Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure
Abstract
:1. Introduction
2. Materials and Methods
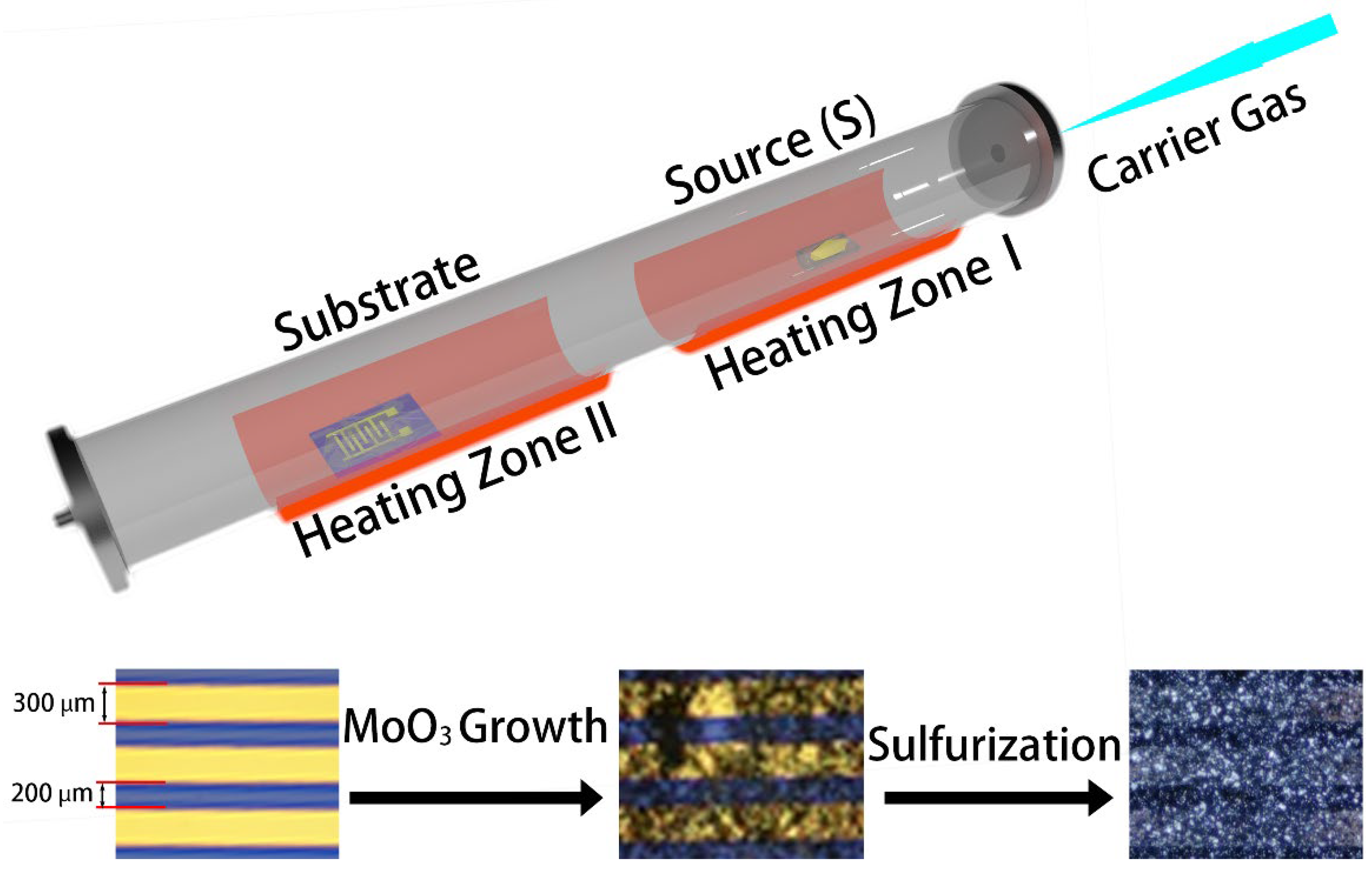
2.1. Synthesis of MoO3
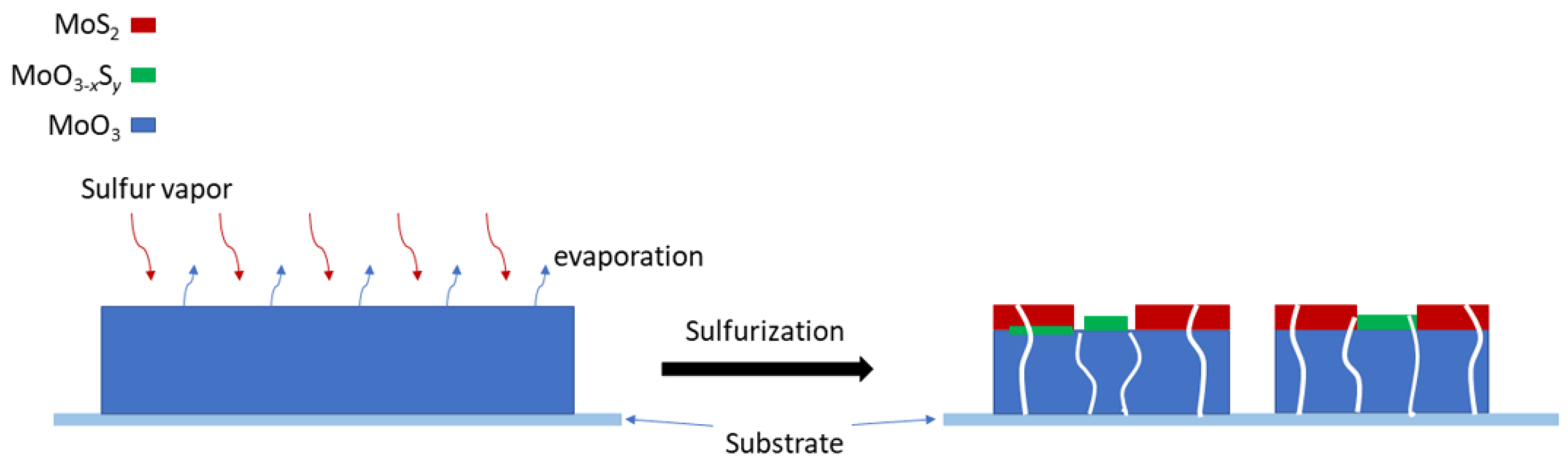
2.2. Sulfurization
2.3. Materials Characterizations
2.4. Characterization of Gas Sensors
3. Results and Discussion
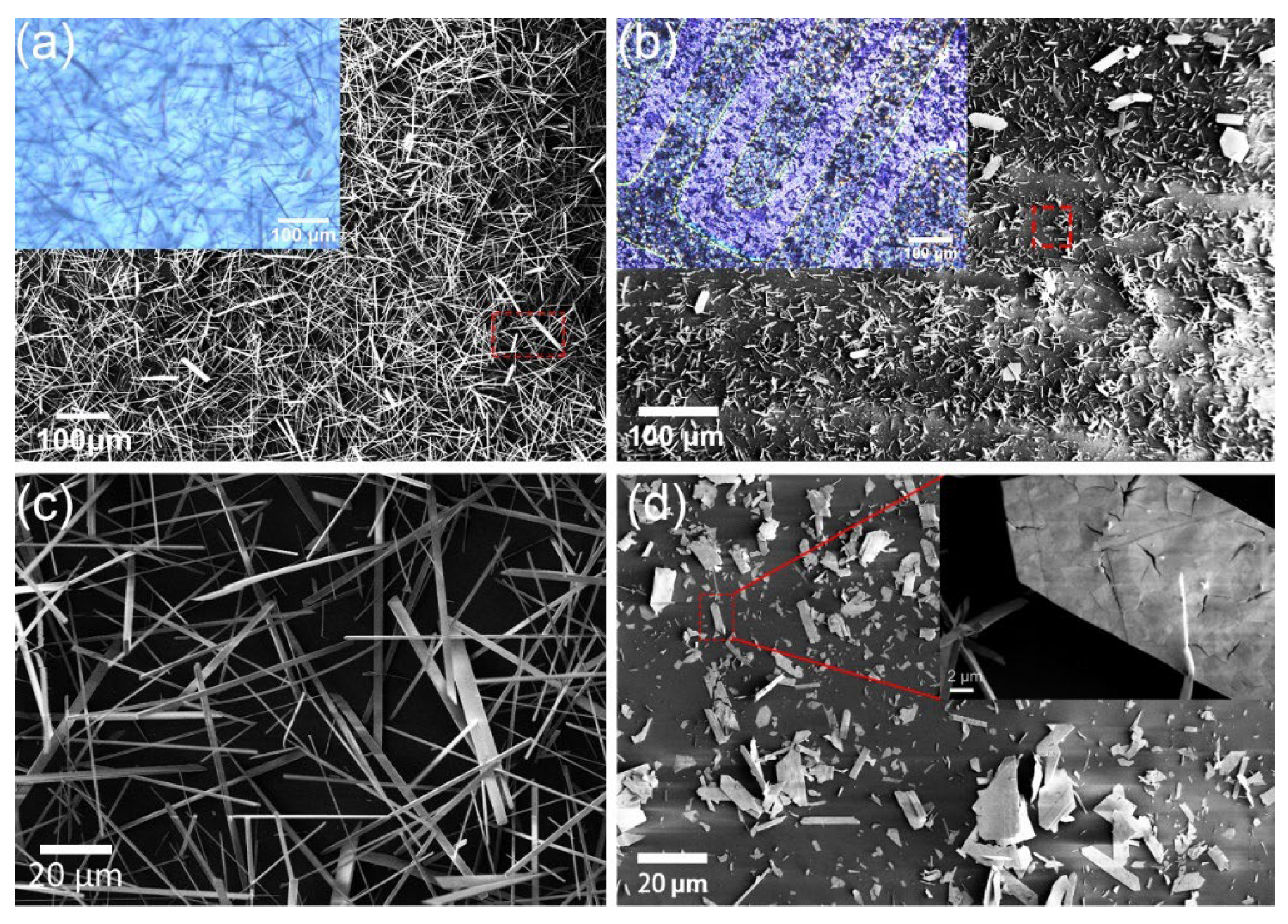
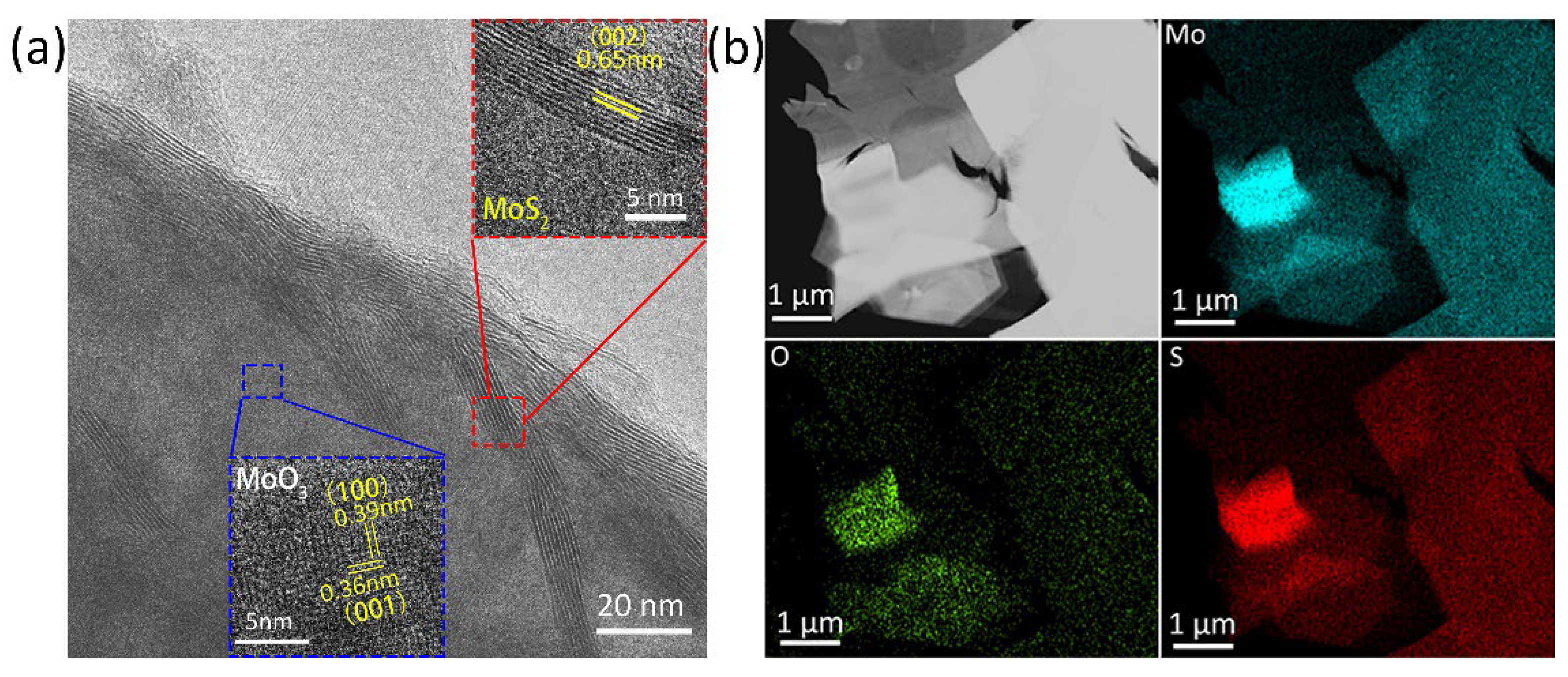
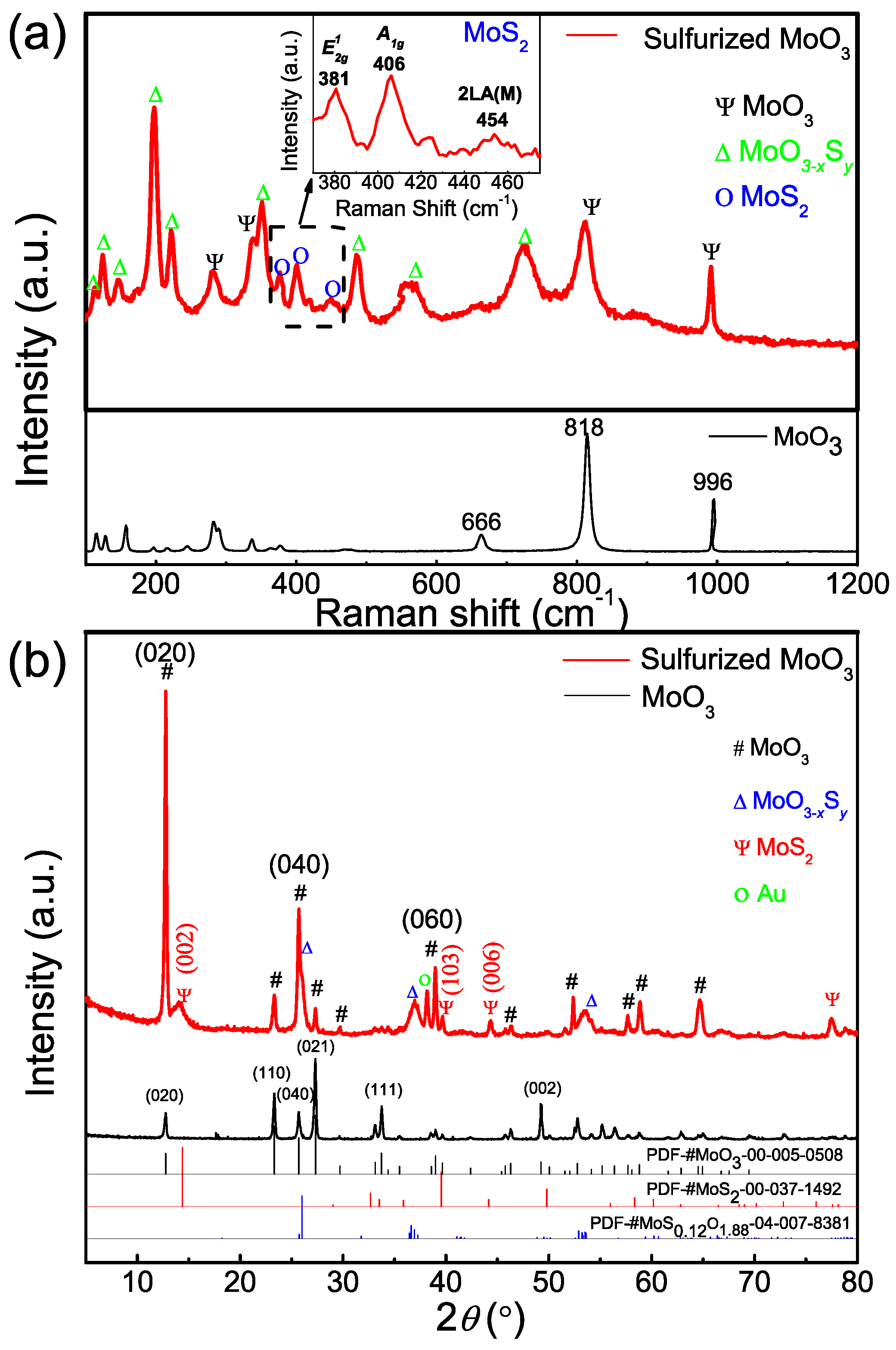
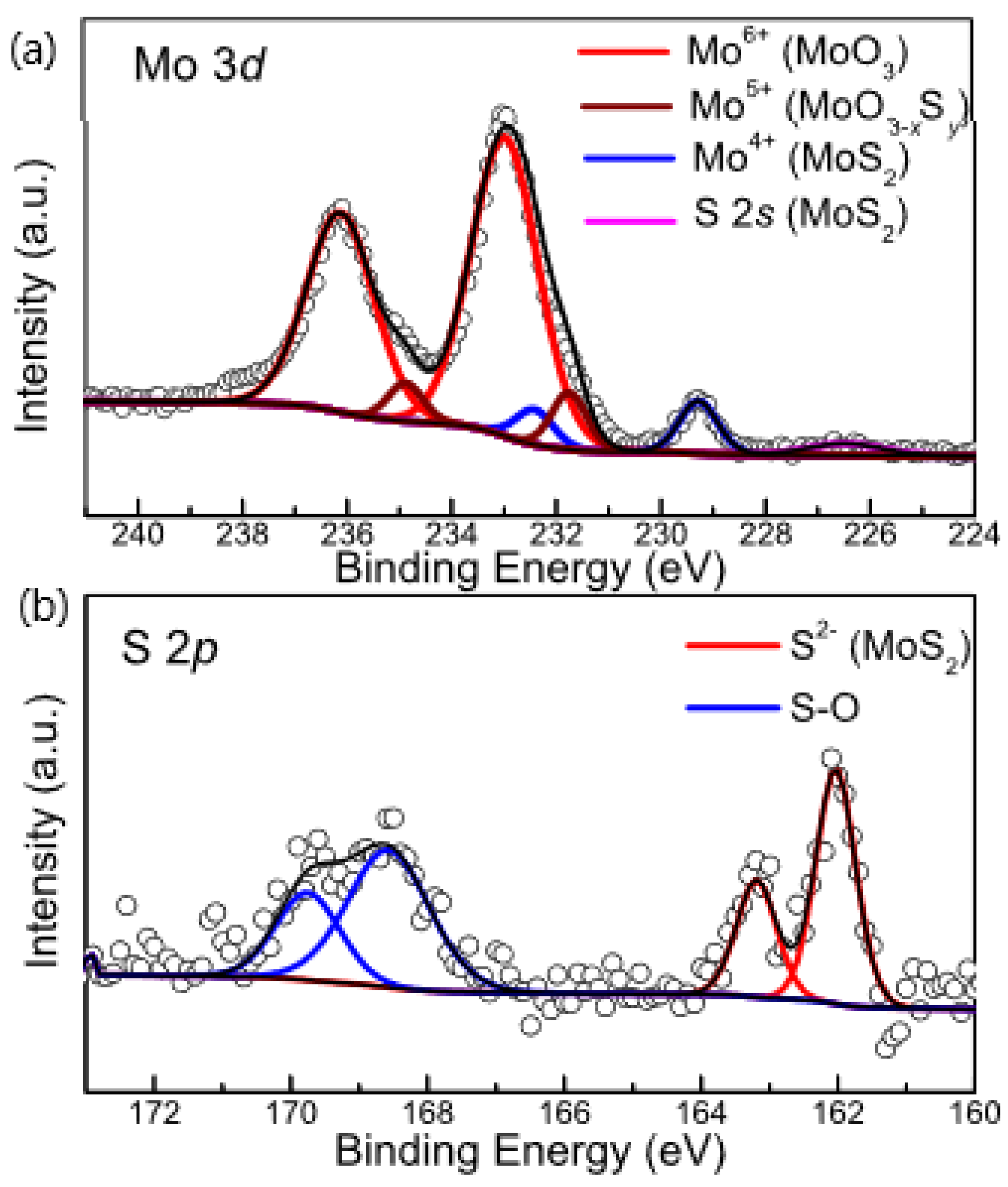
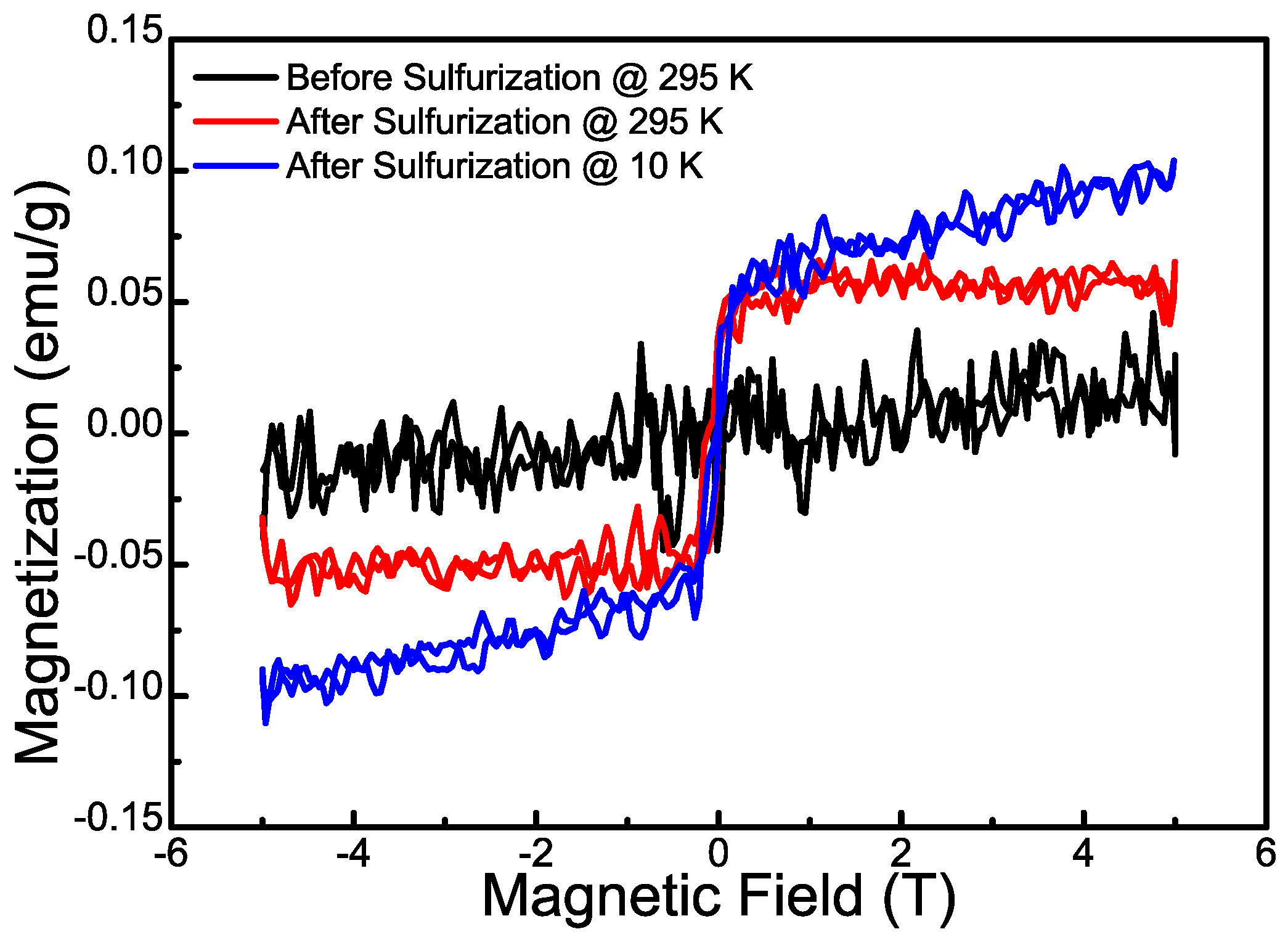
3.1. Materials Characterization
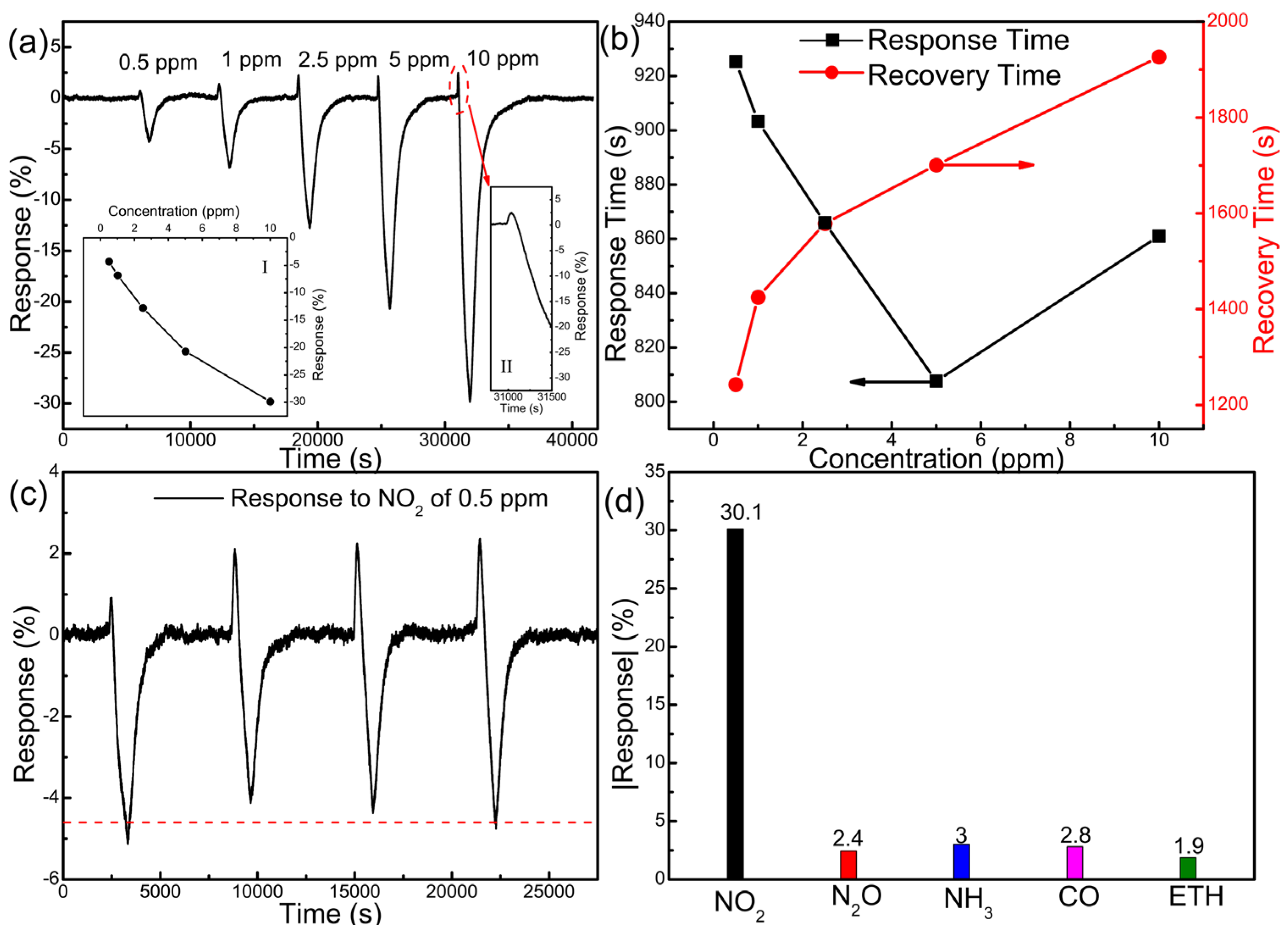
3.2. Gas-Sensing Performance
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B.; Bernstein, J.A. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Alexis, N.; Bacchus, H.; Bernstein, I.L.; Fritz, P.; Horner, E.; Li, N.; Mason, S.; Nel, A.; Oullette, J.; et al. The health effects of nonindustrial indoor air pollution. J. Allergy Clin. Immunol. 2008, 121, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dost, R.; Richardson, T.; Grell, M.; Morrison, J.J.; Turner, M.L. A Nitrogen Dioxide Sensor Based on an Organic Transistor Constructed from Amorphous Semiconducting Polymers. Adv. Mater. 2007, 19, 4018–4023. [Google Scholar] [CrossRef]
- Fernández-Sánchez, J.F.; Fernández, I.; Steiger, R.; Beer, R.; Cannas, R.; Spichiger-Keller, U.E. Second Generation Nanostructured Metal Oxide Matrices to Increase the Thermal Stability of CO and NO2 Sensing Layers Based on Iron(II) Phthalocyanine. Adv. Funct. Mater. 2007, 17, 1188–1198. [Google Scholar] [CrossRef]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Response to NO2 and other gases of resistive chemically exfoliated MoS2-based gas sensors. Sens. Actuator B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Ji, S.; Wang, H.; Wang, T.; Yan, D. A High-Performance Room-Temperature NO2 Sensor Based on An Ultrathin Heterojunction Film. Adv. Mater. 2013, 25, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N.; Sharma, P.; Calle, F. A review on 2D transition metal di-chalcogenides and metal oxide nanostructures based NO2 gas sensors. Mater. Sci. Semicond. Process. 2020, 107, 104865. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Geeta Rani, B.; Saisri, R.; Kailasa, S.; Sai Bhargava Reddy, M.; Maseed, H.; Venkateswara Rao, K. Architectural tailoring of orthorhombic MoO3 nanostructures toward efficient NO2 gas sensing. J. Mater. Sci. 2020, 55, 8109–8122. [Google Scholar] [CrossRef]
