Preparation and Characterization of Blank and Nerolidol-Loaded Chitosan–Alginate Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Blank Chitosan–Alginate Nanoparticles
2.2. Fabrication of Nerolidol-Loaded Chitosan–Alginate Nanoparticles
2.3. Characterization Techniques
2.3.1. Fourier Transform Infrared Spectrometry
2.3.2. Ultraviolet-Visible Spectroscopy
2.3.3. Scanning Electron Microscopy
2.3.4. Transmission Electron Microscopy
2.3.5. In Vitro Release for Oral Absorption
3. Results and Discussion
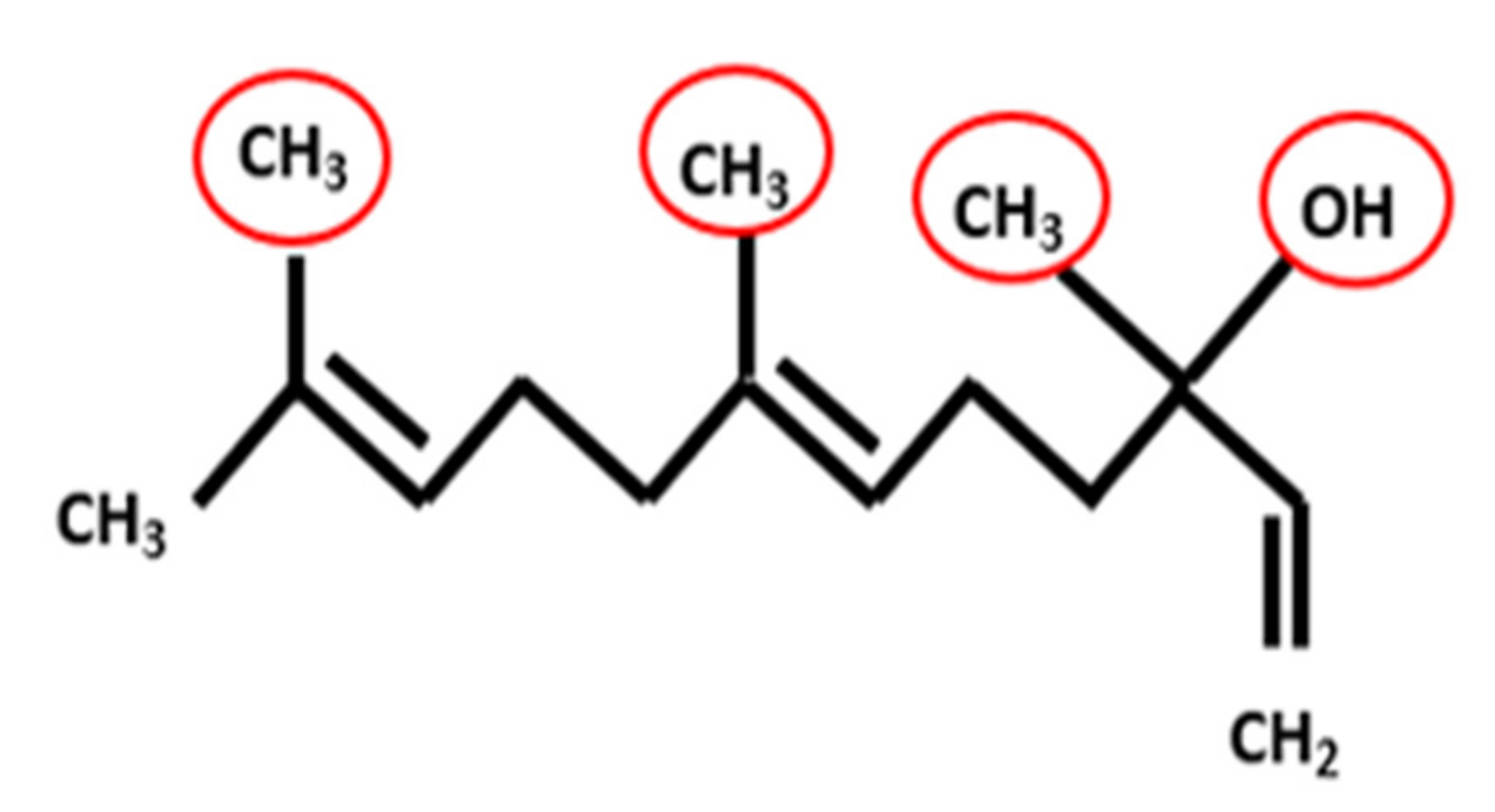
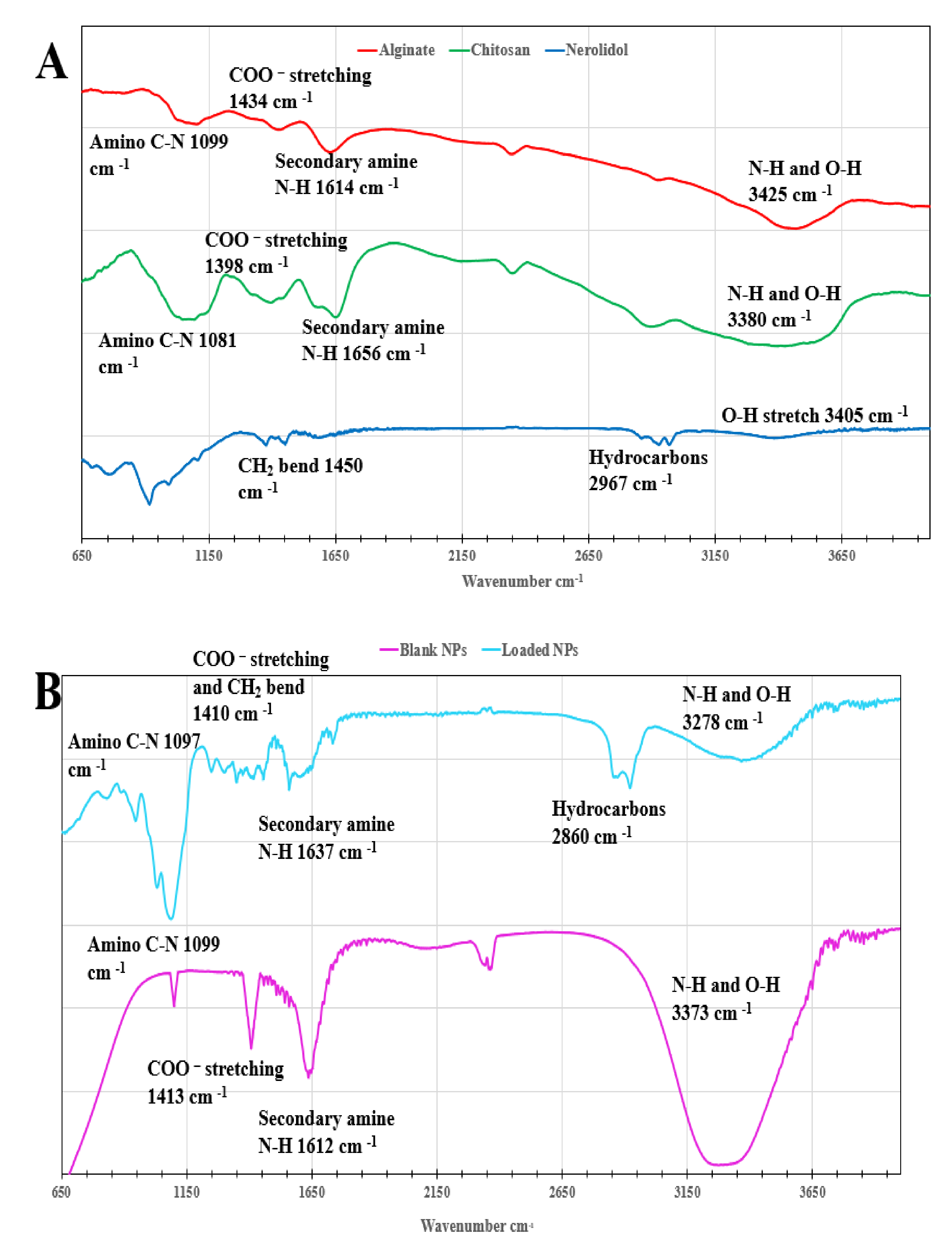
3.1. Fourier Transform Infrared Spectrometry
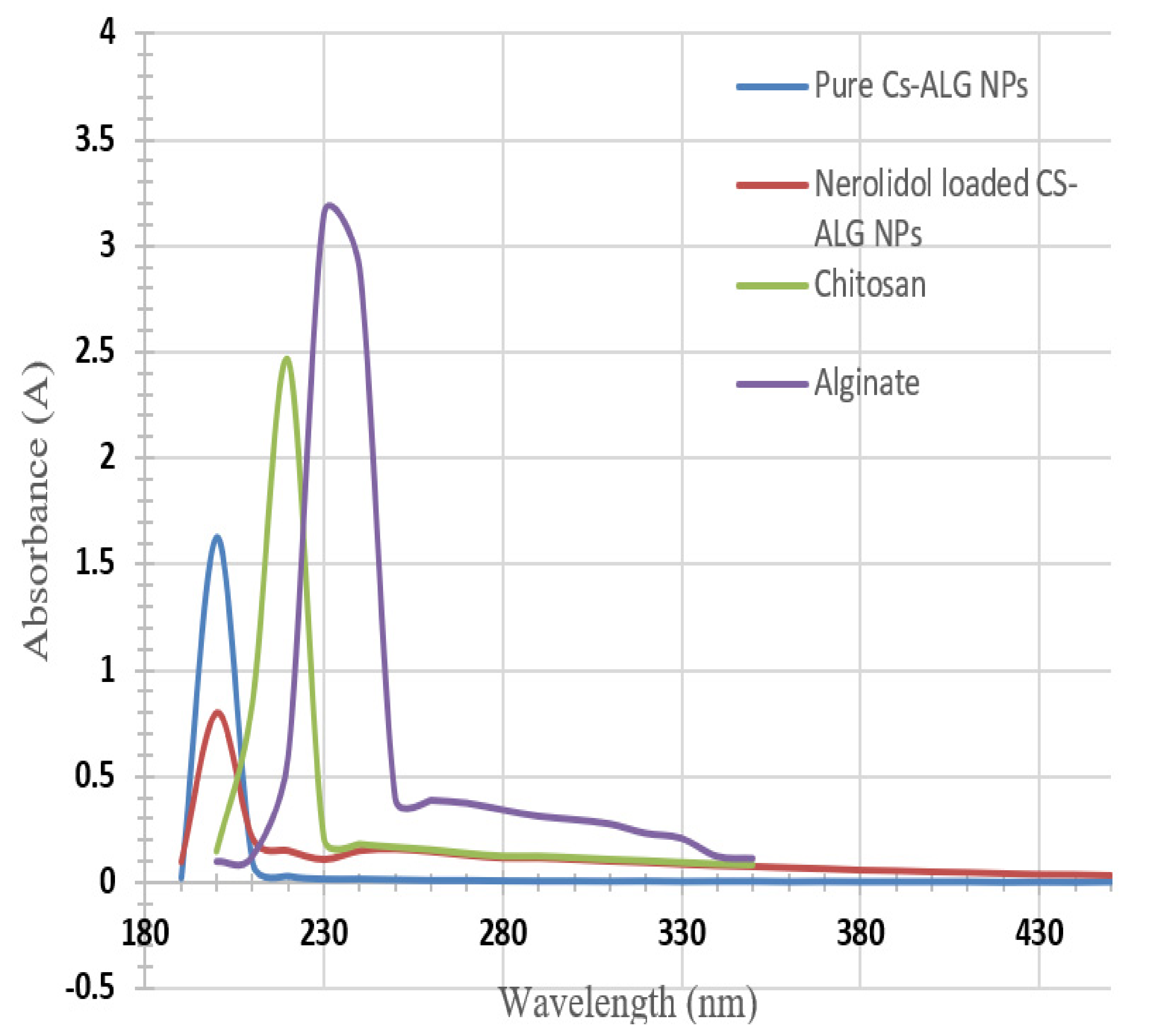
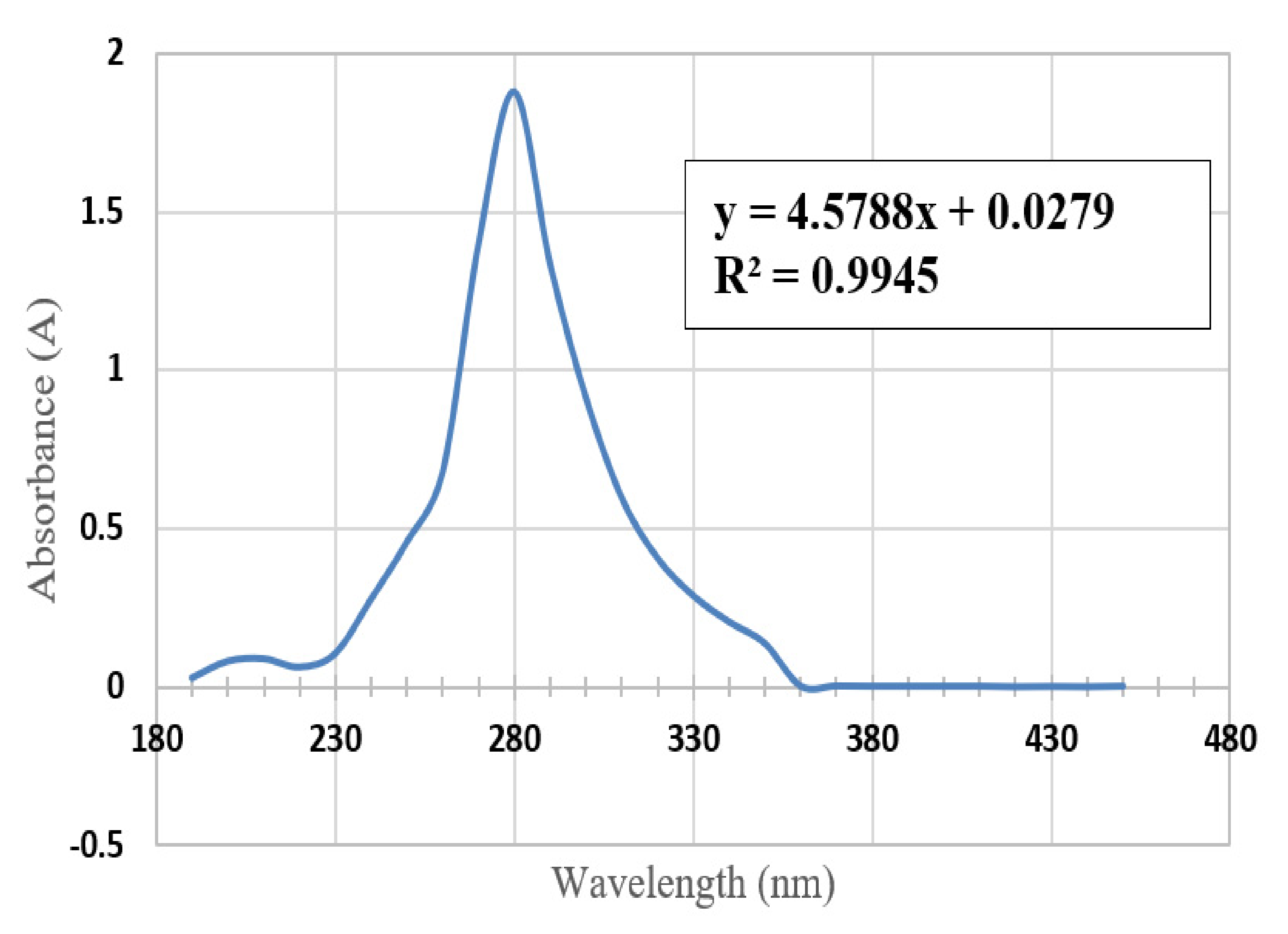
3.2. Ultraviolet-Visible Spectroscopy
3.3. Scanning Electron Microscope
