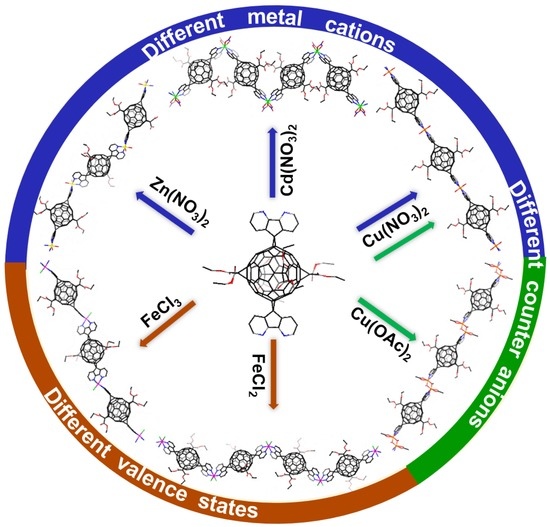
Diversity of Metal—Fullerene Framework Structures Regulated by Metal Salts
Abstract
:1. Introduction
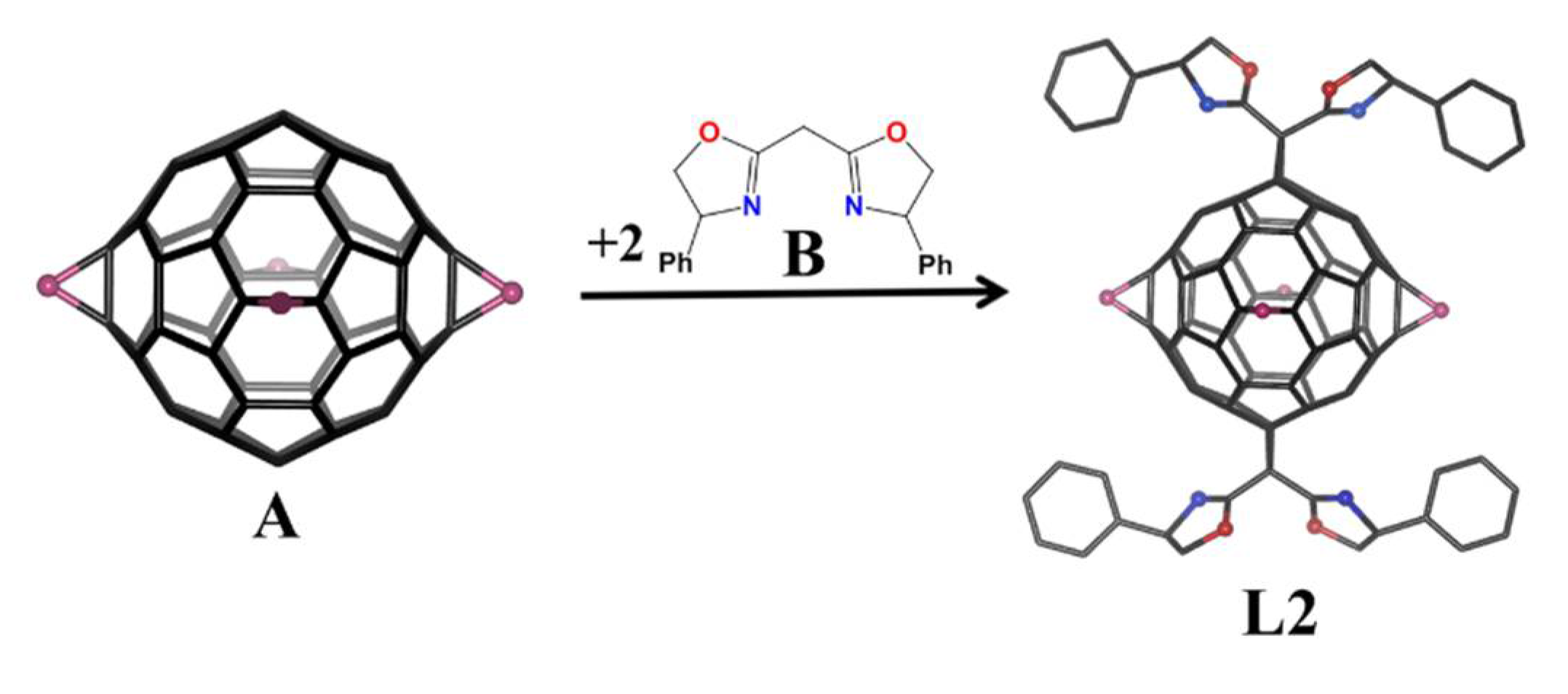
2. Materials and Methods
3. Results
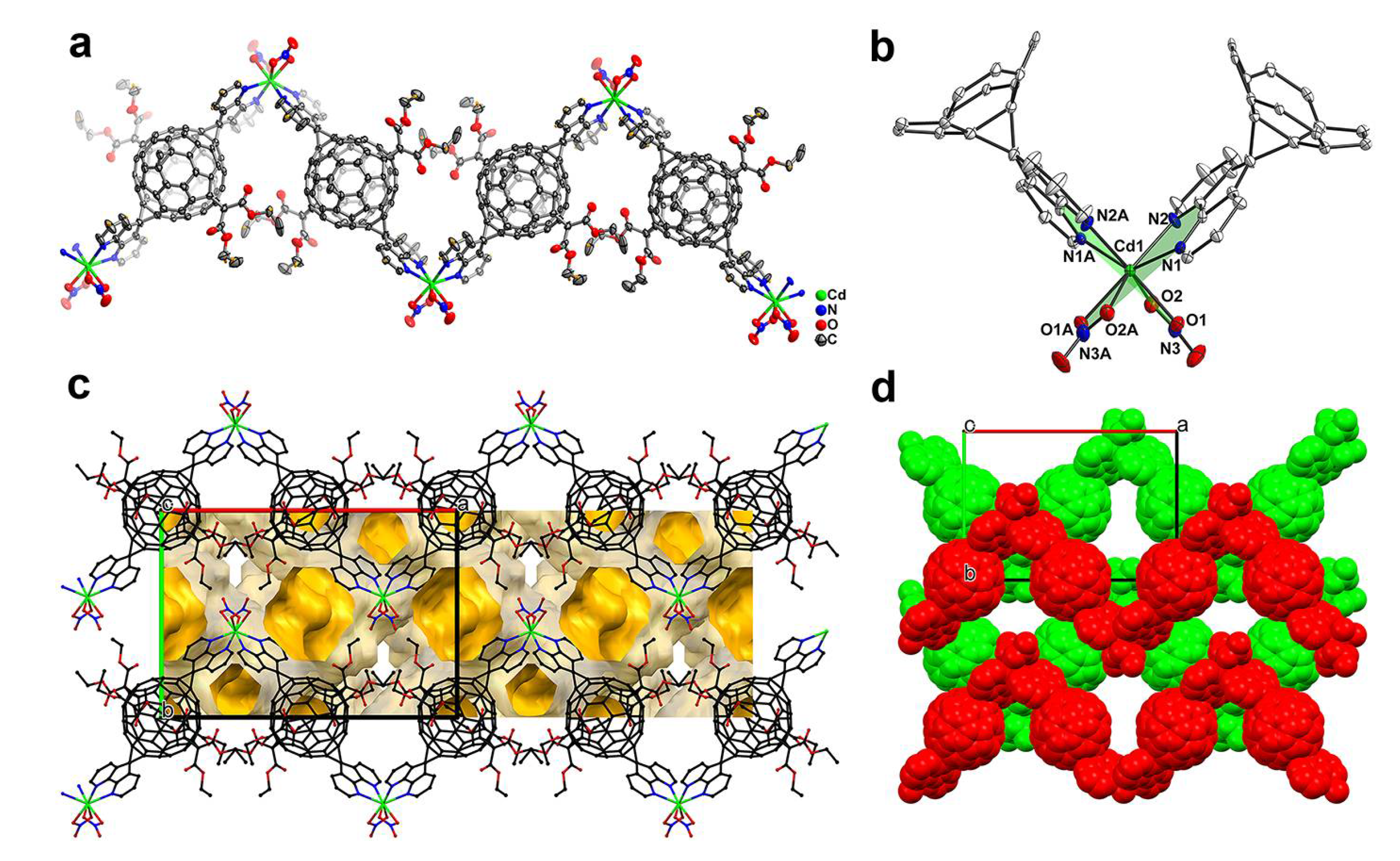
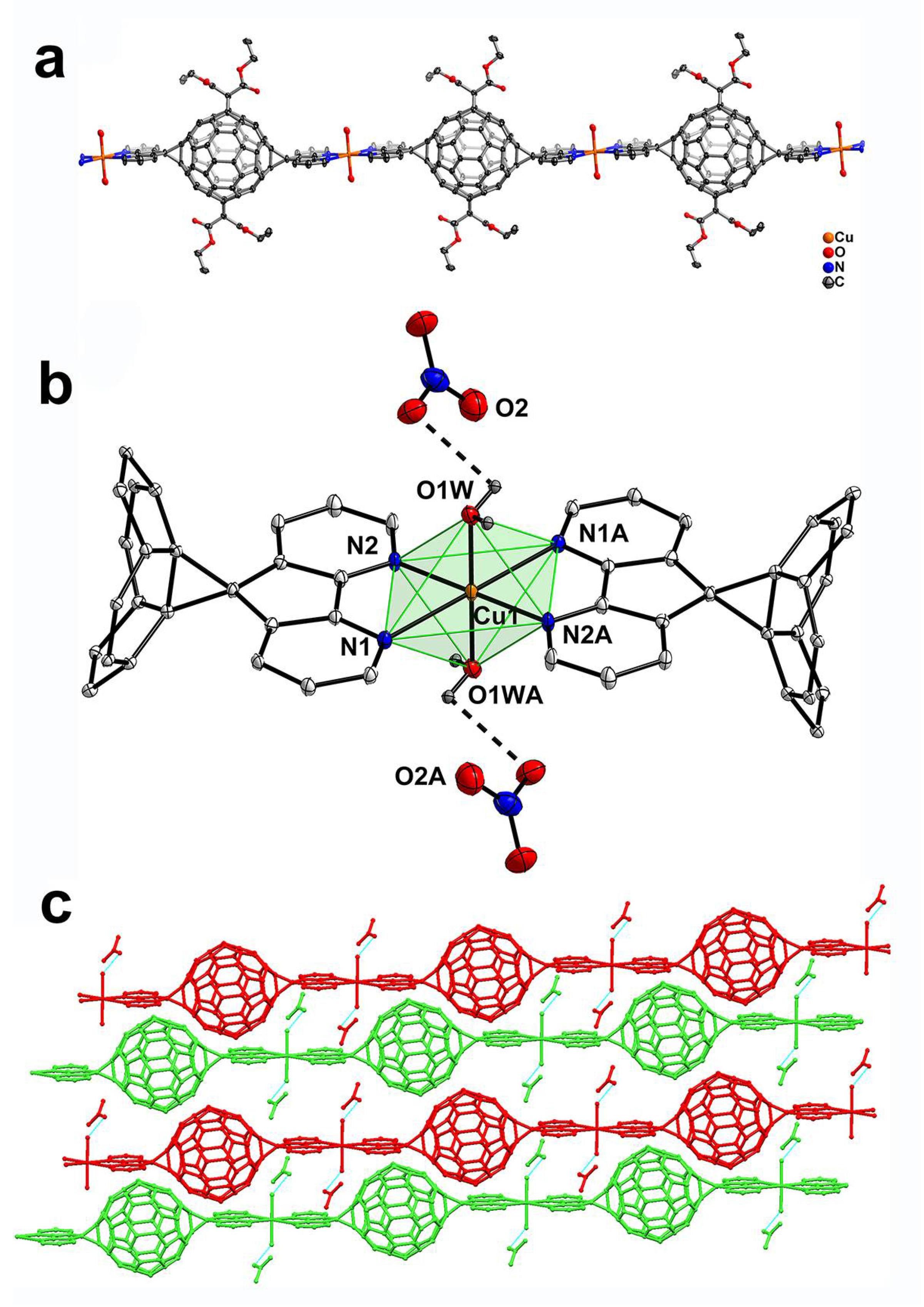
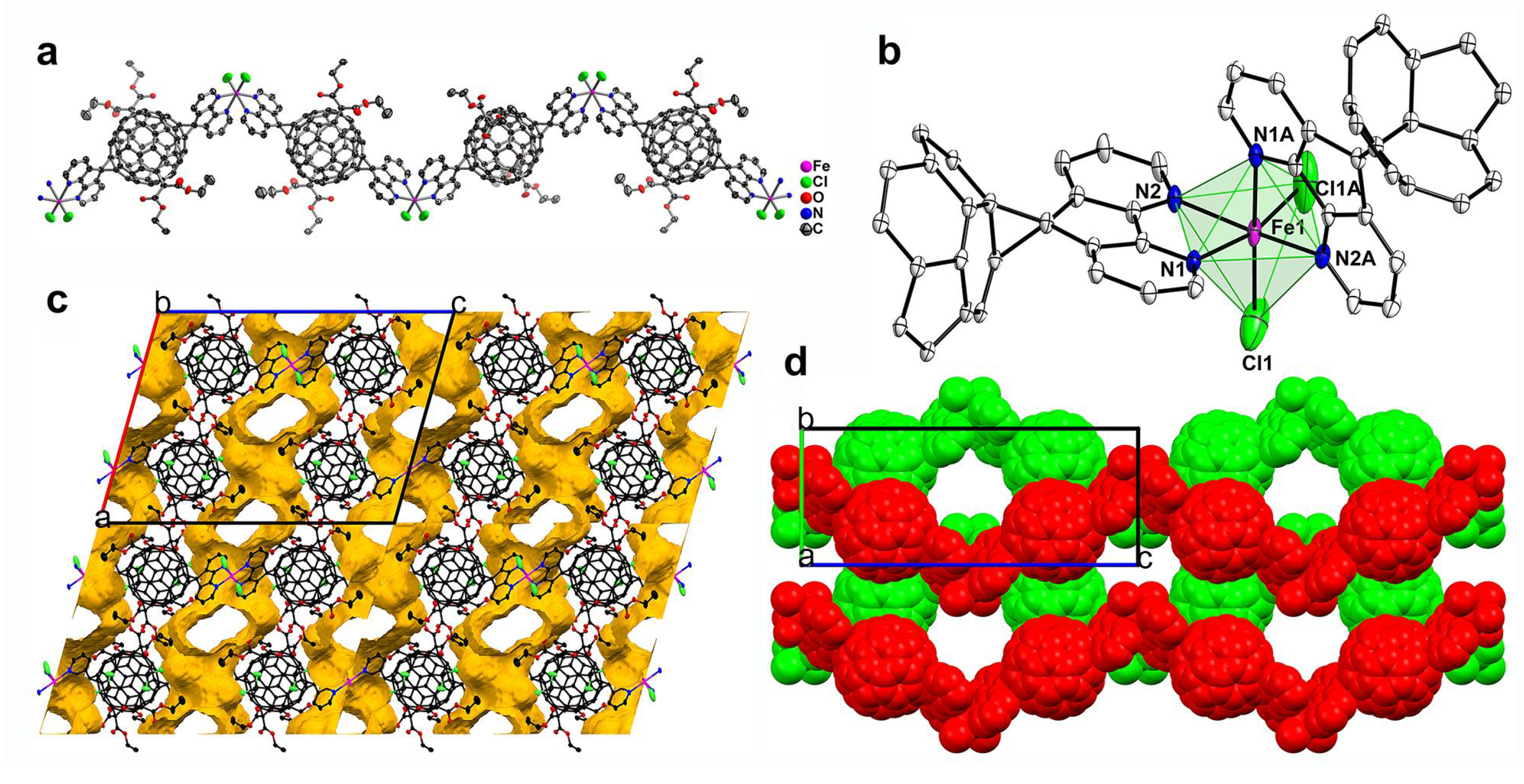
3.1. MFFs from Three Metal Nitrates: Crystal Structures of ZnL1(NO3)2(H2O)2 (1), CdL1(NO3)2 (2) and Cu(L1)(H2O)2(NO3)2 (3)
3.2. MFFs from Copper Salts with Different Counter Anions: Crystal Structure of CuL1(OAc)(CH3O) (4)
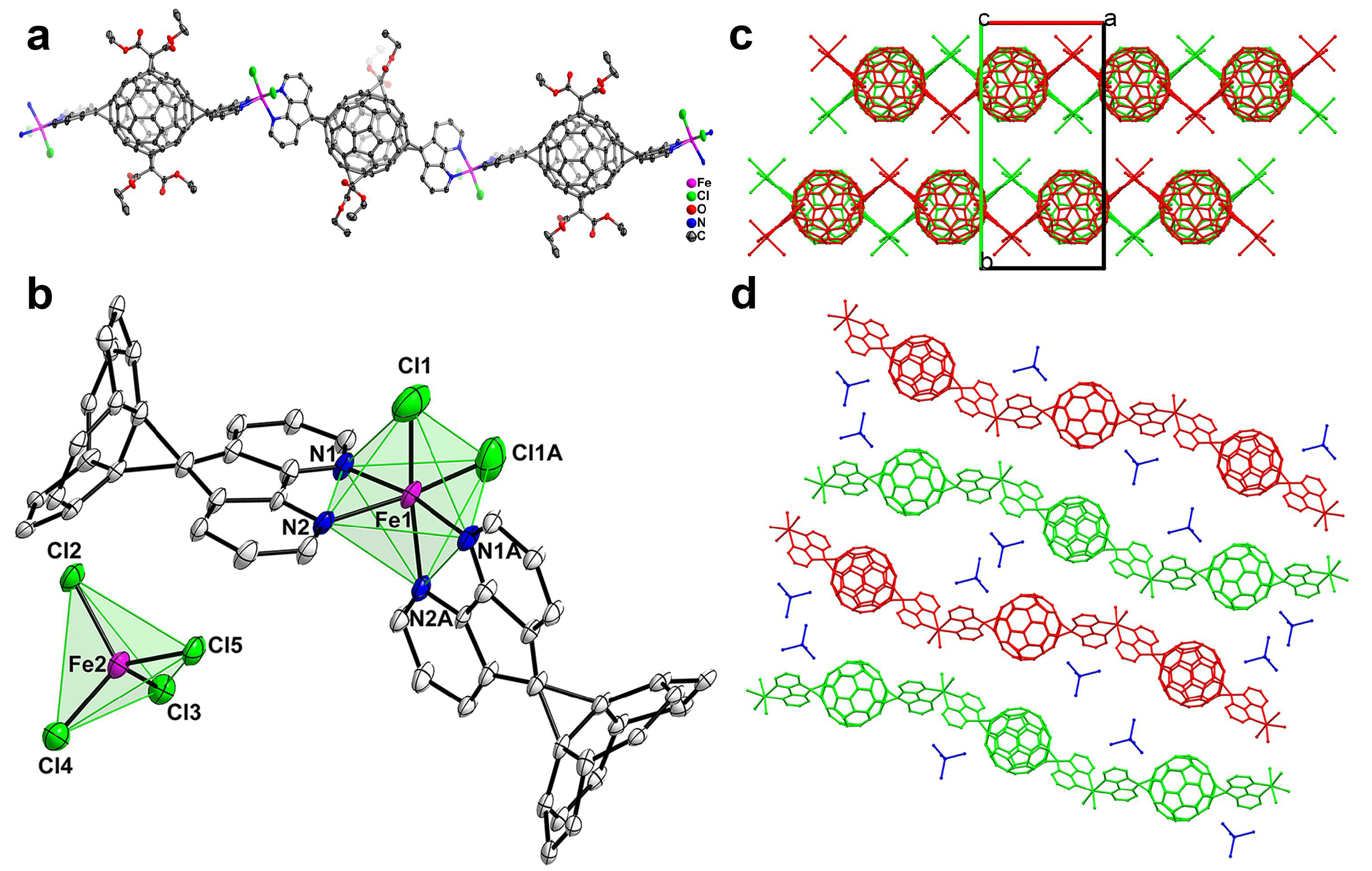
3.3. MFFs from Iron Chloride with Different Oxidation States: Crystal Structures of FeL1Cl2 (5) and FeL1Cl2(FeCl4) (6)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, C.-H.; Chen, N.; Echegoyen, L. Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity. Acc. Chem. Res. 2019, 52, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.-Z.; Zhang, G.-H.; Li, J.-H.; Liu, J.-L.; Zhu, S.-H.; Lu, W.; Zheng, J.; Ng, S.W.; Li, D. Exohedral Cuprofullerene: Sequentially Expanding Metal Olefin Up to a C60@Cu24 Rhombicuboctahedron. J. Am. Chem. Soc. 2020, 142, 5943–5947. [Google Scholar] [CrossRef] [PubMed]