- Rahman, F.; Zavabeti, A.; Rahman, M.A.; Arash, A.; Mazumder, A.; Walia, S.; Sriram, S.; Bhaskaran, M.; Balendhran, S. Dual Selective Gas Sensing Characteristics of 2D α-MoO3−x via a Facile Transfer Process. ACS Appl. Mater. Interfaces 2019, 11, 40189–40195. [Google Scholar] [CrossRef]
- Yang, S.X.; Jiang, C.B.; Wei, S.H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Di Bartolomeo, A. Emerging 2D Materials and Their Van Der Waals Heterostructures. Nanomaterials 2020, 10, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiao, H.; Guo, Z.; Ren, X.; Huang, Z.; Qi, X.; Dhanabalan, S.C.; Ponraj, J.S.; Zhang, D.; Li, J.; et al. High-Performance Photo-Electrochemical Photodetector Based on Liquid-Exfoliated Few-Layered InSe Nanosheets with Enhanced Stability. Adv. Funct. Mater. 2018, 28, 1705237. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Zhou, B.; Jia, C.; Zhang, X.; Jiang, Z. Theoretical study of the NO, NO2, CO, SO2, and NH3 adsorptions on multi-diameter single-wall MoS2 nanotube. J. Phys. D Appl. Phys. 2015, 49, 045106. [Google Scholar] [CrossRef]
- Bermudez, V.M. Computational Study of the Adsorption of NO2 on Monolayer MoS2. J. Phys. Chem. C 2020, 124, 15275–15284. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.; Zheng, L.; Yang, C.; Pinna, N.; Liu, X.; Zhang, J. MoS2 Van der Waals p–n Junctions Enabling Highly Selective Room-Temperature NO2 Sensor. Adv. Funct. Mater. 2020, 30, 2000435. [Google Scholar] [CrossRef]
- Hau, H.H.; Duong, T.T.H.; Man, N.K.; Thi Viet Nga, T.; Thi Xuan, C.; Thi Thanh Le, D.; Van Toan, N.; Hung, C.M.; Van Duy, N.; Van Hieu, N.; et al. Enhanced NO2 gas-sensing performance at room temperature using exfoliated MoS2 nanosheets. Sens. Actuators A Phys. 2021, 332, 113137. [Google Scholar] [CrossRef]
- Kumbhakar, P.; Chowde Gowda, C.; Mahapatra, P.L.; Mukherjee, M.; Malviya, K.D.; Chaker, M.; Chandra, A.; Lahiri, B.; Ajayan, P.M.; Jariwala, D.; et al. Emerging 2D metal oxides and their applications. Mater. Today 2021, 45, 142–168. [Google Scholar] [CrossRef]
- Wu, D.; Qi, D.; Liu, J.; Wang, Z.; Hao, Q.; Hong, G.; Liu, F.; Ouyang, F.; Zhang, W. Growth of centimeter-scale single crystal MoO3 ribbons for high performance ultraviolet photodetectors. Appl. Phys. Lett. 2021, 118, 243101. [Google Scholar] [CrossRef]
- Duraisamy, S.; Ganguly, A.; Sharma, P.K.; Benson, J.; Davis, J.; Papakonstantinou, P. One-Step Hydrothermal Synthesis of Phase-Engineered MoS2/MoO3 Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2021, 4, 2642–2656. [Google Scholar] [CrossRef]
- He, R.; Lai, H.; Wang, S.; Chen, T.; Xie, F.; Chen, Q.; Liu, P.; Chen, J.; Xie, W. Few-layered vdW MoO3 for sensitive, uniform and stable SERS applications. Appl. Surf. Sci. 2020, 507, 145116. [Google Scholar] [CrossRef]