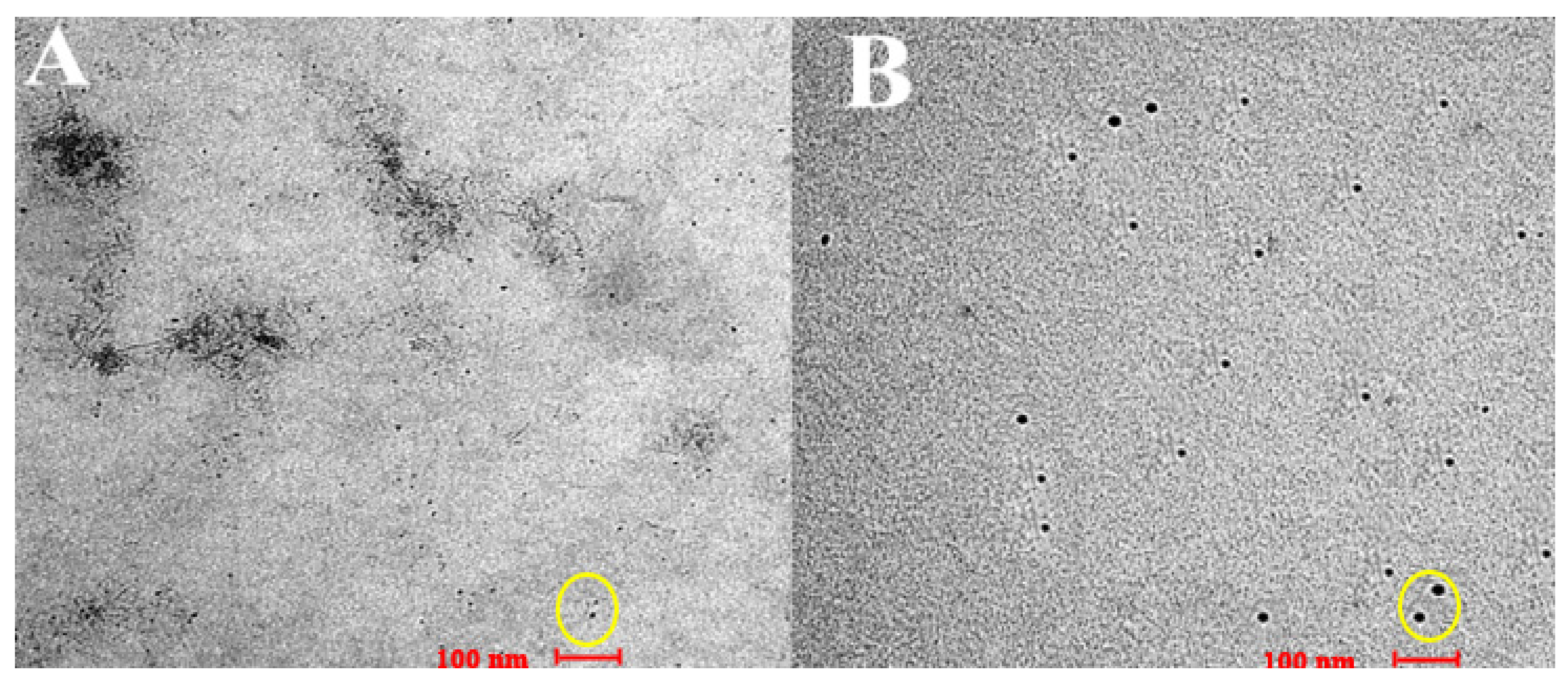
3.4. Transmission Electron Microscopy
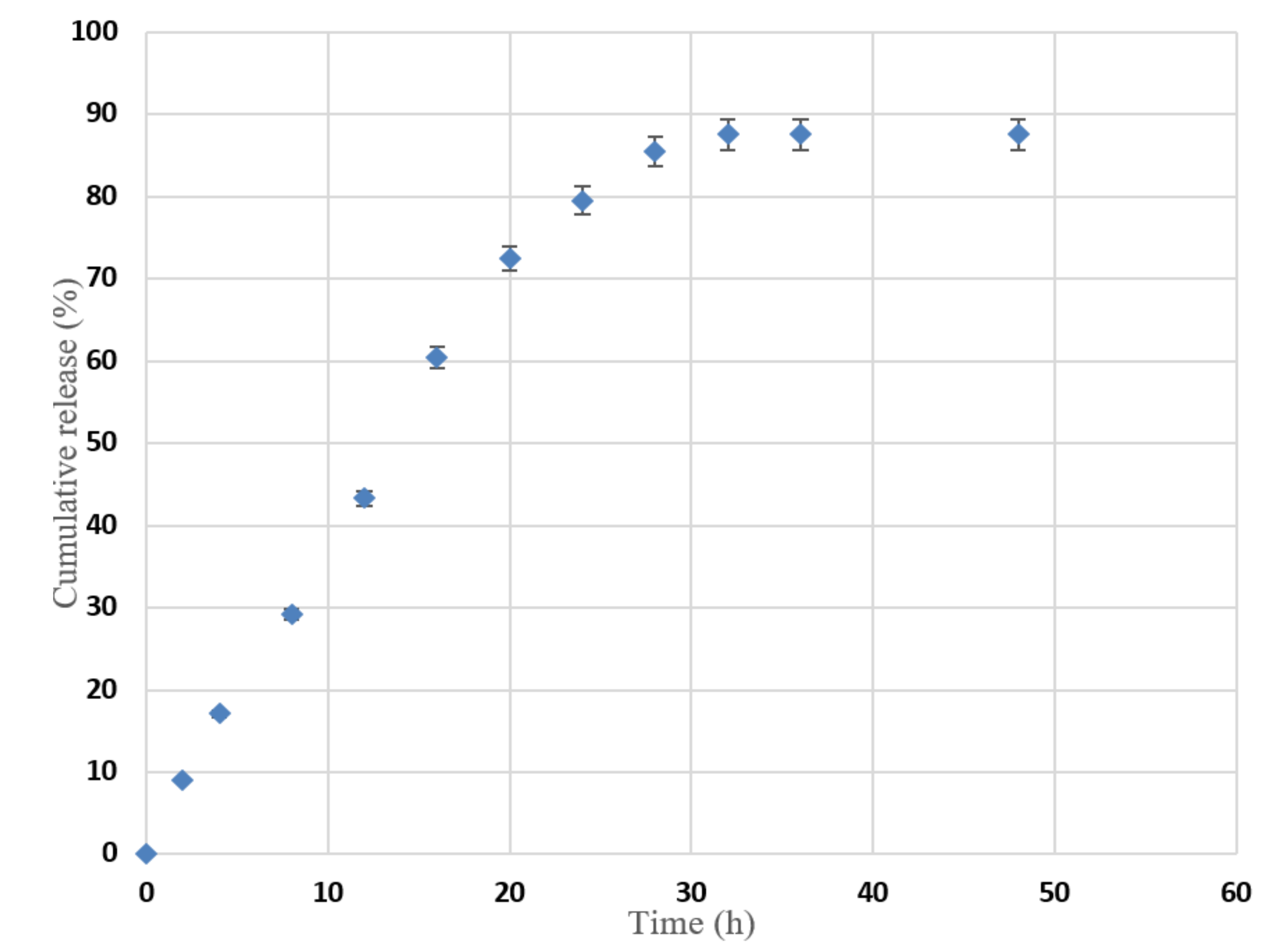
3.5. In Vitro Release for Oral Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klopell, F.C.; Lemos, M.; Sousa, J.P.B.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; De Andrade, S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z. Nat.-Sect. C J. Biosci. 2007, 62, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.M.; Palmeira, C.M.; Oliveira, M.M.; Santos, D.; Simões, A.M.; Rocha, S.M.; Coimbra, M.A.; Peixoto, F. Nerolidol effects on mitochondrial and cellular energetics. Toxicol. Vitr. 2012, 26, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Parreira, N.A.; Magalhães, L.G.; Morais, D.R.; Caixeta, S.C.; De