- Balch, A.L.; Olmstead, M.M. Reactions of Transition Metal Complexes with Fullerenes (C60, C70, etc.) and Related Materials. Chem. Rev. 1998, 98, 2123–2166. [Google Scholar] [CrossRef]
- Balch, A.L.; Winkler, K. Two-Component Polymeric Materials of Fullerenes and the Transition Metal Complexes: A Bridge between Metal-Organic Frameworks and Conducting Polymers. Chem. Rev. 2016, 116, 3812–3882. [Google Scholar] [CrossRef]
- Zhan, S.Z.; Li, J.H.; Zhang, G.H.; Li, M.D.; Sun, S.; Zheng, J.; Ning, G.H.; Li, M.; Kuang, D.B.; Wang, X.D.; et al. Coordination disk-type nano-Saturn complexes. Chem. Commun. 2020, 56, 3325. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Y.; Blake, A.J.; Wilson, C.; Davies, E.S.; Khlobystov, A.N.; Schröder, M. Controlled Assembly of Silver(I)-Pyridylfullerene Networks. Angew. Chem. Int. Ed. 2007, 46, 8013–8016. [Google Scholar] [CrossRef]
- Peng, P.; Li, F.-F.; Neti, V.S.P.K.; Metta-Magana, A.J.; Echegoyen, L. Design, Synthesis, and X-ray Crystal Structure of a Fullerene-Linked Metal–Organic Framework. Angew. Chem. Int. Ed. 2014, 126, 164–167. [Google Scholar] [CrossRef]
- Andreas, K.; Patrick, R.; David, S.; Johannes, S.; Klaus, M.B.; Florian, B. Three-Dimensional Metal-Fullerene Frameworks. Chem. Eur. J. 2016, 22, 5982–5987. [Google Scholar] [CrossRef]
- Kraft, A.; Roger, C.; Schmidt, D.; Stangl, J.; Müller-Buschbaum, K.; Beuerle, F. Metal-Based Diversity for Crystalline Metal-Fullerene Frameworks. Chem. Eur. J. 2017, 23, 15864–15868. [Google Scholar] [CrossRef]
- Kraft, A.; Beuerle, F. Metal–organic hybrid architectures built from functionalized fullerenes and metal ions or clusters. Tetrahedron Lett. 2016, 57, 4651–4663. [Google Scholar] [CrossRef]
- Lebedeva, M.A.; Chamberlain, T.W.; Khlobystov, A.N. Harnessing the Synergistic and Complementary Properties of Fullerene and Transition-Metal Compounds for Nanomaterial Applications. Chem. Rev. 2015, 115, 11301–11351. [Google Scholar] [CrossRef]