- Du, Y.; He, J.; Hou, G.; Yuan, F. α-MoO3 sheets with high exposed plane reinforced by thermal plasma for stable Li-ion storage. Electrochim. Acta 2020, 334, 135593. [Google Scholar] [CrossRef]
- Li, W.; Xing, K.; Liu, P.; Chuang, C.; Lu, Y.-R.; Chan, T.-S.; Tesfamichael, T.; Motta, N.; Qi, D.-C. Ultrasensitive NO2 Gas Sensors Based on Layered α-MoO3 Nanoribbons. Adv. Mater. Technol. 2021, 2100579. [Google Scholar] [CrossRef]
- Balendhran, S.; Walia, S.; Nili, H.; Ou, J.Z.; Zhuiykov, S.; Kaner, R.B.; Sriram, S.; Bhaskaran, M.; Kalantar-zadeh, K. Two-Dimensional Molybdenum Trioxide and Dichalcogenides. Adv. Funct. Mater. 2013, 23, 3952–3970. [Google Scholar] [CrossRef]
- Malik, R.; Joshi, N.; Tomer, V.K. Advances in the designs and mechanisms of MoO3 nanostructures for gas sensors: A holistic review. Mater. Adv. 2021, 2, 4190–4227. [Google Scholar] [CrossRef]
- Wei, Z.; Hai, Z.; Akbari, M.K.; Qi, D.; Xing, K.; Zhao, Q.; Verpoort, F.; Hu, J.; Hyde, L.; Zhuiykov, S. Atomic layer deposition-developed two-dimensional α-MoO3 windows excellent hydrogen peroxide electrochemical sensing capabilities. Sens. Actuators B Chem. 2018, 262, 334–344. [Google Scholar] [CrossRef]
- Li, W.; Ou, Q.; Wang, X.; Xing, K.; Tesfamichael, T.; Motta, N.; Qi, D.-C. Large-sized α-MoO3 layered single crystals for superior NO2 gas sensing. Appl. Surf. Sci. 2022, 586, 152793. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Sun, Z. 2D/2D Heterostructures: Rational Design for Advanced Batteries and Electrocatalysis. Energy Environ. Mater. 2021, 5, 115–132. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Gas sensing with heterostructures based on two-dimensional nanostructured materials: A review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Ren, X.; Yang, L.; Zhu, J.; Wei, B. Optical and Electrical Enhancement of Hydrogen Evolution by MoS2@MoO3 Core–Shell Nanowires with Designed Tunable Plasmon Resonance. Adv. Funct. Mater. 2018, 28, 1802567. [Google Scholar] [CrossRef]
- Hou, X.; Mensah, A.; Zhao, M.; Cai, Y.; Wei, Q. Facile controlled synthesis of monodispersed MoO3-MoS2 hybrid nanospheres for efficient hydrogen evolution reaction. Appl. Surf. Sci. 2020, 529, 147115. [Google Scholar] [CrossRef]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 Nanowires for Hydrogen Evolution: A Functional Design for Electrocatalytic Materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef]
- Zhao, S.; Zha, Z.; Liu, X.; Tian, H.; Wu, Z.; Li, W.; Sun, L.-B.; Liu, B.; Chen, Z. Core-Sheath Structured MoO3@MoS2 Composite for High-Performance Lithium-Ion Battery Anodes. Energy Fuels 2020, 34, 11498–11507. [Google Scholar] [CrossRef]
