Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HNT-Grapefruit Seed Oil Nano-Hybrids
2.3. Preparation of Nano-Hybrid/Pectin Composites
2.4. Methods
3. Results
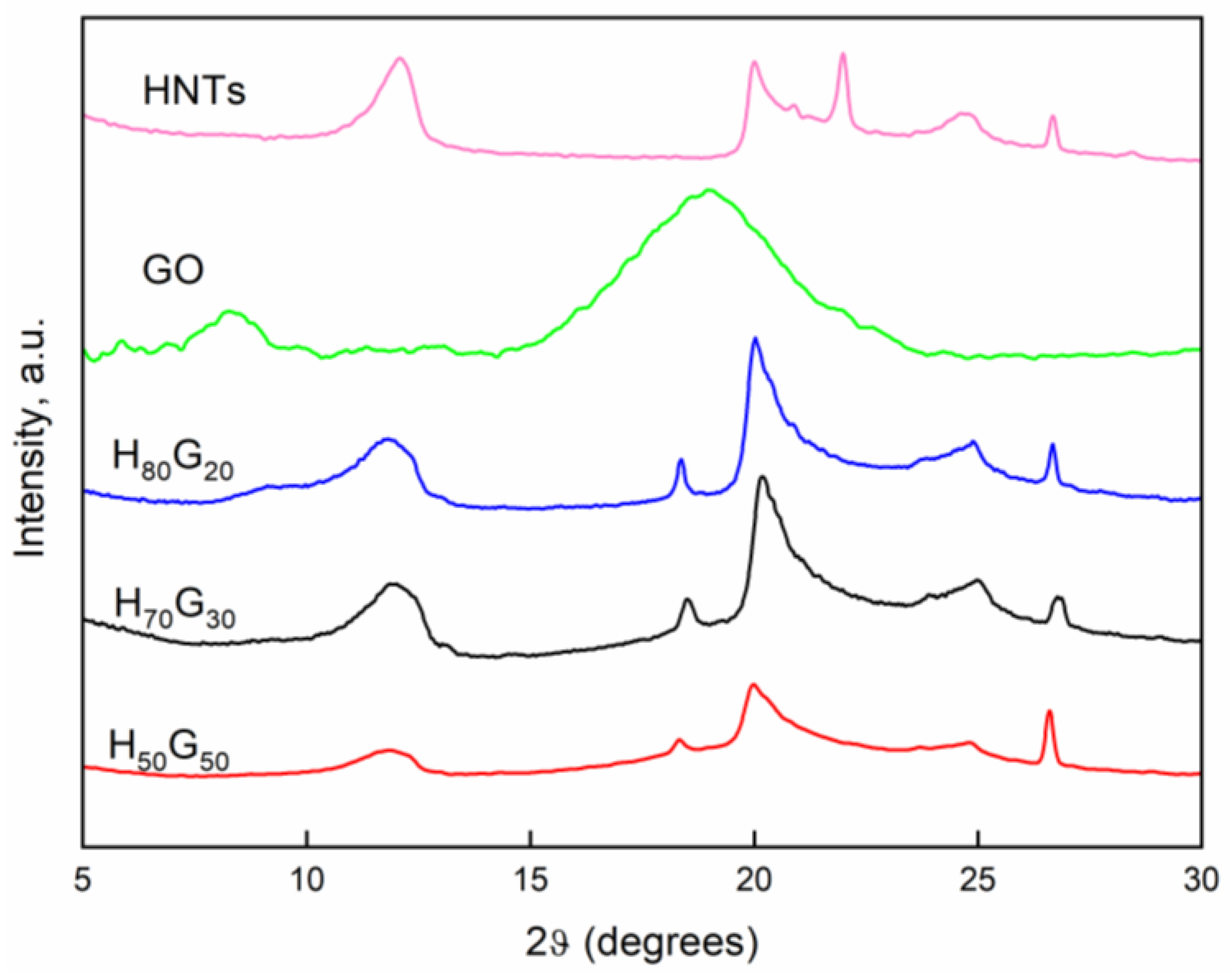
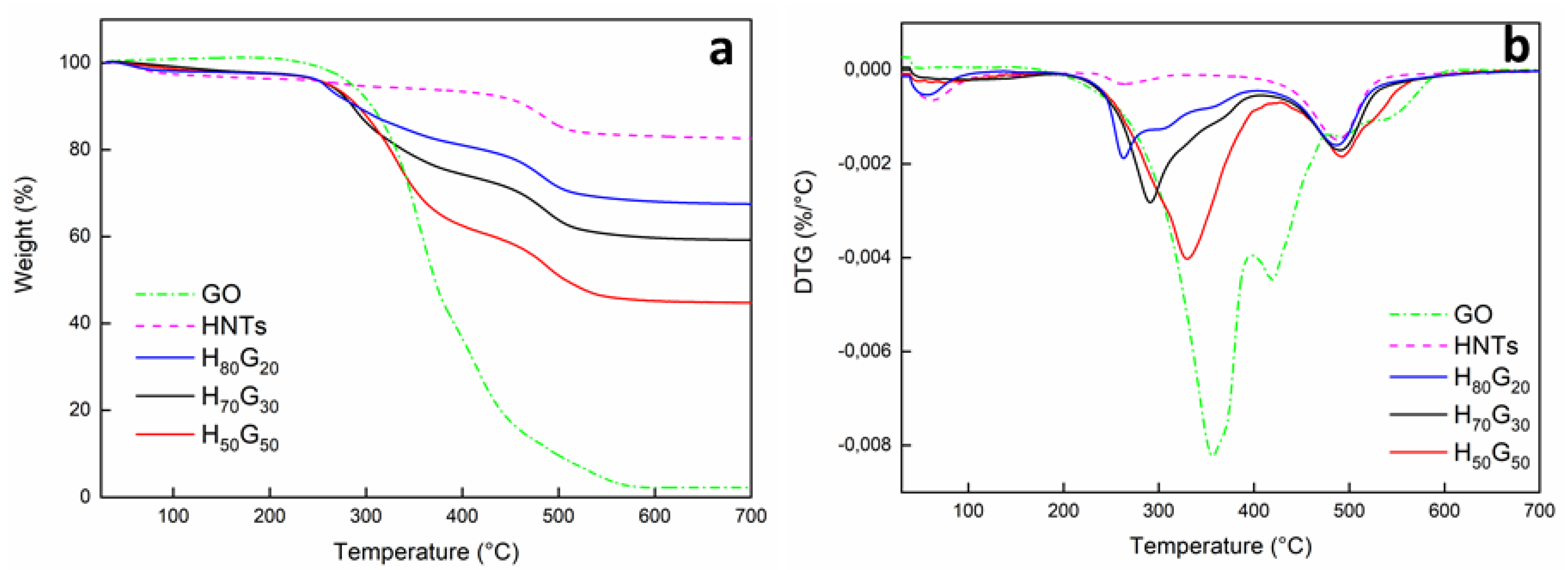
3.1. Characterization of HNT-Grapefruit Seed Oil Nano-Hybrids
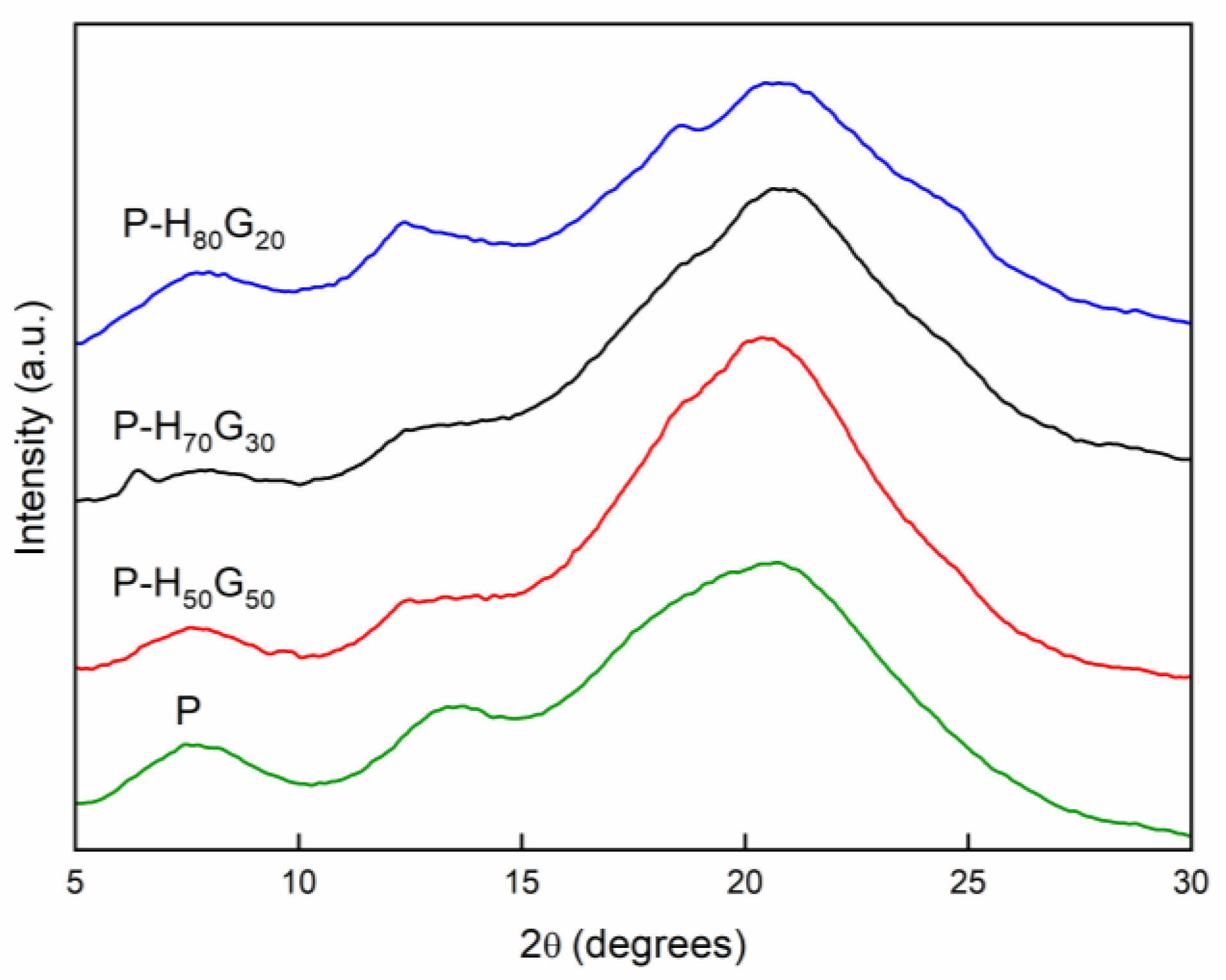
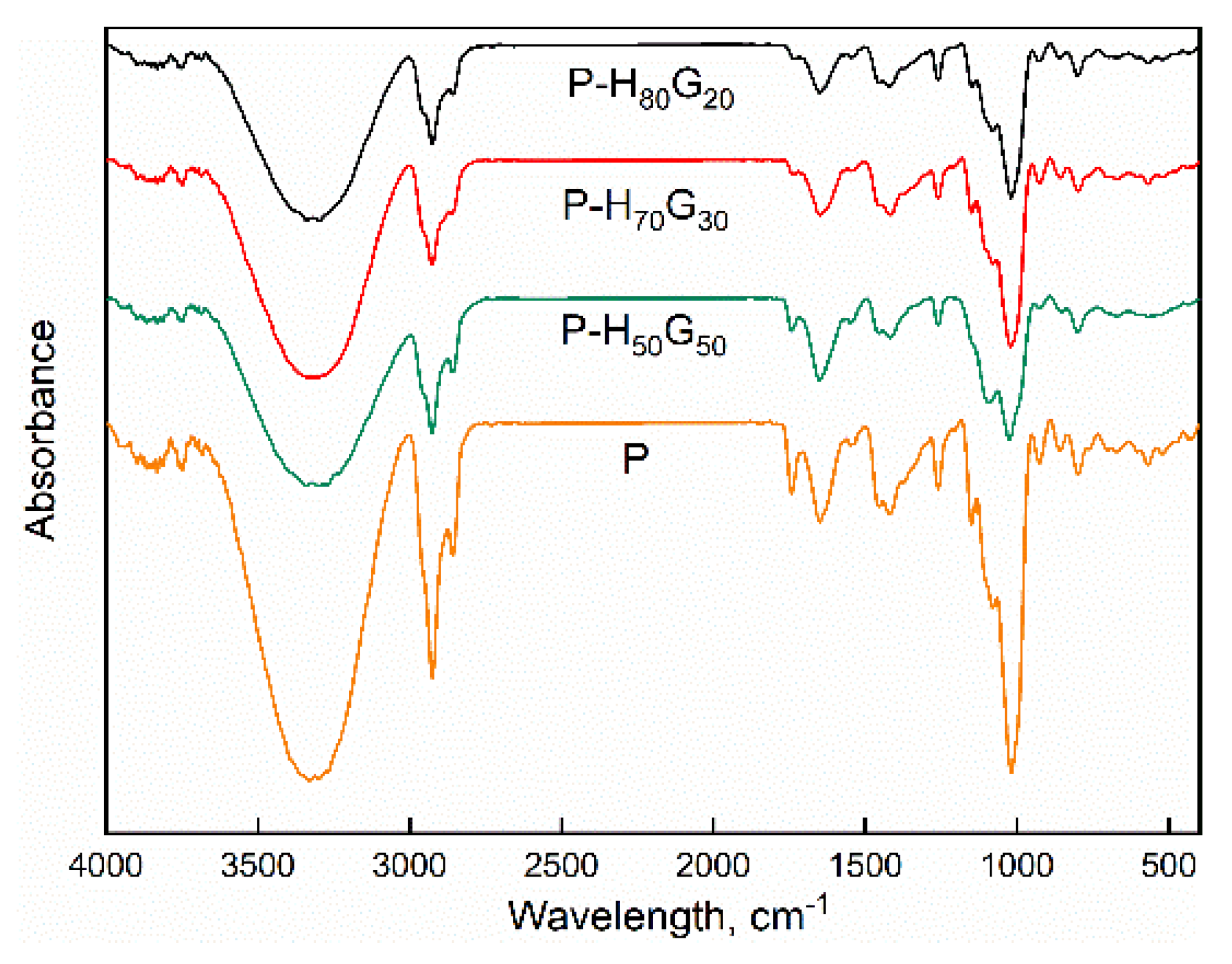
3.2. Characterization of Nano-Hybrid/Pectin Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Yu, Z.f.; Song, S.; Xu, X.l.; Ma, Q.; Lu, Y. Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ. Exp. Bot. 2021, 192, 104635. [Google Scholar] [CrossRef]
- Barani, M.; Zeeshan, M.; Kalantar-Neyestanaki, D.; Farooq, M.A.; Rahdar, A.; Jha, N.K.; Sargazi, S.; Gupta, P.K.; Thakur, V.K. Nanomaterials in the Management of Gram-Negative Bacterial Infections. Nanomaterials 2021, 11, 2535. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; Avena-Bustillos, R.d.J.; Soares, N.d.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Rhim, J.W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Farris, S.; Unalan, I.U.; Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A. Pullulan-based films and coatings for food packaging: Present applications, emerging opportunities, and