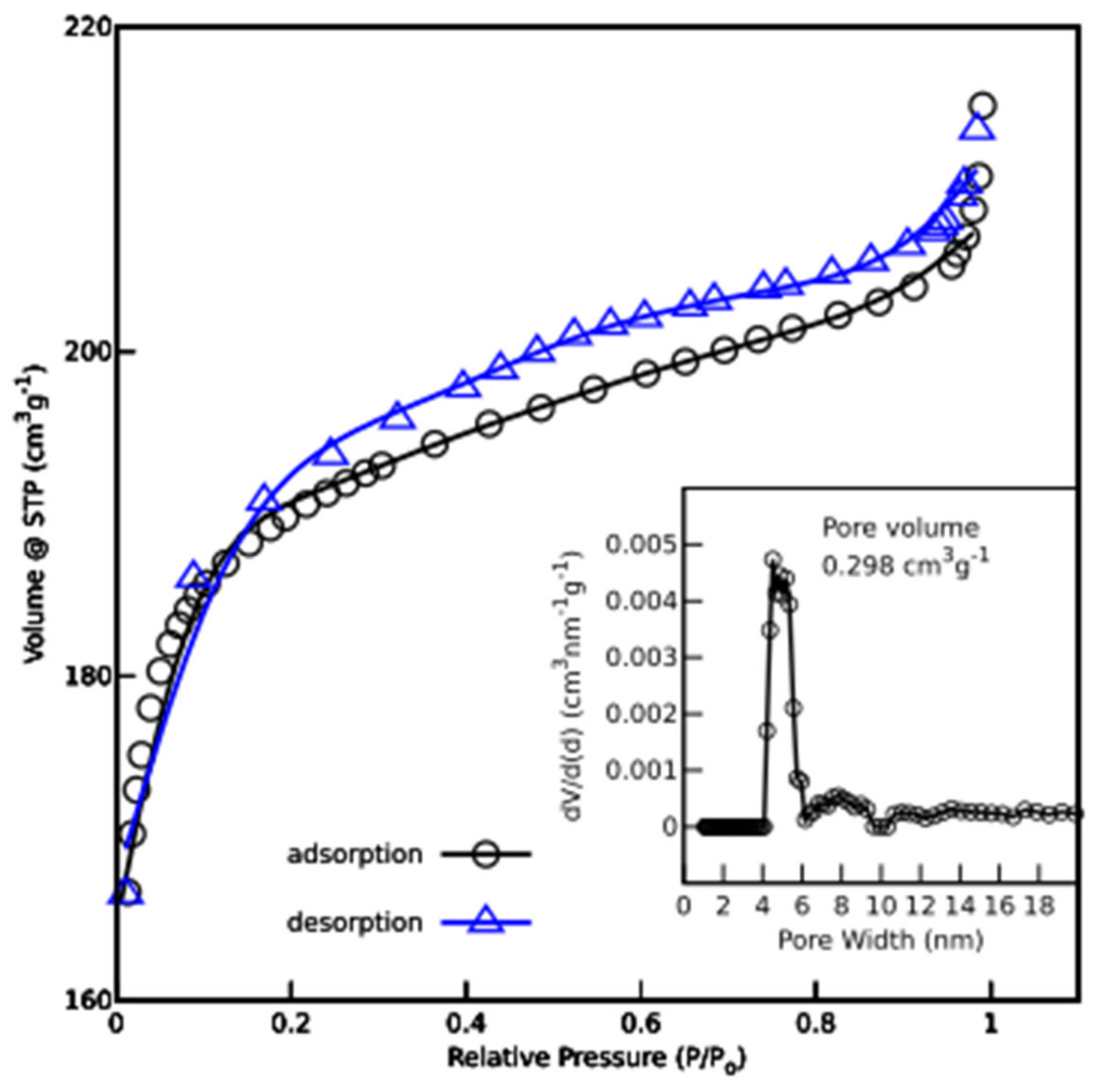

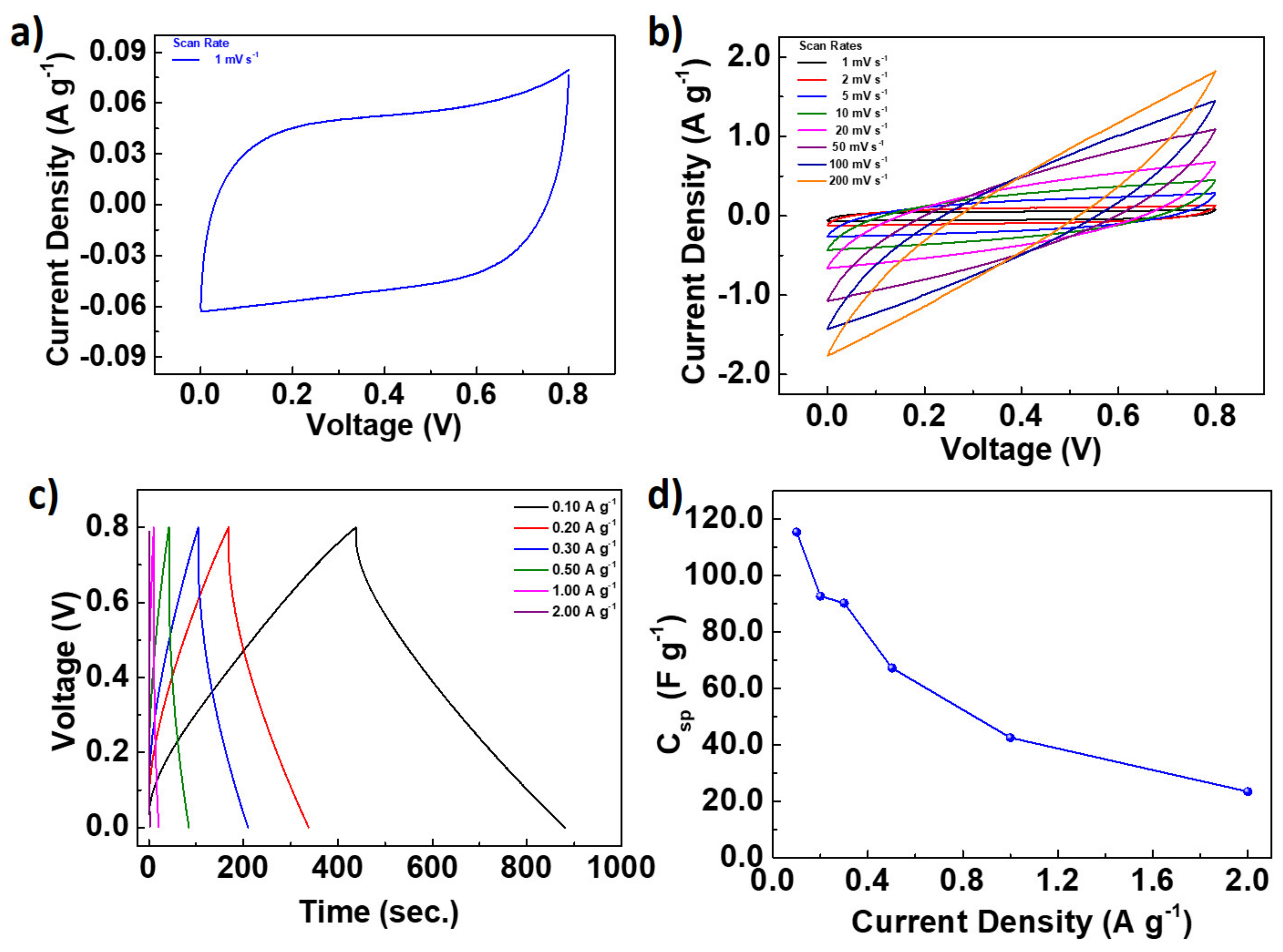
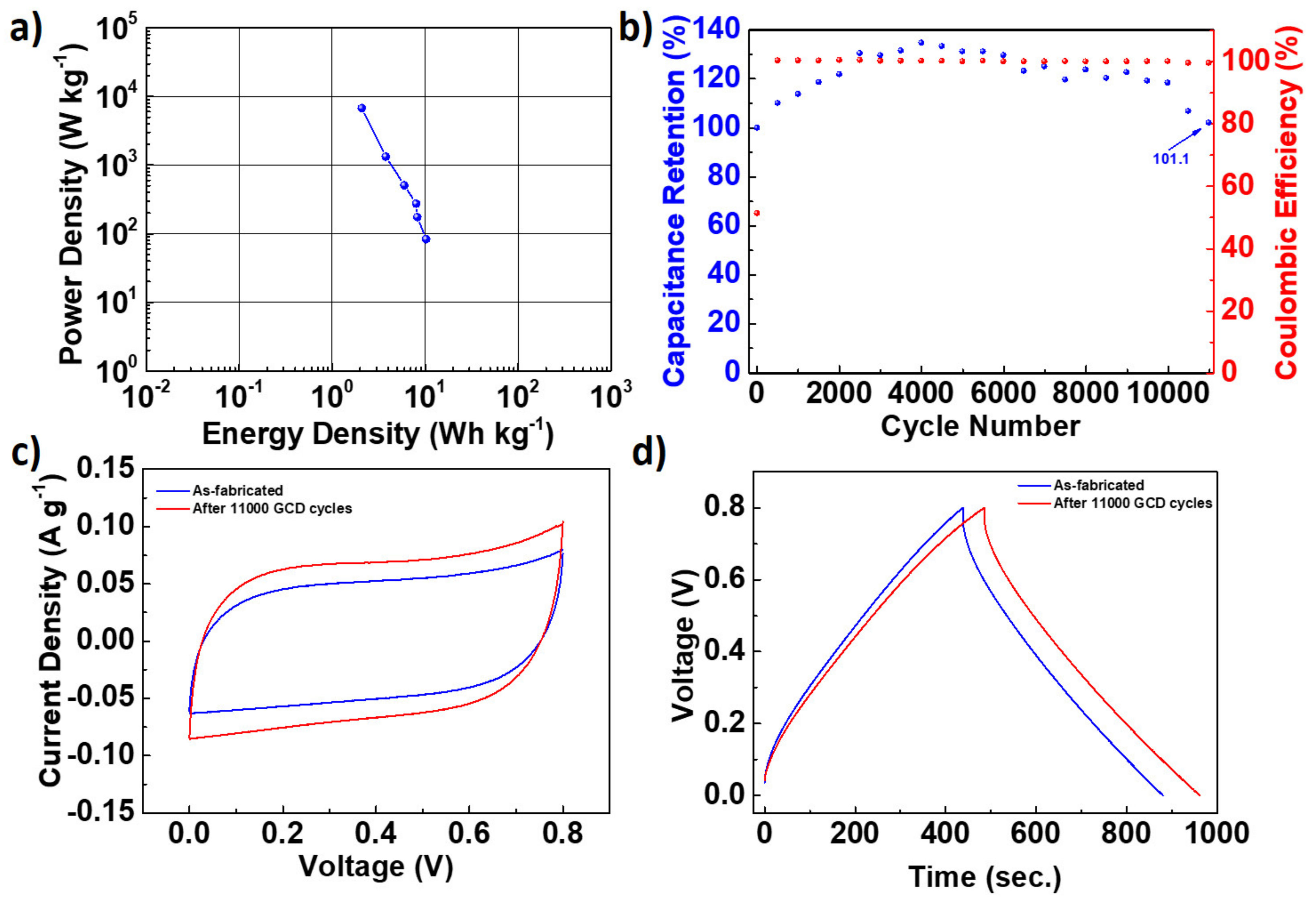
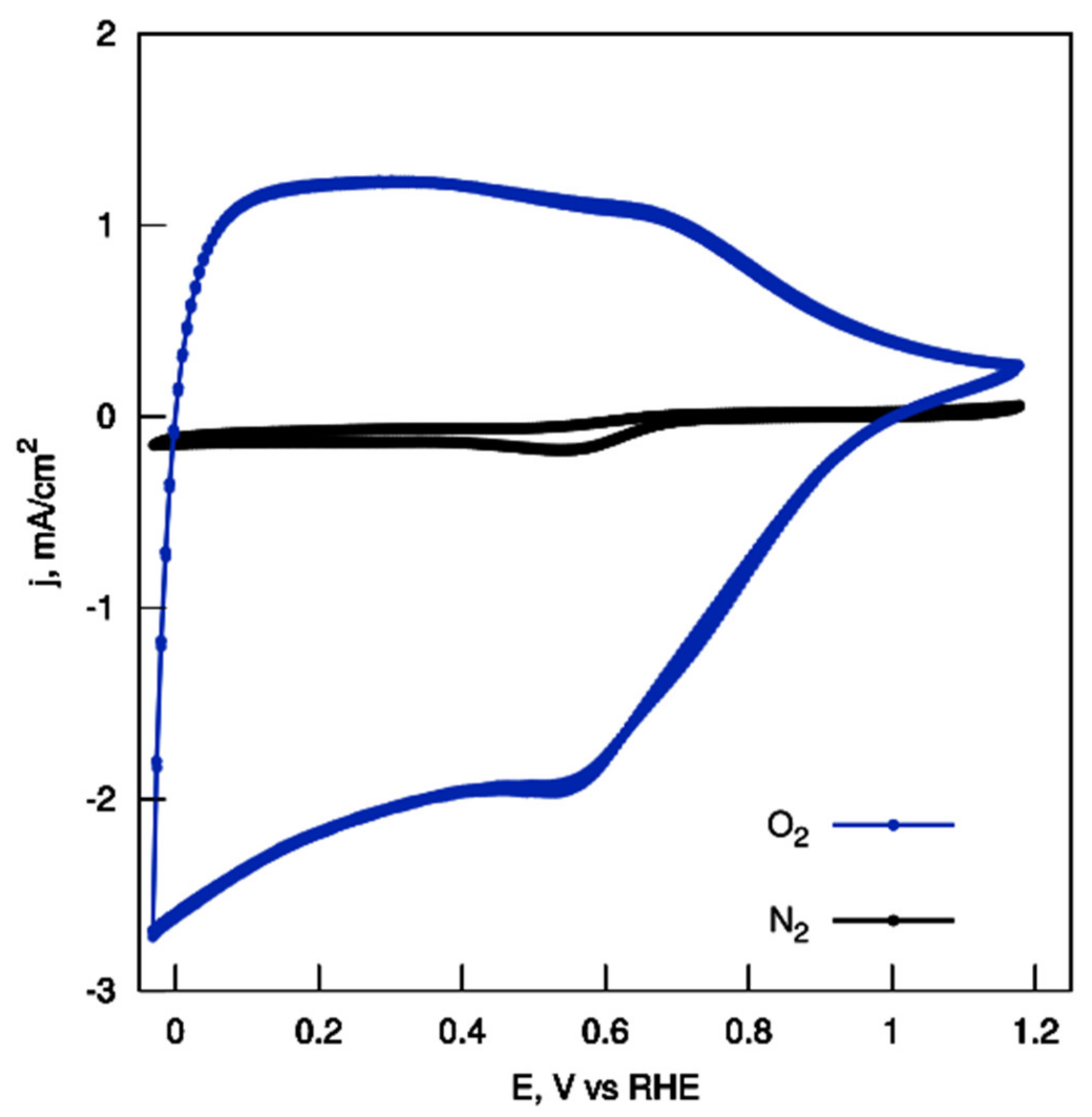
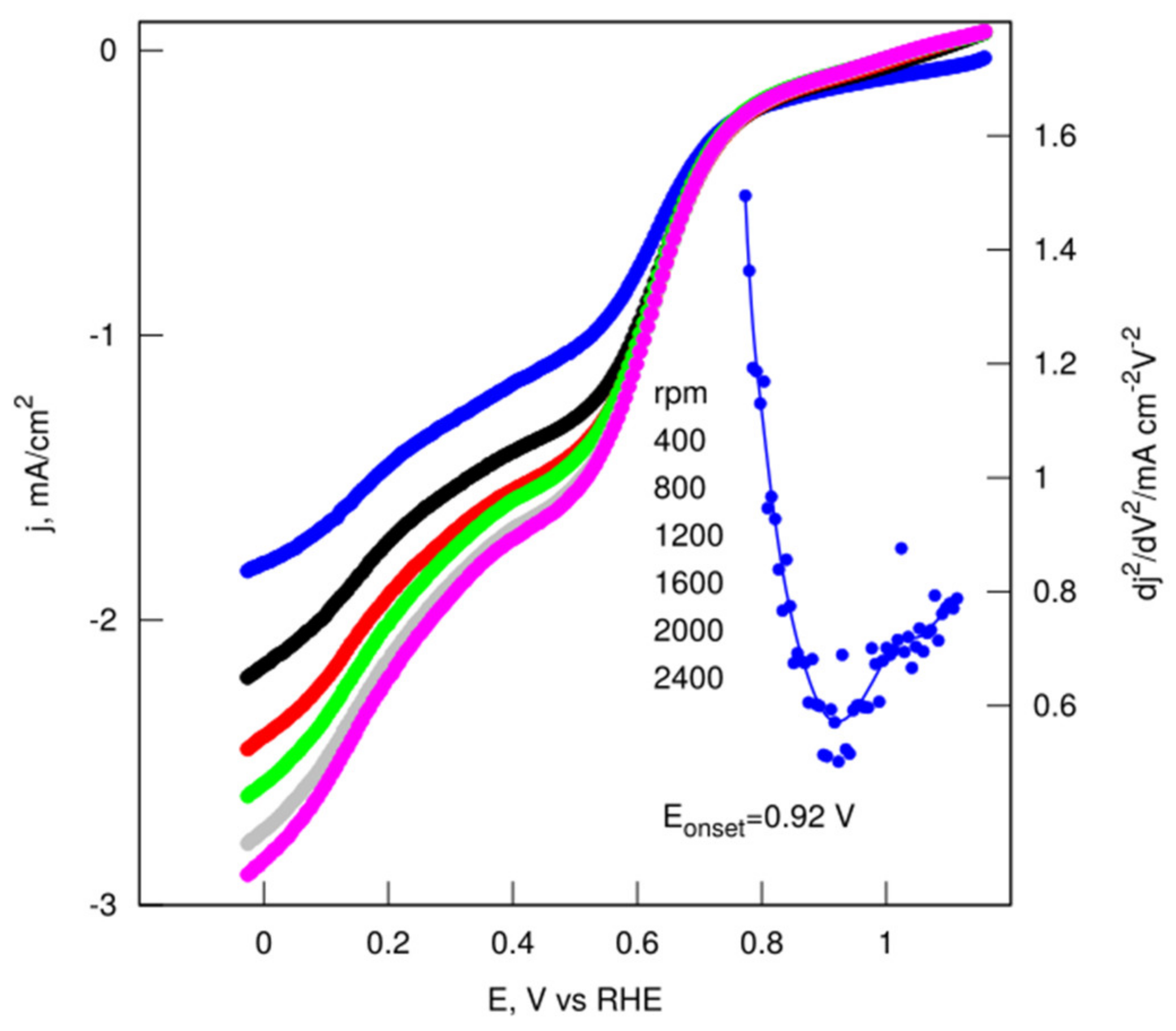
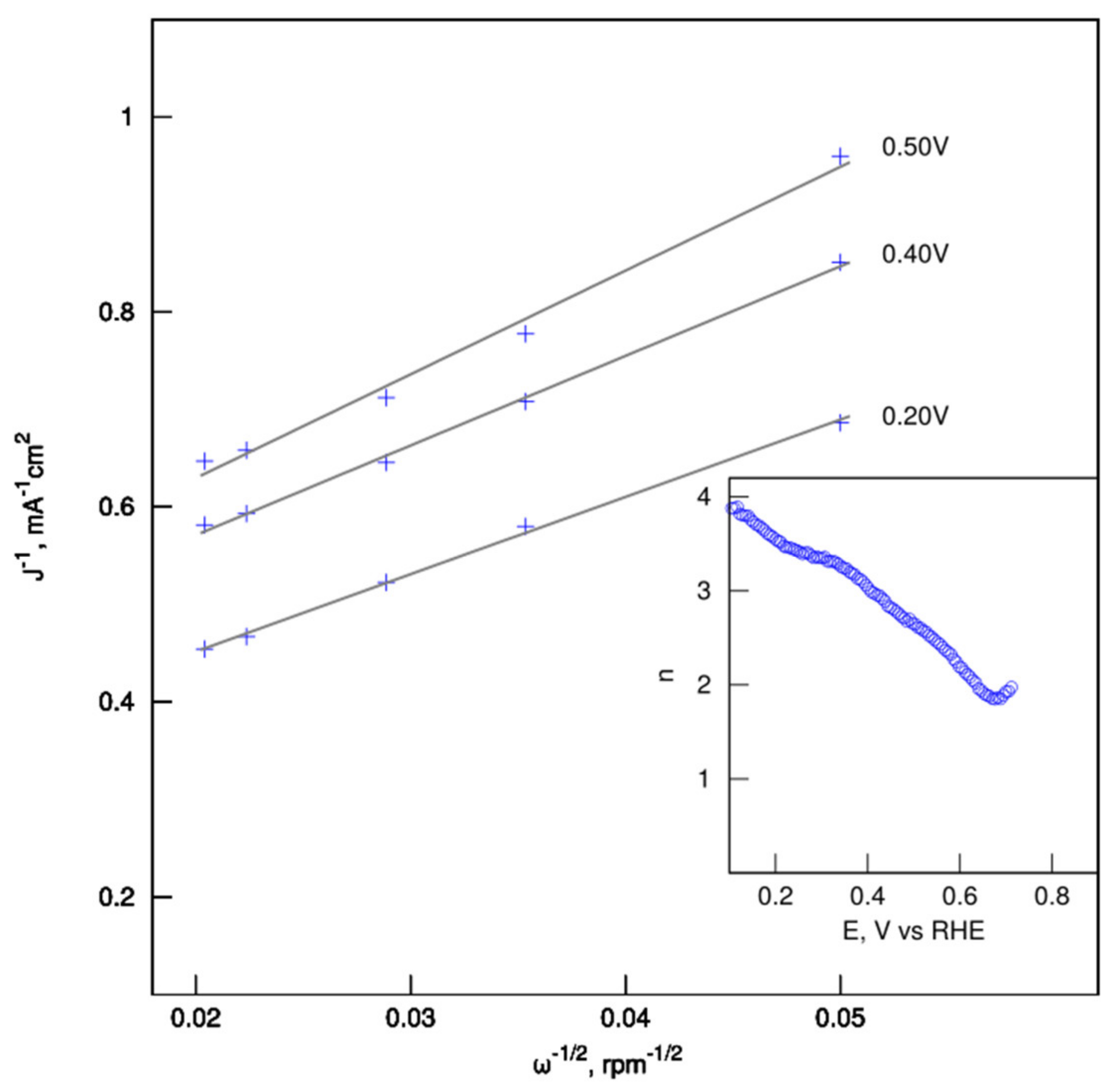
| Name | Total Area m2 g−1 | Micro-Pore Area m2 g−1 | Meso-Pore