One-Step Hydrothermal Synthesis of Nanostructured MgBi2O6/TiO2 Composites for Enhanced Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MBO Nanospheres and MBO/TiO2 Photocatalysts
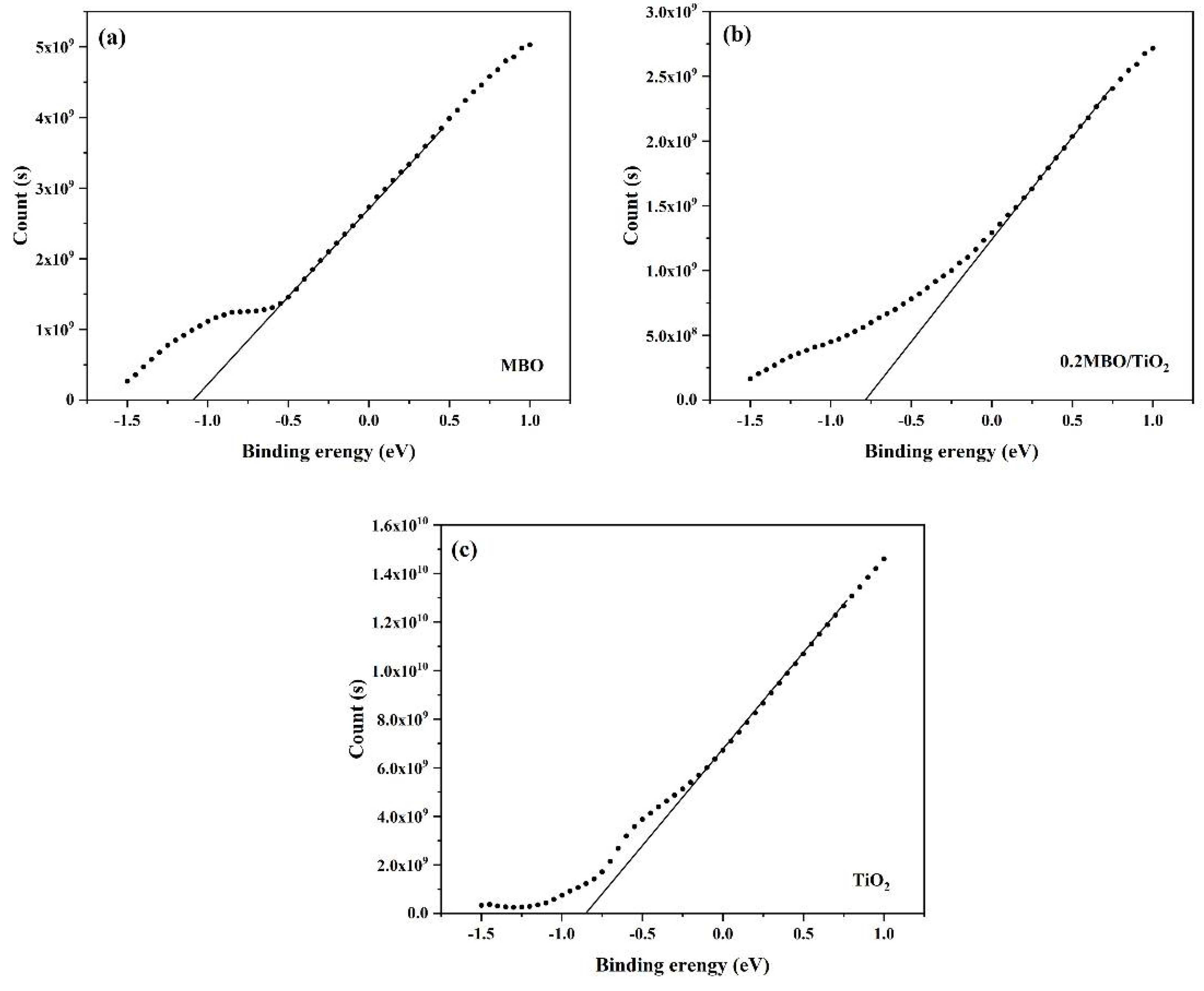
2.2. Characterization of Samples
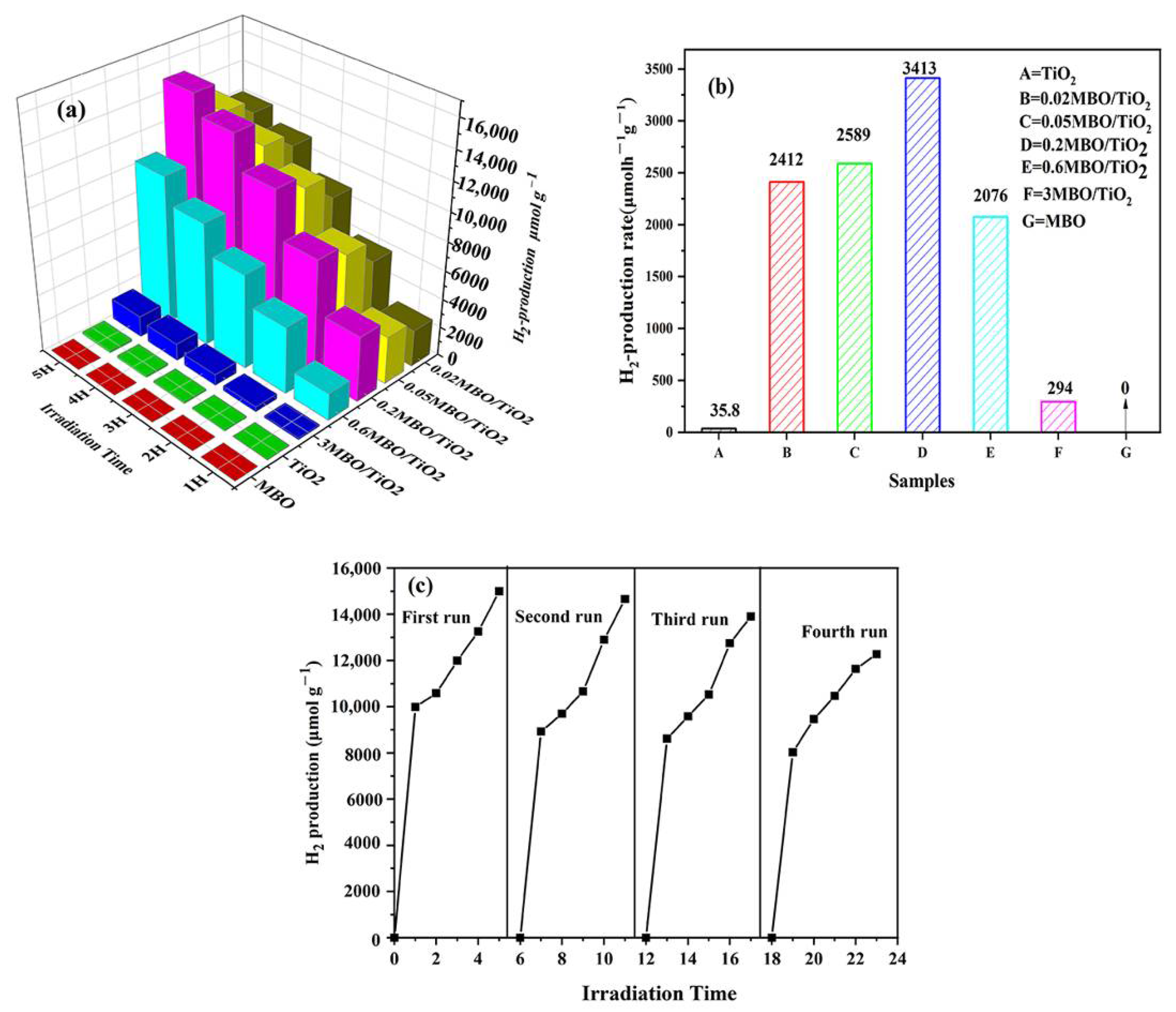
2.3. Photocatalytic Hydrogen Production Test
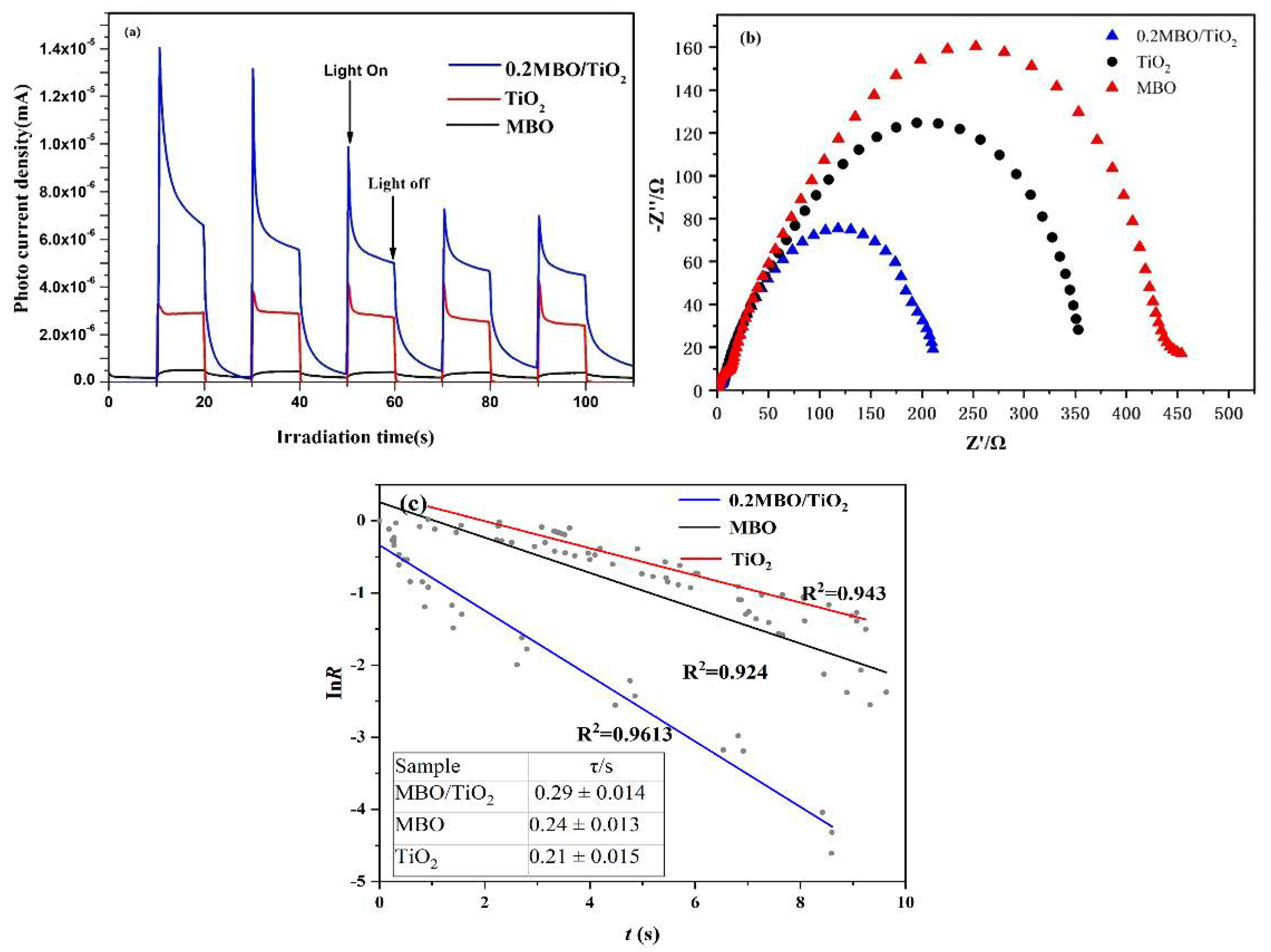
2.4. Photoelectrochemical Studies
3. Results and Discussion
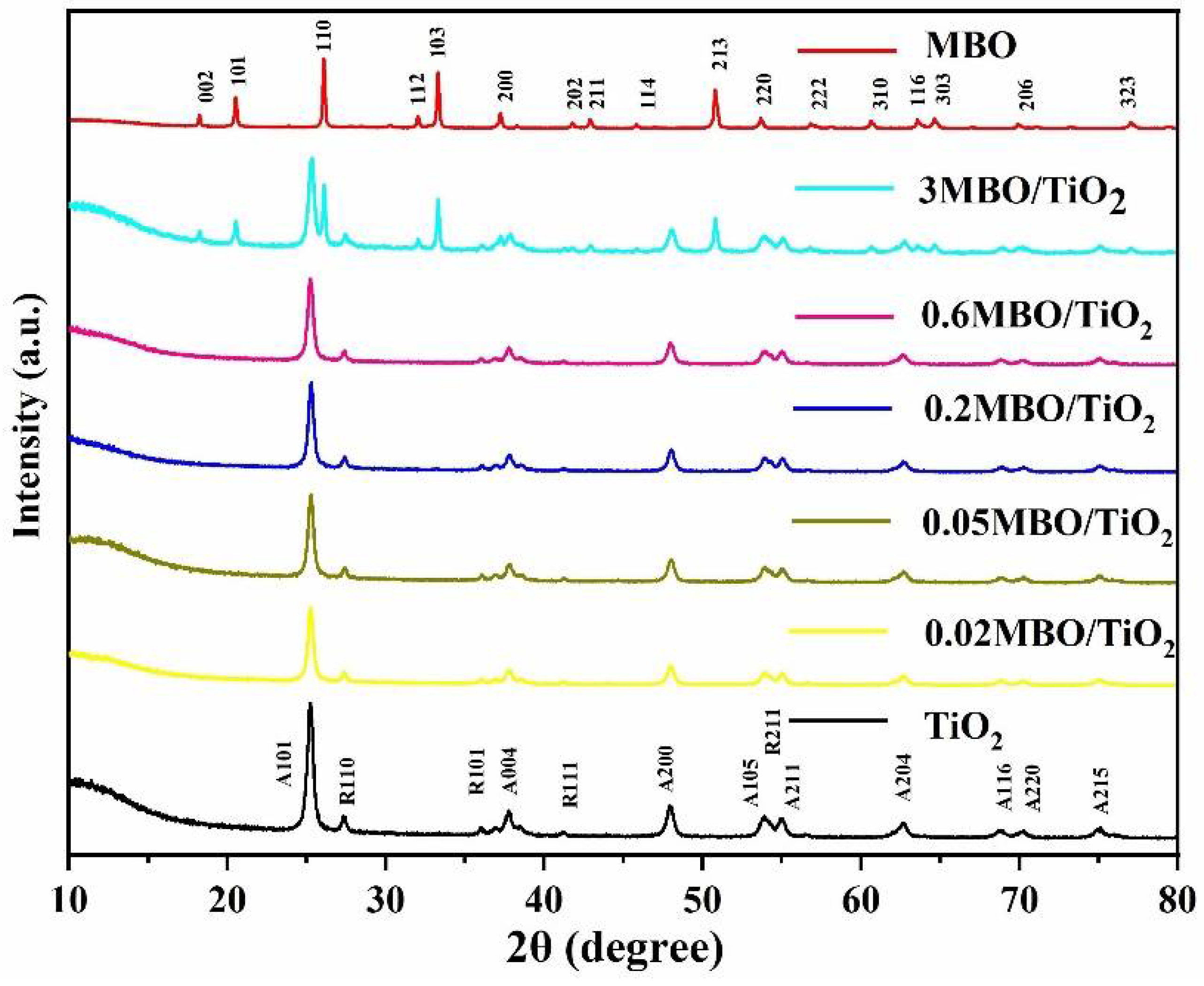
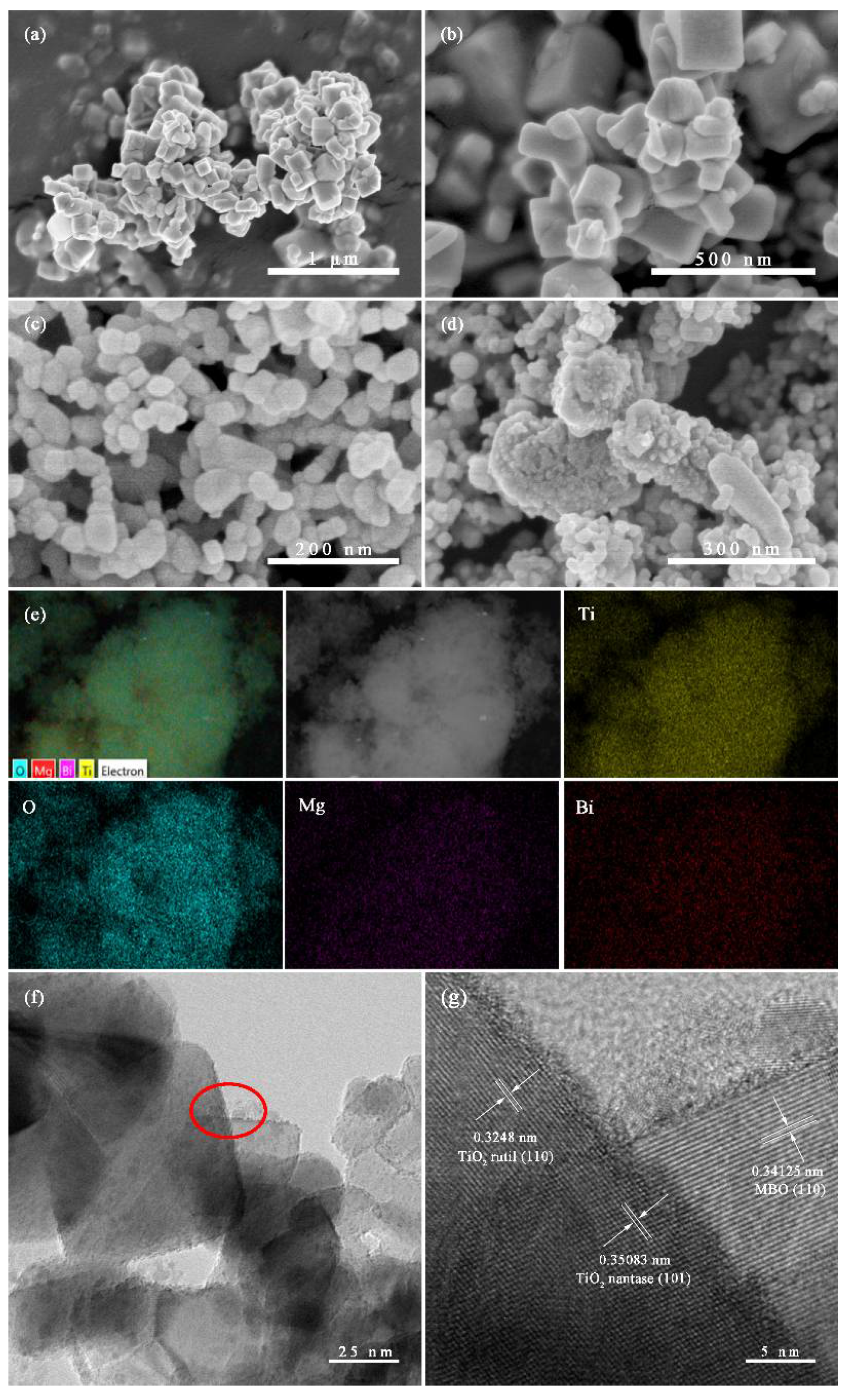
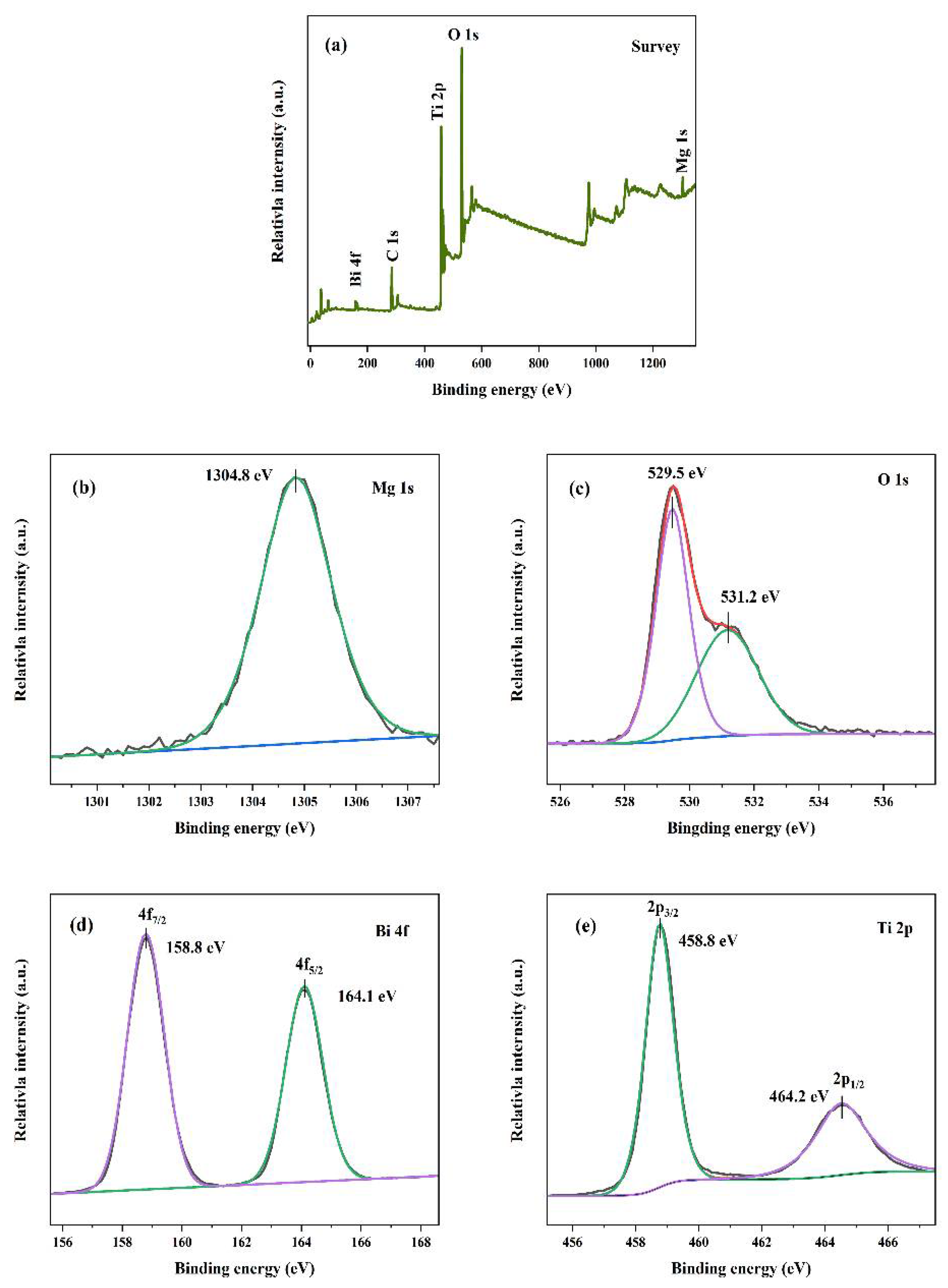
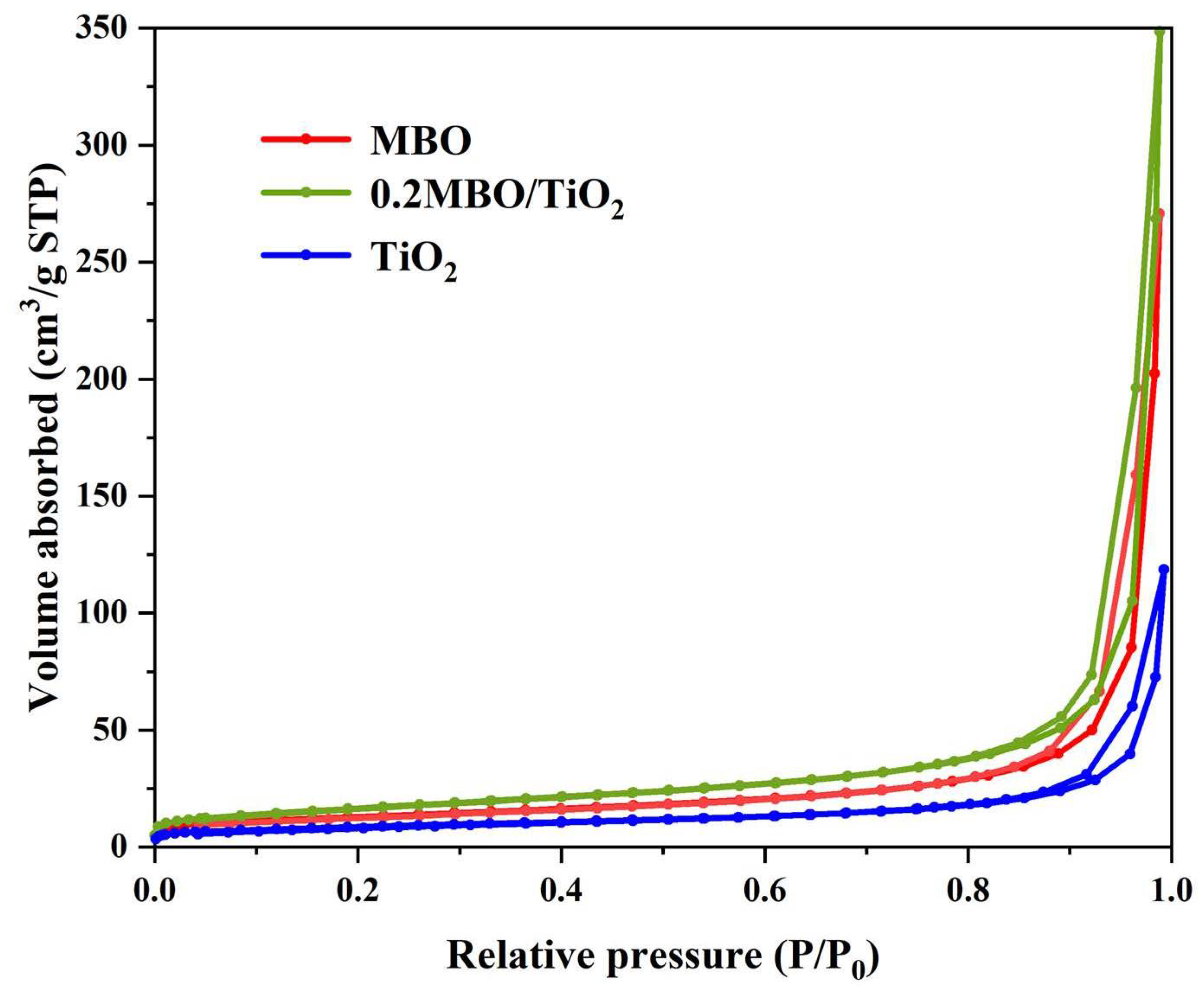
3.1. Phase Structure and Morphological Analysis
3.2. Light Absorption Spectra of MBO/TiO2 Catalysts
3.3. Hydrogen Production Performance
3.4. Possible Mechanism of Hydrogen Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Davies, R.H.; Worsley, D. Water-purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Ma, W.H.; Zhao, J.C. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Meng, X.C.; Li, Z.Z.; Chen, J.; Xie, H.W.; Zhang, Z.S. Enhanced visible light-induced photocatalytic activity of surface-modified BiOBr with Pd nanoparticles. Appl. Surf. Sci. 2018, 433, 76–87. [Google Scholar] [CrossRef]