future challenges. J. Appl. Polym. Sci. 2014, 131, 40539. [Google Scholar] [CrossRef] [Green Version]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The improvement of characteristics of biodegradable films made from kefiran–whey protein by nanoparticle incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 213–255. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. Physical Performance of Biodegradable Films Intended for Antimicrobial Food Packaging. J. Food Sci. 2010, 75, E502–E507. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Gorrasi, G.; Bugatti, V.; Vittoria, V. Pectins filled with LDH-antimicrobial molecules: Preparation, characterization and physical properties. Carbohydr. Polym. 2012, 89, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Bugatti, V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: Preparation and analysis of physical properties. LWT—Food Sci. Technol. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- De Cindio, B.; Gabriele, D.; Lupi, F.R. Pectin: Properties Determination and Uses. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 294–300. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Pacheco, M.M.; Ortega-Ramirez, L.A.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Combinational Approaches for Antimicrobial Packaging: Pectin and Cinnamon Leaf Oil. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 609–617. [Google Scholar] [CrossRef]
- Froiio, F.; Mosaddik, A.; Morshed, M.T.; Paolino, D.; Fessi, H.; Elaissari, A. Edible Polymers for Essential Oils Encapsulation: Application in Food Preservation. Ind. Eng. Chem. Res. 2019, 58, 20932–20945. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L. Anxiolytic Effect of Essential Oils and Their Constituents: A Review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Xu, W.T.; Huang, K.L.; Guo, F.; Qu, W.; Yang, J.J.; Liang, Z.H.; Luo, Y.B. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Technol. 2007, 46, 86–94. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bergaya, F.; Lagaly, G. Handbook of Clay Science; Newnes: Oxford, UK, 2013. [Google Scholar]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Dispersions of nanoclays of different shapes into aqueous and solid biopolymeric matrices. extended physicochemical study. Langmuir 2011, 27, 1158–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mateos, M.; Montero, P.; Gómez-Guillén, M.C. Formulation and stability of biodegradable films made from cod gelatin and sunflower oil blends. Food Hydrocoll. 