- Singh, S.; Guleria, A.; Singh, N.; Sharma, S. Highly responsive room-temperature ammonia sensing properties of MoS2/MoO3 nano-composite. Mater. Today Proc. 2021, 46, 10732–10735. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Huang, B.; Lou, Z.; Li, B. Light-Induced In Situ Formation of a Nonmetallic Plasmonic MoS2/MoO3−x Heterostructure with Efficient Charge Transfer for CO2 Reduction and SERS Detection. ACS Appl. Mater. Interfaces 2021, 13, 10047–10053. [Google Scholar] [CrossRef]
- Pondick, J.V.; Woods, J.M.; Xing, J.; Zhou, Y.; Cha, J.J. Stepwise Sulfurization from MoO3 to MoS2 via Chemical Vapor Deposition. ACS Appl. Nano Mater. 2018, 1, 5655–5661. [Google Scholar] [CrossRef]
- Heo, S.N.; Ishiguro, Y.; Hayakawa, R.; Chikyow, T.; Wakayama, Y. Perspective: Highly ordered MoS2 thin films grown by multi-step chemical vapor deposition process. APL Mater. 2016, 4, 030901. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Kang, L.; Song, P.; Zeng, Q.; Tang, B.; Yang, J.; Wu, Y.; Tian, D.; Xu, M.; Zhao, W.; et al. MoO3–MoS2 vertical heterostructures synthesized via one-step CVD process for optoelectronics 2D. Materials 2021, 8, 035036. [Google Scholar] [CrossRef]
- Weber, T.; Muijsers, J.C.; van Wolput, J.H.M.C.; Verhagen, C.P.J.; Niemantsverdriet, J.W. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2, as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 1996, 100, 14144. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Goel, N.; Mishra, M.; Gupta, G.; Fanetti, M.; Valant, M.; Kumar, M. Growth of MoS2–MoO3 Hybrid Microflowers via Controlled Vapor Transport Process for Efficient Gas Sensing at Room Temperature. Adv. Mater. Interfaces 2018, 5, 1800071. [Google Scholar] [CrossRef]
- Mohammadbeigi, M.; Jamilpanah, L.; Rahmati, B.; Mohseni, S.M. Sulfurization of planar MoO3 optical crystals: Enhanced Raman response and surface porosity. Mater. Res. Bull. 2019, 118, 110527. [Google Scholar] [CrossRef]
- Liu, H.; Lin, M.; Guo, S. Morphological and structural evolutions of α-MoO3 single crystal belts towards MoS2/MoO2 heterostructures upon post-growth thermal vapor sulfurization. Appl. Surf. Sci. 2021, 536, 147956. [Google Scholar] [CrossRef]
- Joya, M.R.; Alfonso, J.E.; Moreno, L.C. Photoluminescence and Raman studies of alpha-MoO3 doped with erbium and neodymium. Curr. Sci. 2019, 116, 1690–1695. [Google Scholar] [CrossRef]
- Diaz-Droguett, D.E.; El Far, R.; Fuenzalida, V.M.; Cabrera, A.L. In situ-Raman studies on thermally induced structural changes of porous MoO3 prepared in vapor phase under He and H2. Mater. Chem. Phys. 2012, 134, 631–638. [Google Scholar] [CrossRef]