Area m2 g−1 | Total Pore Volume cm3 g−1 | Micropore Volume cm3 g−1 | Mesopore Volume cm3 g−1 | Pore with nm |
|---|---|---|---|---|---|---|---|
| CH800 a | 10.5 | n/a | n/a | 0.03 | n/a | n/a | 3.939 |
| CH_K | 606.42 | 553 | 6.775 | 0.298 | 0.285 | 0.013 | 1.007 |
| Precursor | CH_K | ||
|---|---|---|---|
| [%mas.] CHN | XPS | ||
| C | 38.3 | 80.0 | 83.3 |
| H | 6.7 | 2.02 | - |
| N | 5.9 | 8.36 | 8.7 |
| O | 49.1 b | 9.62 b | 8.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybarczyk, M.K.; Cysewska, K.; Yuksel, R.; Lieder, M. Microporous N-Doped Carbon Obtained from Salt Melt Pyrolysis of Chitosan toward Supercapacitor and Oxygen Reduction Catalysts. Nanomaterials 2022, 12, 1162. https://doi.org/10.3390/nano12071162
Rybarczyk MK, Cysewska K, Yuksel R, Lieder M. Microporous N-Doped Carbon Obtained from Salt Melt Pyrolysis of Chitosan toward Supercapacitor and Oxygen Reduction Catalysts. Nanomaterials. 2022; 12(7):1162. https://doi.org/10.3390/nano12071162
Chicago/Turabian StyleRybarczyk, Maria Krystyna, Karolina Cysewska, Recep Yuksel, and Marek Lieder. 2022. "Microporous N-Doped Carbon Obtained from Salt Melt Pyrolysis of Chitosan toward Supercapacitor and Oxygen Reduction Catalysts" Nanomaterials 12, no. 7: 1162. https://doi.org/10.3390/nano12071162
APA StyleRybarczyk, M. K., Cysewska, K., Yuksel, R., & Lieder, M. (2022). Microporous N-Doped Carbon Obtained from Salt Melt Pyrolysis of Chitosan toward Supercapacitor and Oxygen Reduction Catalysts. Nanomaterials, 12(7), 1162. https://doi.org/10.3390/nano12071162