2009, 23, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Viscusi, G.; Adami, R.; Gorrasi, G. Fabrication of rice flour films reinforced with hemp hurd and loaded with grapefruit seed oil: A simple way to valorize agro-waste resources toward low cost materials with added value. Ind. Crops Prod. 2021, 170, 113785. [Google Scholar] [CrossRef]
- Gorrasi, G.; Longo, R.; Viscusi, G. Fabrication and characterization of electrospun membranes based on “poly(ε-caprolactone)”, “poly(3-hydroxybutyrate)” and their blend for tunable drug delivery of curcumin. Polymers 2020, 12, 2239. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Levis, S.R.; Deasy, P.B. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002, 243, 125–134. [Google Scholar] [CrossRef]
- Mellouk, S.; Belhakem, A.; Marouf-Khelifa, K.; Schott, J.; Khelifa, A. Cu(II) adsorption by halloysites intercalated with sodium acetate. J. Colloid Interface Sci. 2011, 360, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Phosphonium ionic liquid as interfacial agent of layered double hydroxide: Application to a pectin matrix. Carbohydr. Polym. 2018, 182, 142–148. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Viscusi, G.; Vittoria, V. Physical and barrier properties of chemically modified pectin with polycaprolactone through an environmentally friendly process. Colloid Polym. Sci. 2021, 299, 429–437. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Improved method for determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. J. Agric. Food Chem. 2001, 49, 2756–2760. [Google Scholar] [CrossRef]
- Kowalonek, J. Studies of chitosan/pectin complexes exposed to UV radiation. Int. J. Biol. Macromol. 2017, 103, 515–524. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Compos. Part A Appl. Sci. Manuf. 2011, 42, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Mahboubifar, M.; Yousefinejad, S.; Alizadeh, M.; Hemmateenejad, B. Prediction of the acid value, peroxide value and the percentage of some fatty acids in edible oils during long heating time by chemometrics analysis of FTIR-ATR spectra. J. Iran. Chem. Soc. 2016, 13, 2291–2299. [Google Scholar] [CrossRef]
- Cai, H.; Bao, F.; Gao, J.; Chen, T.; Wang, S.; Ma, R. Preparation and characterization of novel carbon dioxide adsorbents based on polyethylenimine-modified Halloysite nanotubes. Environ. Technol. 2015, 36, 1273–1280. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.H.A.; Li, W.; Jones, C.W.; Giannelis, E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine -functionalized mesoporous capsules. Energy Environ. Sci. 2011, 4, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Lun, H.; Ouyang, J.; Yang, H. Enhancing dispersion of halloysite nanotubes via chemical modification. Phys. Chem. Miner. 2014, 41, 281–288. [Google Scholar] [CrossRef]
- Shim, H.S.; Hajaligol, M.R.; Baliga, V.L. Oxidation behavior of biomass chars: Pectin and Populus deltoides. Fuel 2004, 83, 1495–1503. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer-clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Mangiacapra, P.; Gorrasi, G.; Sorrentino, A.; Vittoria, V. Biodegradable nanocomposites obtained by ball milling of pectin and montmorillonites. Carbohydr. Polym. 2006, 64, 516–523. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Response surface modeling and analysis of barrier and optical properties of maize starch edible films. Int. J. Biol. Macromol. 2013, 60, 412–421. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; el Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Bartolomeu, B.G.; Pinheiro, A.C.; Carneiro-Da-Cunha, M.G.; Vicente, A.A. Development and characterization of a nanomultilayer coating of pectin and chitosan—Evaluation of its gas barrier properties and application on ‘Tommy Atkins’ mangoes. J. Food Eng. 2012, 110, 457–464. [Google Scholar] [CrossRef] [Green Version]