- Camacho-López, M.A.; Escobar-Alarcón, L.; Picquart, M.; Arroyo, R.; Córdoba, G.; Haro-Poniatowski, E. Micro-Raman study of the m-MoO2 to α-MoO3 transformation induced by cw-laser irradiation. Opt. Mater. 2011, 33, 480–484. [Google Scholar] [CrossRef]
- Silveira, J.V.; Batista, J.A.; Saraiva, G.D.; Mendes Filho, J.; Souza Filho, A.G.; Hu, S.; Wang, X. Temperature dependent behavior of single walled MoO3 nanotubes: A Raman spectroscopy study. Vib. Spectrosc. 2010, 54, 179–183. [Google Scholar] [CrossRef]
- Singh, S.; Deb, J.; Sarkar, U.; Sharma, S. MoS2/MoO3 Nanocomposite for Selective NH3 Detection in a Humid Environment. ACS Sustain. Chem. Eng. 2021, 9, 7328–7340. [Google Scholar] [CrossRef]
- Windom, B.C.; Sawyer, W.G.; Hahn, D.W. A Raman Spectroscopic Study of MoS2 and MoO3: Applications to Tribological Systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Bera, A.; Sood, A.K. Insights into Vibrational and Electronic Properties of MoS2 Using Raman, Photoluminescence, and Transport Studies. In MoS2: Materials, Physics, and Devices; Wang, Z.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 155–215. [Google Scholar]
- Wang, D.; Li, J.-N.; Zhou, Y.; Xu, D.-H.; Xiong, X.; Peng, R.-W.; Wang, M. Van der Waals epitaxy of ultrathin α-MoO3 sheets on mica substrate with single-unit-cell thickness. Appl. Phys. Lett. 2016, 108, 053107. [Google Scholar] [CrossRef]
- Song, L.X.; Xia, J.; Dang, Z.; Yang, J.; Wang, L.B.; Chen, J. Formation, structure and physical properties of a series of α-MoO3 nanocrystals: From 3D to 1D and 2D. Cryst. Eng. Comm. 2012, 14, 2675–2682. [Google Scholar] [CrossRef]
- Song, S.; Wang, J.; Peng, T.; Fu, W.; Zan, L. MoS2-MoO3−x hybrid cocatalyst for effectively enhanced H2 production photoactivity of AgIn5S8 nano-octahedrons. Appl. Catal. B Environ. 2018, 228, 39–46. [Google Scholar] [CrossRef]
- Szoszkiewicz, R.; Rogala, M.; Dąbrowski, P. Surface-Bound and Volatile Mo Oxides Produced During Oxidation of Single MoS2 Crystals in Air and High Relative Humidity. Materials 2020, 13, 3067. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Yang, B.; Gao, Y. Chemical vapor deposition growth and characterization of drop-like MoS2/MoO2 granular films. Phys. Status Solidi 2017, 254, 1600245. [Google Scholar] [CrossRef]
- Wang, L.; Ji, X.; Wang, T.; Zhang, Q. Novel Red Emission from MoO3/MoS2–MoO2–MoO3 Core–Shell Belt Surface. ACS Appl. Mater. Interfaces 2018, 10, 36297–36303. [Google Scholar] [CrossRef]