Sousa, J.P.B.; Bastos, J.K.; Cunha, W.R.; Silva, M.L.; Nanayakkara, N.P.; Rodrigues, V.; et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of baccharis dracunculifolia. Chem. Biodivers. 2010, 7, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Asaikumar, L.; Vennila, L.; Akila, P.; Sivasangari, S.; Kanimozhi, K.; Premalatha, V.; Sindhu, G. Preventive effect of nerolidol on isoproterenol induced myocardial damage in Wistar rats: Evidences from biochemical and histopathological studies. Drug Dev. Res. 2019, 80, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, D.; Bao, Y.; Shi, Y.; Cui, Y.; Guo, M. Nerolidol Protects Against LPS-induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Signaling. Phyther. Res. 2017, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, N.B.; Yüce, H.; Çetin, A.; Şahin, Y.; Ciftci, O. The Ameliorate Effects of Nerolidol on Thioasteamide-Induced Oxidative Damage in Heart and Kidney Tissue. Turk. J. Pharm. Sci. 2022, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Boris, R.; Elena, T.; Erich, S.; Walter, J.; Gerhard, B.; Leopold, J. Cytotoxic properties of selected sesquiterpene alcohols on human cervix carcinoma cell lines. J. Essent. Oil-Bearing Plants 2011, 14, 316–319. [Google Scholar] [CrossRef]
- Ryabchenkoa, B.; Elena Tulupovab, E.; Erich Schmidtc, E.; Wlcekd, K.; Buchbauerd, G.; Jirovetz, L. Investigation of Anticancer and Antiviral Properties of Selected Aroma Samples. Nat. Prod. Commun. 2008, 3, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Prasanthi, D.; Lakshmi, P.K. Terpenes: Effect of lipophilicity in enhancing transdermal delivery of alfuzosin