- Cheng, L.J.; Kang, Y. Synthesis and characterization of Bi2O3/NaBiO3 composite visible light-driven photocatalyst. Mater. Lett. 2013, 97, 125–128. [Google Scholar] [CrossRef]
- Shen, J.; Wang, R.; Liu, Q.Q.; Yang, X.F.; Tang, H.; Yang, J. Accelerating photocatalytic hydrogen evolution and pollutant degradation by coupling organic co-catalysts with TiO2. Chin. J. Catal. 2019, 40, 380–389. [Google Scholar] [CrossRef]
- Kako, T.; Zou, Z.; Katagiri, M.; Ye, J. Decomposition of organic compounds over NaBiO3 under visible light irradiation. Chem. Mater. 2007, 19, 198–202. [Google Scholar] [CrossRef]
- Takei, T.; Haramoto, R.; Dong, Q.; Kumada, N.; Yonesaki, Y.; Kinomura, N.; Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Photocatalytic activities of various pentavalent bismuthates under visible light irradiation. J. Solid State Chem. 2011, 184, 2017–2022. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, C.H.; Zhuang, J.; Zhong, Y.; Wang, D.H.; Zhou, H.Y. AgBr/MgBi2O6 heterostructured composites with highly efficient visible-light-driven photocatalytic activity. J. Phys. Chem. Solids 2018, 117, 94–100. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, X.L.; An, H.T.; Zhong, Y.; Wang, D.H.; Tang, C.Y.; Hu, C.H. Facile one-step hydrothermal fabrication of (Sr0.6Bi0.305)2Bi2O7/SnO2 heterojunction with excellent photocatalytic activity. Nanomaterials 2020, 10, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.; Zhang, J.; Chen, S.; Zhang, S.; Huang, W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Hu, C.H.; Zhuang, J.; Zhong, L.S.; Zhong, Y.; Wang, D.H.; Zhou, H.Y. Significantly enhanced photocatalytic activity of visible light responsive AgBr/Bi2Sn2O7 heterostructured composites. Appl. Surf. Sci. 2017, 426, 1173–1181. [Google Scholar] [CrossRef]
- Nanu, M.; Schoonman, J.; Goossens, A. Solar-energy conversion in TiO2/CuInS2 nanocomposites. Adv. Funct. Mater. 2005, 15, 95–100. [Google Scholar] [CrossRef]
- Ju, P.; Wang, Y.; Sun, Y.; Zhang, D. Controllable one-pot synthesis of a nest-like Bi2WO6/BiVO4 composite with enhanced photocatalytic antifouling performance under visible light irradiation. Dalton Trans. 2016, 45, 4588–4602. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.L.; Zhang, X.H.; Peng, T.Y.; Li, X.G. Low-temperature preparation of AgIn5S8/TiO2 heterojunction nanocomposite with efficient visible-light-driven hydrogen production. Int. J. Hydrog. Energy 2013, 38, 15965–15975. [Google Scholar] [CrossRef]