- Yoon, A.; Kim, J.H.; Yoon, J.; Lee, Y.; Lee, Z. van der Waals Epitaxial Formation of Atomic Layered α-MoO3 on MoS2 by Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 22029–22036. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Brown, N.M.D.; Cui, N.; McKinley, A. An XPS study of the surface modification of natural MoS2 following treatment in an RF-oxygen plasma. Appl. Surf. Sci. 1998, 134, 11–21. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-Based Charge Transfer in Two-Dimensional SnS2 for Selective and Reversible NO2 Gas Sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Wang, R.; Meng, L.J.; Xu, X.Y.; Hu, J.G.; Pan, J. Achieving half-metallicity in zigzag MoS2 nanoribbon with a sulfur vacancy by edge passivation. J. Phys. D Appl. Phys. 2018, 51, 265005. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, Y.; Kwon, Y.H.; Choi, S.H.; Yang, W.; Kim, D.Y.; Lee, S. Room-Temperature Ferromagnetic Ultrathin α-MoO3:Te Nanoflakes. ACS Nano 2019, 13, 8717–8724. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, A.; Huijben, M.; van Zalk, M.; Huijben, J.; Zeitler, U.; Maan, J.C.; van der Wiel, W.G.; Rijnders, G.; Blank, D.H.A.; Hilgenkamp, H. Magnetic effects at the interface between non-magnetic oxides. Nat. Mater. 2007, 6, 493–496. [Google Scholar] [CrossRef]
- Shidpour, R.; Manteghian, M. A density functional study of strong local magnetism creation on MoS2 nanoribbon by sulfur vacancy. Nanoscale 2010, 2, 1429–1435. [Google Scholar] [CrossRef]
- Rusydi, A.; Dhar, S.; Barman, A.R.; Ariando, n.; Qi, D.C.; Motapothula, M.; Yi, J.B.; Santoso, I.; Feng, Y.P.; Yang, K.; et al. Cationic-vacancy-induced room-temperature ferromagnetism in transparent, conducting anatase Ti1−xTaxO2 (x∼0.05) thin films. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 4927–4943. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Li, W.; Sun, X.; Ma, Y.; Xia, J.; Zhao, Y.; Lu, X.; Gan, J.; Liu, Z.; Chen, J.; et al. Room-temperature ferromagnetism in hierarchically branched MoO3 nanostructures. Crystengcomm 2012, 14, 1419–1424. [Google Scholar] [CrossRef]
- Botello-Méndez, A.R.; López-Urías, F.; Terrones, M.; Terrones, H. Metallic and ferromagnetic edges in molybdenum disulfide nanoribbons. Nanotechnology 2009, 20, 325703. [Google Scholar] [CrossRef]
- Li, Y.; Song, Z.; Li, Y.; Li, S.; Wang, H.; Song, B.; Gao, B.; Du, X. Synthesis of Mo4O11@MoO3 nanobelts and their improved sensing performance to NO2 gas. Mater. Res. Express 2019, 6, 055041. [Google Scholar] [CrossRef]
- Analytical Methods, C. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar] [CrossRef]