- Lee, K.; Song, H.; Park, J.T. Fullerene-Metal Cluster Complexes: Novel Bonding Modes and Electronic Communication. Acc. Chem. Res. 2003, 36, 78. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Zeng, X.C. Designs of fullerene-based frameworks for hydrogen storage. J. Mater. Chem. A 2014, 2, 5910–5914. [Google Scholar] [CrossRef] [Green Version]
- Rio, Y.; Sánchez-García, D.; Seitz, W.; Torres, T.; Sessler, J.L.; Guldi, D.M. A Bisfullerene—Bis(dipyrrinato)zinc Complex: Electronic Coupling and Charge Separation in an Easy-to-Assemble Synthetic System. Chem. Eur. J. 2009, 15, 3956–3959. [Google Scholar] [CrossRef]
- Habicher, T.; Nierengarten, J.F.; Gramlich, V.; Diederich, F. PtII-Directed Self-Assembly of a Dinuclear Cyclophane Containing Two Fullerenes. Angew. Chem. Int. Ed. 1998, 37, 1916–1919. [Google Scholar] [CrossRef]
- Fu, K.; Henbest, K.; Zhang, Y.J.; Valentin, S.; Sun, Y.-P. Synthesis and optical properties of metal-centered dimeric fullerene macromolecules. J. Photochem. Photobiol. A Chem. 2002, 150, 143–152. [Google Scholar] [CrossRef]
- Zhou, Z.; Sarova, G.H.; Zhang, S.; Ou, Z.; Tat, F.T.; Kadish, K.M.; Echegoyen, L.; Guldi, D.M.; Schuster, D.I.; Wilson, S.R. Fullerene Polypyridine Ligands: Synthesis, Ruthenium Complexes, and Electrochemical and Photophysical Properties. Chem. A Eur. J. 2006, 12, 4241–4248. [Google Scholar] [CrossRef]