- Khojasteh, F.; Mersagh, M.R.; Hashemipour, H. The influences of Ni, Ag-doped TiO2 and SnO2, Ag-doped SnO2/TiO2 nanocomposites on recombination reduction in dye synthesized solar cells. J. Alloy. Compd. 2022, 890, 161709. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L.; Liao, C.H. N-Doped TiO2 prepared from microwave-assisted titanate nanotubes (NaxH2-xTi3O7): The effect of microwave irradiation during TNT synthesis on the visible light photoactivity of N-Doped TiO2. J. Phys. Chem. C 2011, 115, 4000–4007. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, J. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.L.; Liu, L.; An, H.T.; Zhong, Y.; Wang, D.H.; Tang, C.Y.; Hu, C.H. (Sr0.6Bi0.305)2Bi2O7 as a new visible-light-responsive photocatalyst: An experimental and theoretical study. Mater. Res. Bull. 2019, 118, 110484. [Google Scholar] [CrossRef]
- Zhang, W.N.; Zhang, Q.G.; Wang, X.H.; Yan, X.X.; Xu, J.Q.; Zeng, Z.G. Lead-free organic-inorganic hybrid perovskite heterojunction composites for photocatalytic applications. Catal. Sci. Technol. 2017, 7, 2753–2762. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Vadivel, S. Novel TiO2/Ag2CrO4 nanocomposites: Efficient visible-light-driven photocatalysts with n-n heterojunctions. J. Photochem. Photobiol. A Chem. 2017, 341, 57–68. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Bhuvanesh, N.S.P.; Woodward, P.M. Optical and electrical properties of the wide gap, n-type semiconductors: ZnBi2O6 and MgBi2O6. Chem. Commun. 2003, 9, 1084–1085. [Google Scholar] [CrossRef]
- Wang, X.L.; Hu, C.H.; An, H.T.; Zhu, D.; Zhong, Y.; Wang, D.H.; Tang, C.Y.; Sun, L.X.; Zhou, H.Y. Photocatalytic removal of MB and hydrogen evolution in water by (Sr0.6Bi0.305)2Bi2O7/TiO2 heterostructures under visible-light irradiation. Appl. Surf. Sci. 2021, 544, 148920. [Google Scholar] [CrossRef]
- Spadavecchia, F.; Ardizzone, S.; Cappelleetti, G.; Falciola, L.; Ceotto, M.; Lotti, D. Investigation and optimization of photocurrent transient measurements on nano-TiO2. J. Appl. Electrchem. 2013, 43, 217–225. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Smitha, C.; Abbubakkar, B.M.; Govindhan, P.N.; Krishnan, A. Synthesis and characterization of TiO2/ZnO–Ag nanocomposite for photocatalytic degradation of dyes and anti-microbial activity. Mater. Today Proc. 2021, 45, 3357–3364. [Google Scholar] [CrossRef]