- Shafiei, M.; Bradford, J.; Khan, H.; Piloto, C.; Wlodarski, W.; Li, Y.; Motta, N. Low-operating temperature NO2 gas sensors based on hybrid two-dimensional SnS2-reduced graphene oxide. Appl. Surf. Sci. 2018, 462, 330–336. [Google Scholar] [CrossRef]
- Acharyya, D.; Huang, K.Y.; Chattopadhyay, P.P.; Ho, M.S.; Fecht, H.J.; Bhattacharyya, P. Hybrid 3D structures of ZnO nanoflowers and PdO nanoparticles as a highly selective methanol sensor. Analyst 2016, 141, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.A.O.; Cao, L.; Qi, D.C.; Tallaire, A.; Crawford, K.G.; Wee, A.T.S.; Moran, D.A.J. Surface transfer doping of diamond by MoO3: A combined spectroscopic and Hall measurement study. Appl. Phys. Lett. 2013, 103, 202112. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ma, Y.; Ouedraogo, N.A.N.; Liu, F.; You, C.; Deng, W.; Zhang, Y. p-/n-Type modulation of 2D transition metal dichalcogenides for electronic and optoelectronic devices. Nano Res. 2022, 15, 123–144. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuator B-Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Xing, K.; Xiang, Y.; Jiang, M.; Creedon, D.L.; Akhgar, G.; Yianni, S.A.; Xiao, H.; Ley, L.; Stacey, A.; McCallum, J.C.; et al. MoO3 induces p-type surface conductivity by surface transfer doping in diamond. Appl. Surf. Sci. 2020, 509, 144890. [Google Scholar] [CrossRef] [Green Version]
- Xing, K.; Li, W.; Della Gaspera, E.; van Embden, J.; Zhang, L.; Yianni, S.A.; Creedon, D.L.; Wang, T.; McCallum, J.C.; Wang, L.; et al. Surface transfer doping of diamond using solution-processed molybdenum trioxide. Carbon 2021, 175, 20–26. [Google Scholar] [CrossRef]
- Keyshar, K.; Berg, M.; Zhang, X.; Vajtai, R.; Gupta, G.; Chan, C.K.; Beechem, T.E.; Ajayan, P.M.; Mohite, A.D.; Ohta, T. Experimental Determination of the Ionization Energies of MoSe2, WS2, and MoS2 on SiO2 Using Photoemission Electron Microscopy. ACS Nano 2017, 11, 8223–8230. [Google Scholar] [CrossRef]
- McDonnell, S.; Azcatl, A.; Addou, R.; Gong, C.; Battaglia, C.; Chuang, S.; Cho, K.; Javey, A.; Wallace, R.M. Hole Contacts on Transition Metal Dichalcogenides: Interface Chemistry and Band Alignments. ACS Nano 2014, 8, 6265–6272. [Google Scholar] [CrossRef]
- Pal, S.; Mukherjee, S.; Nand, M.; Srivastava, H.; Mukherjee, C.; Jha, S.N.; Ray, S.K. Si compatible MoO3/MoS2 core-shell quantum dots for wavelength tunable photodetection in wide visible range. Appl. Surf. Sci. 2020, 502, 144196. [Google Scholar] [CrossRef]
- Hung, C.M.; Vuong, V.A.; Duy, N.V.; An, D.V.; Hieu, N.V.; Kashif, M.; Hoa, N.D. Controlled Growth of Vertically Oriented Trilayer MoS2 Nanoflakes for Room-Temperature NO2 Gas Sensor Applications. Phys. Status Solidi 2020, 217, 2000004. [Google Scholar] [CrossRef]