- Pierrat, P.; Réthoré, C.; Muller, T.; Bräse, S. Design and Efficient Synthesis of Fullerene Bismalonates as Building Blocks for Metal Organic Frameworks and Organic Nanostructures. Synlett 2008, 2008, 1706–1710. [Google Scholar]
- Rodríguez-Morgade, M.S.; Plonska-Brzezinska, M.E.; Athans, A.J.; Carbonell, E.; de Miguel, G.; Guldi, D.M.; Echegoyen, L.; Torres, T. Synthesis, Characterization, and Photoinduced Electron Transfer Processes of Orthogonal Ruthenium Phthalocyanine−Fullerene Assemblies. J. Am. Chem. Soc. 2009, 131, 10484–10496. [Google Scholar] [CrossRef]
- Castro, E.; Azmani, K.; Garcia, A.H.; Aghabali, A.; Liu, S.; Metta-Magana, A.J.; Olmstead, M.M.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Unusual C2h-Symmetric trans-1-(Bis-pyrrolidine)-tetra-malonate Hexa-Adducts of C60: The Unexpected Regio- and Stereocontrol Mediated by Malonate–Pyrrolidine Interaction. Chem. A Eur. J. 2017, 23, 15937–15944. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, C.J.; Olmstead, M.M.; Balch, A.L. Formation of Crystalline Polymers from the Reaction of Amine-Functionalized C60 with Silver Salts. Inorg. Chem. 2009, 48, 1339. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Li, F.F.; Bowles, F.L.; Neti, V.S.P.K.; Metta-Magana, A.J.; Olmstead, M.M.; Balch, A.L.; Echegoyen, L. High Yield Synthesis of a New Fullerene Linker and its Use in the Formation of a Linear Coordination Polymer by Silver Complexation. Chem. Commun. 2013, 49, 3209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Aghabali, A.; Suarez, C.; Olmstead, M.M.; Balch, A.L.; Echegoyen, L. Synthesis and Characterization of Bis-triruthenium Cluster Derivatives of an all Equatorial Fullerene Tetramalonate. Chem. Commun. 2015, 51, 6489. [Google Scholar] [CrossRef]
- Aghabali, A.; Jun, S.; Olmstead, M.M.; Balch, A.L. The directional character of the piperazine double addition product, e{N(CH2CH2)2N}2C60, as a building block for forming crystalline fullerene polymers. CrystEngComm 2018, 20, 924–929. [Google Scholar] [CrossRef]