- Ding, Y.B.; Zhang, G.L.; Wang, X.R.; Zhu, L.H.; Tang, H.Q. Chemical and photocatalytic oxidative degradation of carbamazepine by using metastable Bi3+ self-doped NaBiO3 nanosheets as a bifunctional material. Appl. Catal. B Environ. 2017, 202, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Devi, L.G.; Kavitha, R. A review on plasmonic metal-TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogenerated electrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhang, Y.; Li, Q.; Du, H.; Liu, F.; Zhang, X.; Mu, H.; Duan, J. Enhanced photocatalytic antibacterial and degradation performance by p-n-p type CoFe2O4/CoFe2S4/MgBi2O6 photocatalyst under visible light irradiation. Chem. Eng. J. 2022, 429, 132270. [Google Scholar] [CrossRef]
- Ren, F.M.; Ma, H.H.; Hu, W.; Zhou, Z.F.; Xu, W.B. Preparation of Sn-doped CdS/TiO2/ conducting polymer fiber composites for efficient photocatalytic hydrogen production under visible light irradiation. J. Appl. Polym. Sci. 2015, 132, 42300. [Google Scholar] [CrossRef]
- Xu, X.; Yang, G.R.; Liang, J.; Ding, S.J.; Tang, C.L.; Yang, H.H.; Yan, W.; Yang, G.D.; Yu, D.M. Fabrication of one-dimensional heterostructured TiO2@SnO2 with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 116–122. [Google Scholar] [CrossRef]
- Chen, W.; Liu, T.Y.; Huang, T.; Liu, X.H.; Duan, G.R.; Yang, X.J.; Chen, S.M. A novel yet simple strategy to fabricate visible light responsive C, N-TiO2/g-C3N4 heterostructures with significantly enhanced photocatalytic hydrogen generation. RSC Adv. 2015, 5, 101214–101220. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.-H.; Zhao, L.; Yang, G.-D. Synthesis of TiO2@ZnIn2S4 hollow nanospheres with enhanced photocatalytic hydrogen evolution. Rare Met. 2019, 38, 420–427. [Google Scholar]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]


| Photocatalyst | Light Source | Reactant Solution and Sacrificial Reagents | H2 Evolution Rate | Ref. |
|---|---|---|---|---|
| (Sr0.6Bi0.305)2Bi2O7/TiO2 | PLS-SXE 300 | Methanol aqueous solution | 3.18 mmol h−1 g−1 | [28] |
| AgIn5S8/TiO2 | 300 W Xe lamp | Na2SO3 and Na2S aqueous solution | 371.1 μmol h−1 | [20] |
| CdS/TiO2 | 350 W Xenon lamp | Na2S aqueous solution | 2885 μmol h−1 g−1 | [34] |