hydrochloride. J. Adv. Pharm. Technol. Res. 2012, 3, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, P.A.; Barry, B.W. Sesquiterpene Components of Volatile Oils as Skin Penetration Enhancers for the Hydrophilic Permeant 5-Fluorouracil. J. Pharm. Pharmacol. 1994, 46, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.G.; Silva, A.S.B.; Souza, G.E.P.; Figueiredo, J.G.; Cunha, F.Q.; Lahlou, S.; Da Silva, J.K.; Maia, J.G.; Sousa, P.J. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J. Ethnopharmacol. 2011, 138, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, D.K.S.; Ballico, L.J.; Rocha Lapa, F.; Gonçalves, H.P.; De Souza, L.M.; Iacomini, M.; de Paula Werner, M.F.; Baggio, C.H.; Pereira, I.T.; da Silva, L.M.; et al. Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C.DC in rodents. J. Ethnopharmacol. 2012, 142, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Hoet, S.; Stévigny, C.; Hérent, M.F.; Quetin-Leclercq, J. Antitrypanosomal compounds from the leaf essential oil of Strychnos spinosa. Planta Med. 2006, 72, 480–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Han, J.I.; Lee, G.S.; Park, M.J.; Choi, I.G.; Na, K.J.; Jeung, E.B. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull. 2007, 30, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curvelo, J.A.R.; Marques, A.M.; Barreto, A.L.S.; Romanos, M.T.V.; Portela, M.B.; Kaplan, M.A.C.; Soares, R.M. A novel nerolidol-rich essential oil from Piper claussenianum modulates Candida albicans biofilm. J. Med. Microbiol. 2014, 63, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Goulart, H.R.; Kimura, E.A.; Peres, V.J.; Couto, A.S.; Duarte, F.A.A.; Katzin, A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 2502–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, N.P.; Kato, M.J.; Andrade, E.H.D.A.; Maia, J.G.S.; Yoshida, M.; Planchart, A.R.; Katzin, A.M. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiapi Amazon Indians. J. Ethnopharmacol. 1999, 67, 313–319. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Dai, Y.N.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Makamh, M. Nanotechnology Based Approach to Enhance the Potential of Chemopreventive Agent Berberine Hydrochloride in Cancer Therapy. Int. J. Biol. Pharm. Allied Sci. 2017, 6, 953–975. Available online: https://ijbpas.com/pdf/2017/May/1493557391MSIJBPAS20174159.pdf (accessed on 27 September 2021).