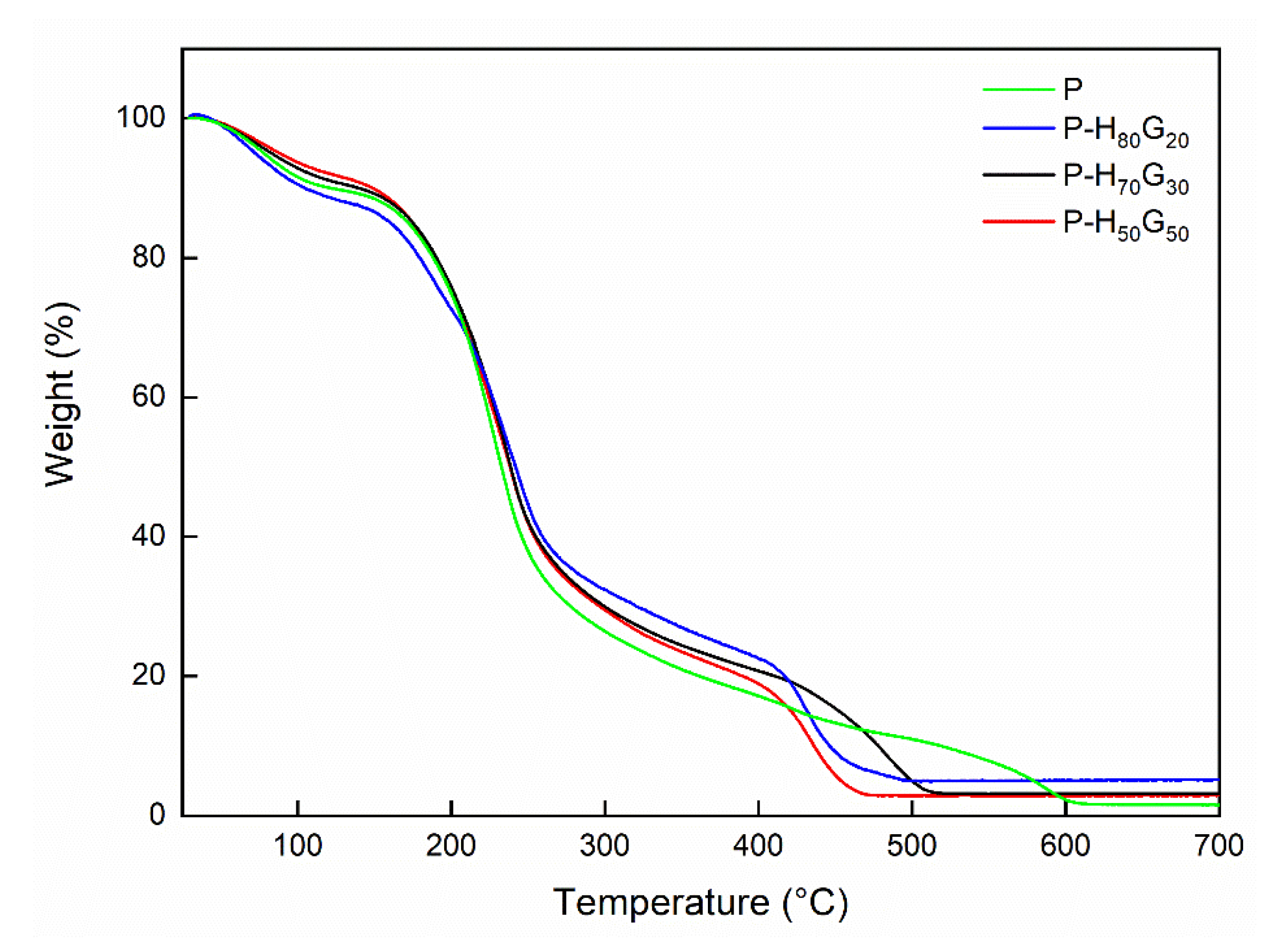

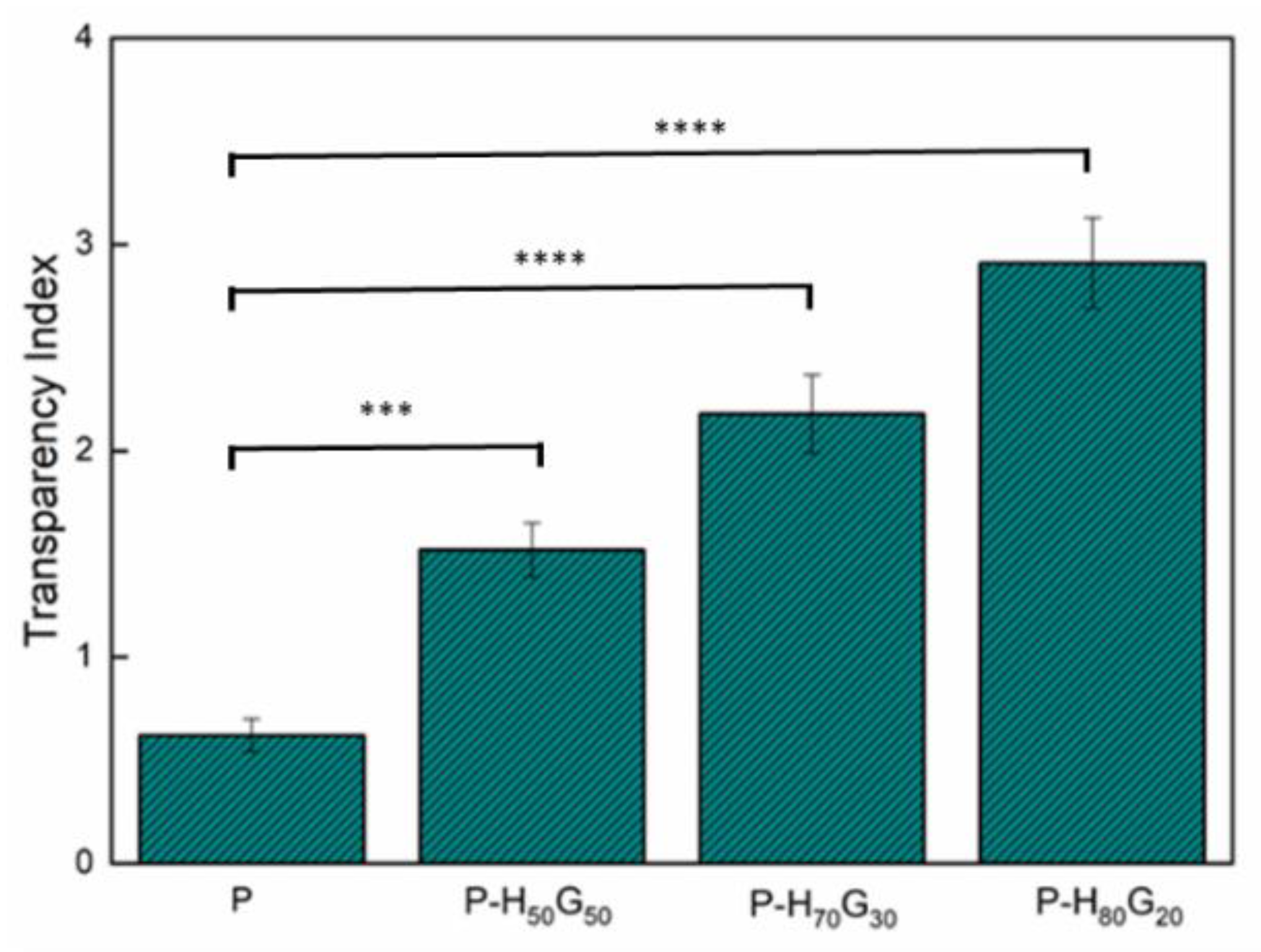
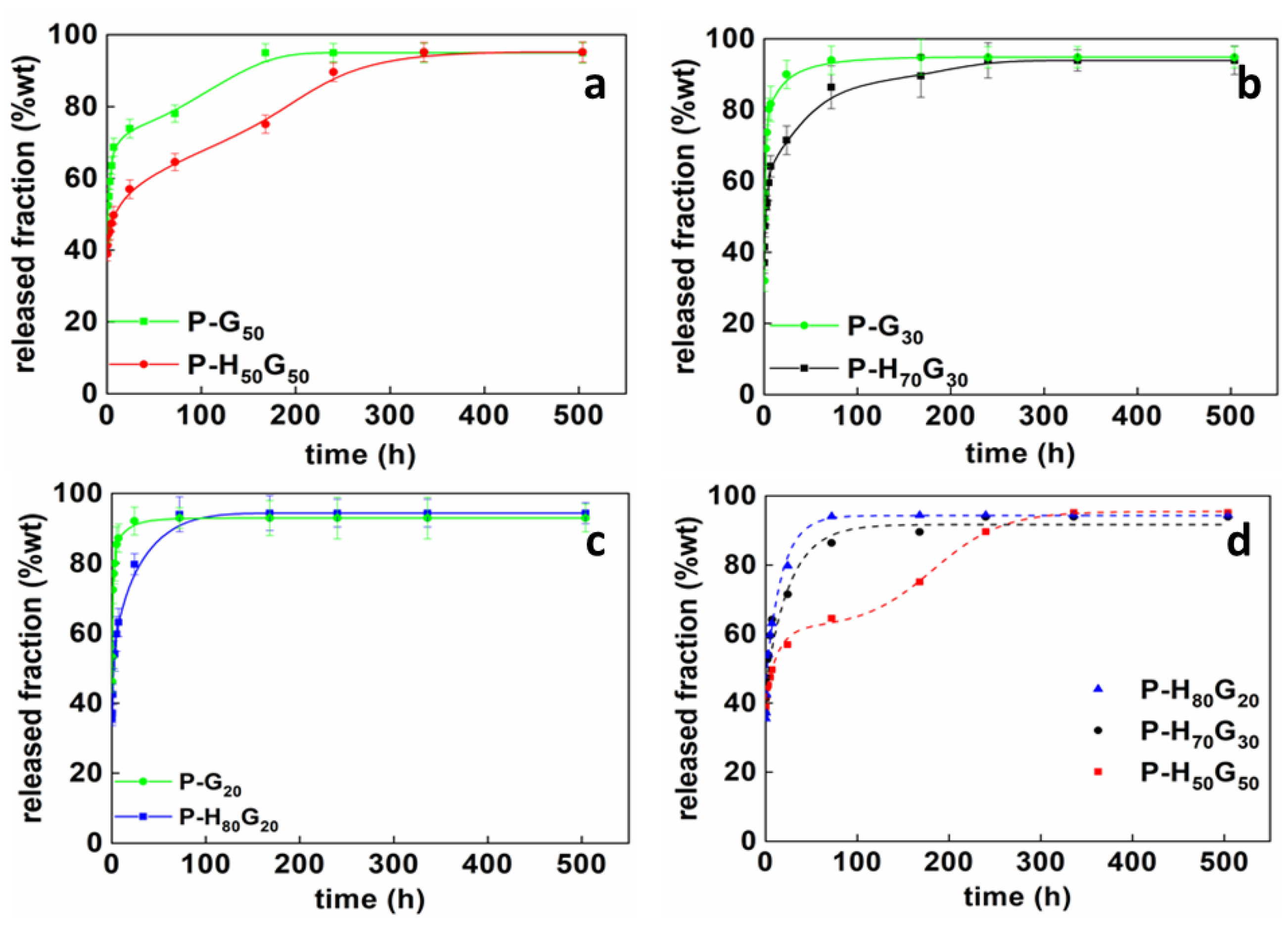
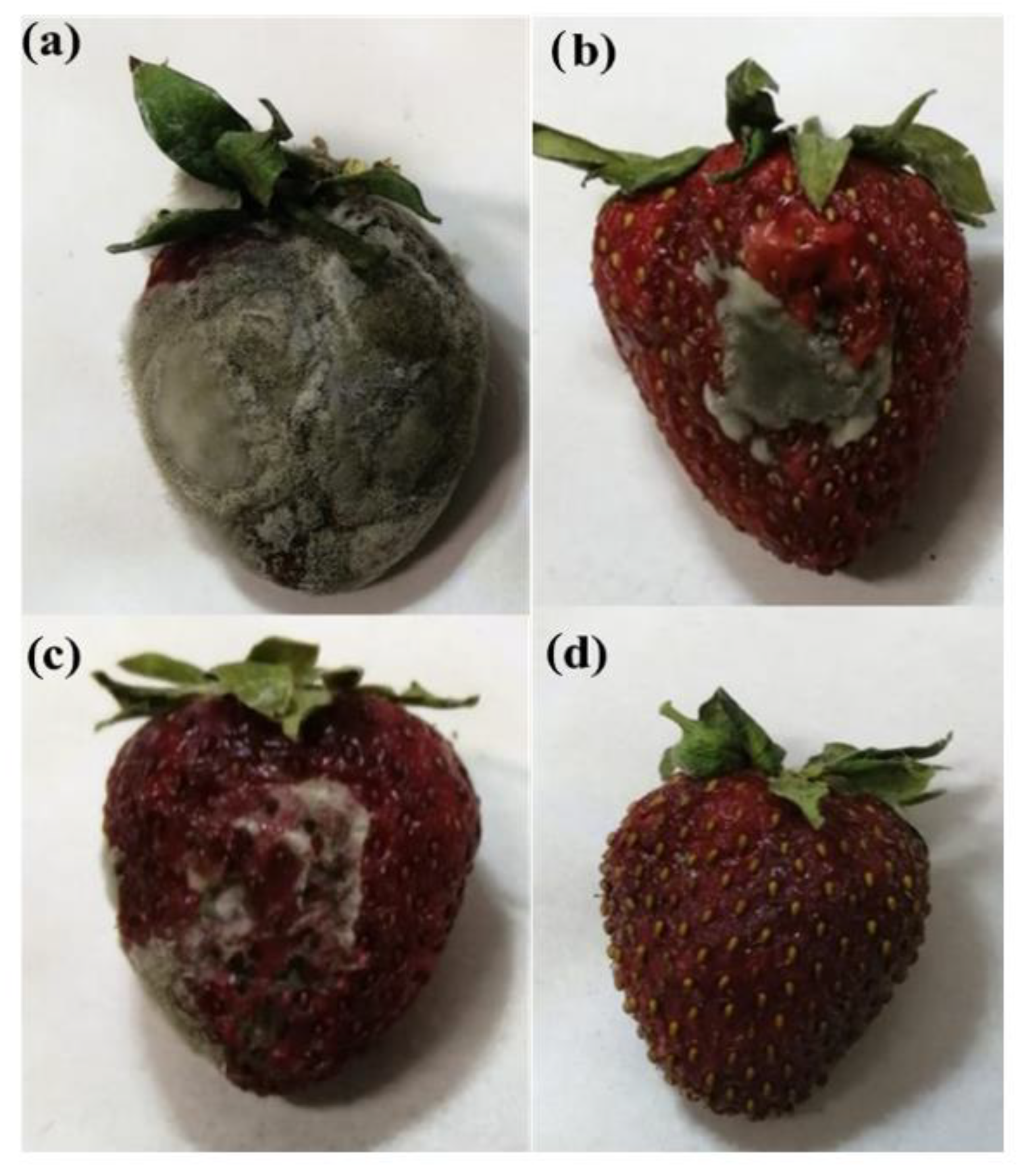
| Sample | P (wt%) | HxGy (wt%) | GO (wt%) |
|---|---|---|---|
| P-H80G20 | 95 | 5 | - |
| P-H70G30 | 95 | 5 | - |
| P-H50G50 P-G20 P-G30 P-G50 | 95 99 98.5 97.5 | 5 - - - | - 1 1.5 2.5 |
| Sample | (001) | (100) | ||
|---|---|---|---|---|
| 2θ | d (Å) | 2θ | d (Å) | |
| HNTs | 12.09 | 7.31 | 20.0 | 4.43 |
| H80G20 | 11.82 | 7.48 | 20.02 | 4.43 |
| H70G30 | 11.87 | 7.45 | 20.18 | 4.40 |
| H50G50 | 11.87 | 7.45 | 19.97 | 4.44 |
| Sample | HNTs Content (% w/w) | GO Content (% w/w) | Mass Loss (% w/w) | Td1(DTG) (°C) | Td2(DTG) (°C) |
|---|---|---|---|---|---|
| HNTs | 100 | 0 | 82.6 | - | 488 |
| GO | 0 | 100 | 2.2 | 356 | - |
| H80G20 | 80 | 20 | 67.5 | 262 | 487 |
| H70G30 | 70 | 30 | 59.2 | 291 | 491 |
| H50G50 | 50 | 50 | 44.8 | 330 | 492 |
| Parameter | P | P-H50G50 | P-H70G30 | P-H80G20 |
|---|---|---|---|---|
| E [MPa] | 32.53 ± 5.22 c | 47.87 ± 6.27 bc | 64.25 ± 6.95 b | 98.54 ± 6.03 a |
| σbreak [MPa] | 1.78 ± 0.15 c | 2.41 ± 0.19 b | 3.05 ± 0.21 a | 3.45 ± 0.26 a |
| εbreak [%] | 29.68 ± 3.12 b | 39.95 ± 3.78 a | 41.32 ± 4.05 a | 37.12 ± 3.89 ab |