- Yan, W.; Yan, W.; Chen, T.; Xu, J.; Tian, Q.; Ho, D. Size-Tunable Flowerlike MoS2 Nanospheres Combined with Laser-Induced Graphene Electrodes for NO2 Sensing. ACS Appl. Nano Mater. 2020, 3, 2545–2553. [Google Scholar] [CrossRef]
- Thang, N.T.; Hong, L.T.; Thoan, N.H.; Hung, C.M.; Van Duy, N.; Van Hieu, N.; Hoa, N.D. Controlled synthesis of ultrathin MoS2 nanoflowers for highly enhanced NO2 sensing at room temperature. RSC Adv. 2020, 10, 12759–12771. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.I. Low-temperature-operated sensitive NO2 gas sensors based on p-type SnO thin-film and thin-film transistors. In Proceedings of the 2020 27th International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 1–4 September 2020; pp. 55–58. [Google Scholar]
- Yu, L.; Guo, F.; Liu, S.; Qi, J.; Yin, M.; Yang, B.; Liu, Z.; Fan, X.H. Hierarchical 3D flower-like MoS2 spheres: Post-thermal treatment in vacuum and their NO2 sensing properties. Mater. Lett. 2016, 183, 122–126. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, H.; Yang, J.; Xie, C.; Zeng, D.; Zhao, N. Red Phosphorus: An Elementary Semiconductor for Room-Temperature NO2 Gas Sensing. ACS Sens. 2018, 3, 2629–2636. [Google Scholar] [CrossRef]
- Yamada, Y.; Ogita, M. Transient response of resistive-type NO2 sensor on temperature change. Sens. Actuators B Chem. 2003, 93, 546–551. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.; Zhang, G.; Zhao, J.; Yu, X.; Zeng, D.; Zhang, S. Enhanced response to NO2 with CuO/ZnO laminated heterostructured configuration. Sens. Actuators B Chem. 2014, 195, 500–508. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Shahbazi, M.; Xing, K.; Tesfamichael, T.; Motta, N.; Qi, D.-C. Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure. Nanomaterials 2022, 12, 1303. https://doi.org/10.3390/nano12081303
Li W, Shahbazi M, Xing K, Tesfamichael T, Motta N, Qi D-C. Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure. Nanomaterials. 2022; 12(8):1303. https://doi.org/10.3390/nano12081303
Chicago/Turabian StyleLi, Wei, Mahboobeh Shahbazi, Kaijian Xing, Tuquabo Tesfamichael, Nunzio Motta, and Dong-Chen Qi. 2022. "Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure" Nanomaterials 12, no. 8: 1303. https://doi.org/10.3390/nano12081303
APA StyleLi, W., Shahbazi, M., Xing, K., Tesfamichael, T., Motta, N., & Qi, D.-C. (2022). Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure. Nanomaterials, 12(8), 1303. https://doi.org/10.3390/nano12081303