- Crowley, D.C.; Lynch, D.; Maguire, A.R. Copper-Mediated, Heterogeneous, Enantioselective Intramolecular Buchner Reactions of α-Diazoketones Using Continuous Flow Processing. J. Org. Chem. 2018, 83, 3794–3805. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.-L.; Zhao, J.-S.; Gao, X.; Shi, Q.-Z.; He, S.-Y. Crystal structure, thermal analysis and theoretical calculation of a one-dimensional chain complex [Zn(dafo)2(H2O)2](NO3)2. Chin. J. Chem. 2004, 22, 837–840. [Google Scholar] [CrossRef]
- Kawano, S.-I.; Fukushima, T.; Tanaka, K. Specific and Oriented Encapsulation of Fullerene C70 into a Supramolecular Double-Decker Cage Composed of Shape-Persistent Macrocycles. Angew. Chem. Int. Ed. 2018, 130, 15043–15047. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Chakraborty, P.; Adhikary, J.; Ghosh, B.; Sanyal, R.; Chattopadhyay, S.K.; Bauzá, A.; Frontera, A.; Zangrando, E.; Das, D. Relation between the Catalytic Efficiency of the Synthetic Analogues of Catechol Oxidase with Their Electrochemical Property in the Free State and Substrate-Bound State. Inorg. Chem. 2014, 53, 8257–8269. [Google Scholar] [CrossRef]