| SnO2/TiO2 | Ultraviolet light (UV) | Na2S and Na2SO3 aqueous solution | 150 μmol h−1 g−1 | [35] |
| TiO2/g-C3N4 | 300 W Xenon arc lamp | Triethanolamine aqueous solution | 39.18 mmol h−1 g−1 | [36] |
| TiO2/ZnIn2S4 | 300 W Xenon lamp | Lactic acid aqueous solution | 4958 μmol h−1 g−1 | [37] |
| MBO/TiO2 | PLS-SXE 300 | Methanol aqueous solution | 3413 μmol h−1 g−1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Hu, C.; Zhu, D.; Wang, D.; Zhong, Y.; Tang, C.; Zhou, H. One-Step Hydrothermal Synthesis of Nanostructured MgBi2O6/TiO2 Composites for Enhanced Hydrogen Production. Nanomaterials 2022, 12, 1302. https://doi.org/10.3390/nano12081302
Xu F, Hu C, Zhu D, Wang D, Zhong Y, Tang C, Zhou H. One-Step Hydrothermal Synthesis of Nanostructured MgBi2O6/TiO2 Composites for Enhanced Hydrogen Production. Nanomaterials. 2022; 12(8):1302. https://doi.org/10.3390/nano12081302
Chicago/Turabian StyleXu, Feng, Chaohao Hu, Di Zhu, Dianhui Wang, Yan Zhong, Chengying Tang, and Huaiying Zhou. 2022. "One-Step Hydrothermal Synthesis of Nanostructured MgBi2O6/TiO2 Composites for Enhanced Hydrogen Production" Nanomaterials 12, no. 8: 1302. https://doi.org/10.3390/nano12081302