- Sachan, N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Shu, X.Z.; Zhu, K.J.; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug-controlled release. Int. J. Pharm. 2001, 212, 19–28. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mater. Sci. Eng. C 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Setty, C.M.; Sahoo, S.S.; Sa, B. Alginate-coated alginate-polyethyleneimine beads for prolonged release of furosemide in simulated intestinal fluid. Drug Dev. Ind. Pharm. 2005, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Katuwavila, N.P.; Perera, A.D.L.C.; Samarakoon, S.R.; Soysa, P.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D. Chitosan-Alginate Nanoparticle System Efficiently Delivers Doxorubicin to MCF-7 Cells. J. Nanomater. 2016, 2016, 3178904. [Google Scholar] [CrossRef] [Green Version]
- Thwala, L.N. Preparation and Characterization of Alginate-Chitosan Nanoparticles as a Drug Delivery System for Lipophilic Compounds. 2012. Available online: https://ujcontent.uj.ac.za/vital/access/services/Download/uj:2752/CONTENT1 (accessed on 2 October 2021).
- Parupudi, A.; Mulagapati, A.H.R.; Subramony, J.A. Nanoparticle technologies: Recent state of the art and emerging opportunities. In Nanoparticle Therapeutics: Production Technologies, Types of Nanoparticles, and Regulatory Aspects; Kesharwani, P., Singh, K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 3–46. [Google Scholar]
- Mehn, D.; Caputo, F.; Rösslein, M.; Calzolai, L.; Saint-Antonin, F.; Courant, T.; Wick, P.; Gilliland, D. Larger or more? Nanoparticle characterisation methods for recognition of dimers. RSC Adv. 2017, 7, 27747–27754. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.Y.; Sussmann, R.A.C.; Kimura, E.A.; Cassera, M.B.; Katzin, A.M. Quantification of nerolidol in mouse plasma using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 100–103. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.M.; Greish, Y.E.; El-Maghraby, H.F.; Lubbad, L.; Makableh, Y.; Hammad, F.T. Preparation and Characterization of Blank and Nerolidol-Loaded Chitosan–Alginate Nanoparticles. Nanomaterials 2022, 12, 1183. https://doi.org/10.3390/nano12071183
Ahmad RM, Greish YE, El-Maghraby HF, Lubbad L, Makableh Y, Hammad FT. Preparation and Characterization of Blank and Nerolidol-Loaded Chitosan–Alginate Nanoparticles. Nanomaterials. 2022; 12(7):1183. https://doi.org/10.3390/nano12071183
Chicago/Turabian StyleAhmad, Rahaf M., Yaser E. Greish, Hesham F. El-Maghraby, Loay Lubbad, Yahia Makableh, and Fayez T. Hammad. 2022. "Preparation and Characterization of Blank and Nerolidol-Loaded Chitosan–Alginate Nanoparticles" Nanomaterials 12, no. 7: 1183. https://doi.org/10.3390/nano12071183
APA StyleAhmad, R. M., Greish, Y. E., El-Maghraby, H. F., Lubbad, L., Makableh, Y., & Hammad, F. T. (2022). Preparation and Characterization of Blank and Nerolidol-Loaded Chitosan–Alginate Nanoparticles. Nanomaterials, 12(7), 1183. https://doi.org/10.3390/nano12071183