- Shahbazi-Raz, F.; Notash, B.; Amani, V.; Safari, N. 4,4′-Dimethyl-2,2′-bithiazole: Potent co-former in coordination compounds. Polyhedron 2016, 119, 227–237. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yao, Y.-R.; Yang, S.; Zhou, X.; Yu, A.; Peng, P.; Li, F.-F. Diversity of Metal—Fullerene Framework Structures Regulated by Metal Salts. Nanomaterials 2022, 12, 1314. https://doi.org/10.3390/nano12081314
Wang J, Yao Y-R, Yang S, Zhou X, Yu A, Peng P, Li F-F. Diversity of Metal—Fullerene Framework Structures Regulated by Metal Salts. Nanomaterials. 2022; 12(8):1314. https://doi.org/10.3390/nano12081314
Chicago/Turabian StyleWang, Jingjing, Yang-Rong Yao, Shaoting Yang, Xinyi Zhou, Ao Yu, Ping Peng, and Fang-Fang Li. 2022. "Diversity of Metal—Fullerene Framework Structures Regulated by Metal Salts" Nanomaterials 12, no. 8: 1314. https://doi.org/10.3390/nano12081314
APA StyleWang, J., Yao, Y.-R., Yang, S., Zhou, X., Yu, A., Peng, P., & Li, F.-F. (2022). Diversity of Metal—Fullerene Framework Structures Regulated by Metal Salts. Nanomaterials, 12(8), 1314. https://doi.org/10.3390/nano12081314