| Parameter | P | P-H50G50 | P-H70G30 | P-H80G20 |
|---|---|---|---|---|
| S (g/g∗atm−1) | 4.40 ± 0.35 a | 3.61 ± 0.22 ab | 3.51 ± 0.17 abc | 3.46 ± 0.26 abc |
| D0 × 107 (cm2/s) | 0.25 ± 0.03 b | 1.59 ± 0.19 a | 1.81 ± 0.26 a | 1.89 ± 0.15 a |
| P × 107 (g/g∗atm−1∗cm2/s) | 1.12 ± 0.08 b | 5.74 ± 0.47 a | 6.36 ± 0.79 a | 6.54 ± 0.52 a |
| Sample | θ | A1 (hb1) | b1 | A2 (hb2) | b2 | tm (h) |
|---|---|---|---|---|---|---|
| P-H50G50 | 0.66 | 1.86 | 0.14 | 7.5 | 0.53 | 128.6 |
| P-H70G30 | 1 | 1.60 | 0.24 | - | - | - |
| P-H80G20 | 1 | 2.03 | 0.35 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscusi, G.; Lamberti, E.; D’Amico, F.; Tammaro, L.; Gorrasi, G. Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection. Nanomaterials 2022, 12, 1265. https://doi.org/10.3390/nano12081265
Viscusi G, Lamberti E, D’Amico F, Tammaro L, Gorrasi G. Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection. Nanomaterials. 2022; 12(8):1265. https://doi.org/10.3390/nano12081265
Chicago/Turabian StyleViscusi, Gianluca, Elena Lamberti, Francesca D’Amico, Loredana Tammaro, and Giuliana Gorrasi. 2022. "Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection" Nanomaterials 12, no. 8: 1265. https://doi.org/10.3390/nano12081265






