[2+2] Cyclo-Addition Reactions for Efficient Polymerization on a HOPG Surface at Ambient Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 1,4-Bis(4′-vinylphenyl)-2,5-bis(octadecyloxy)benzene (Vinyl-OC18)
2.2. STM Experiments
3. Results
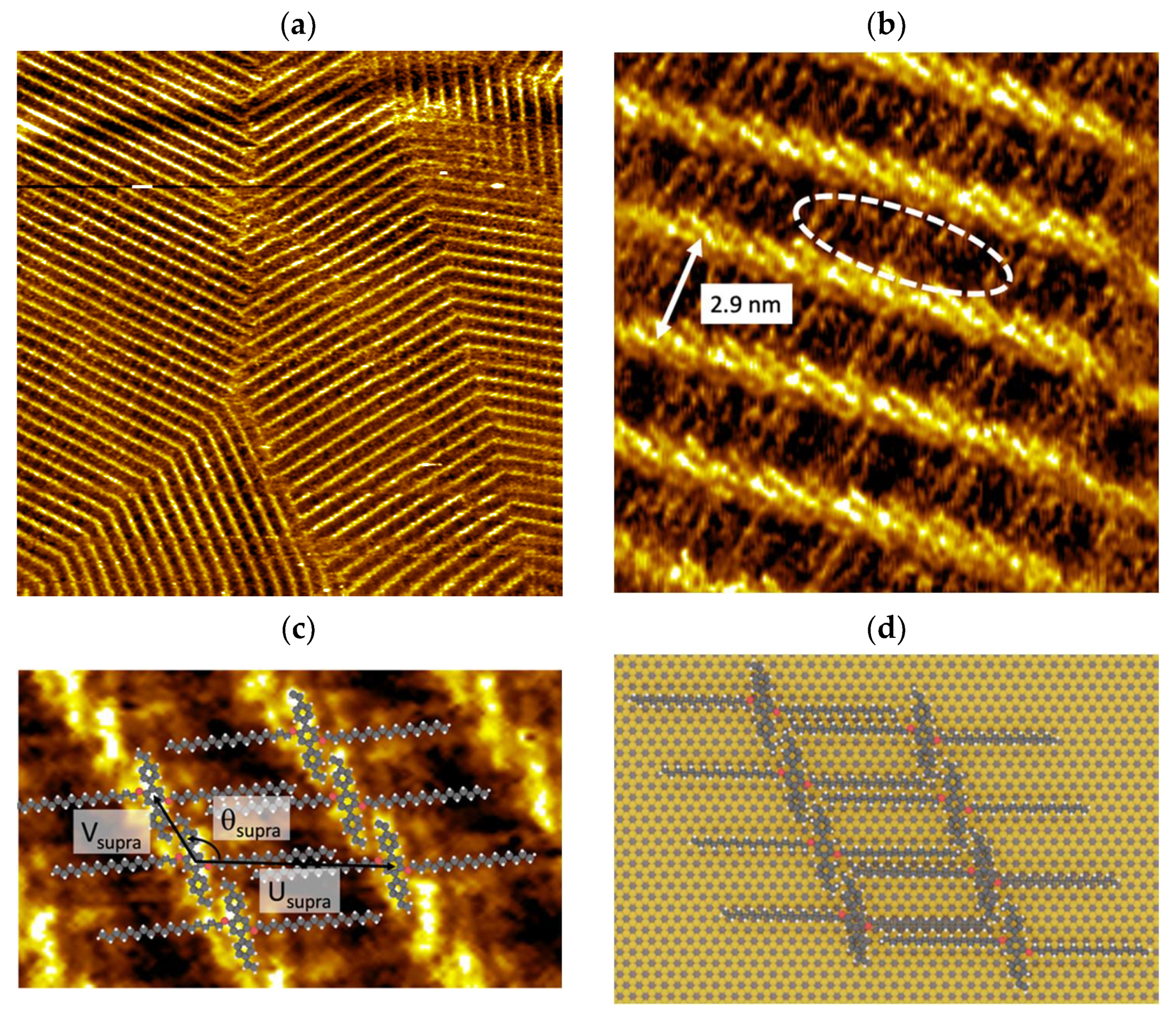
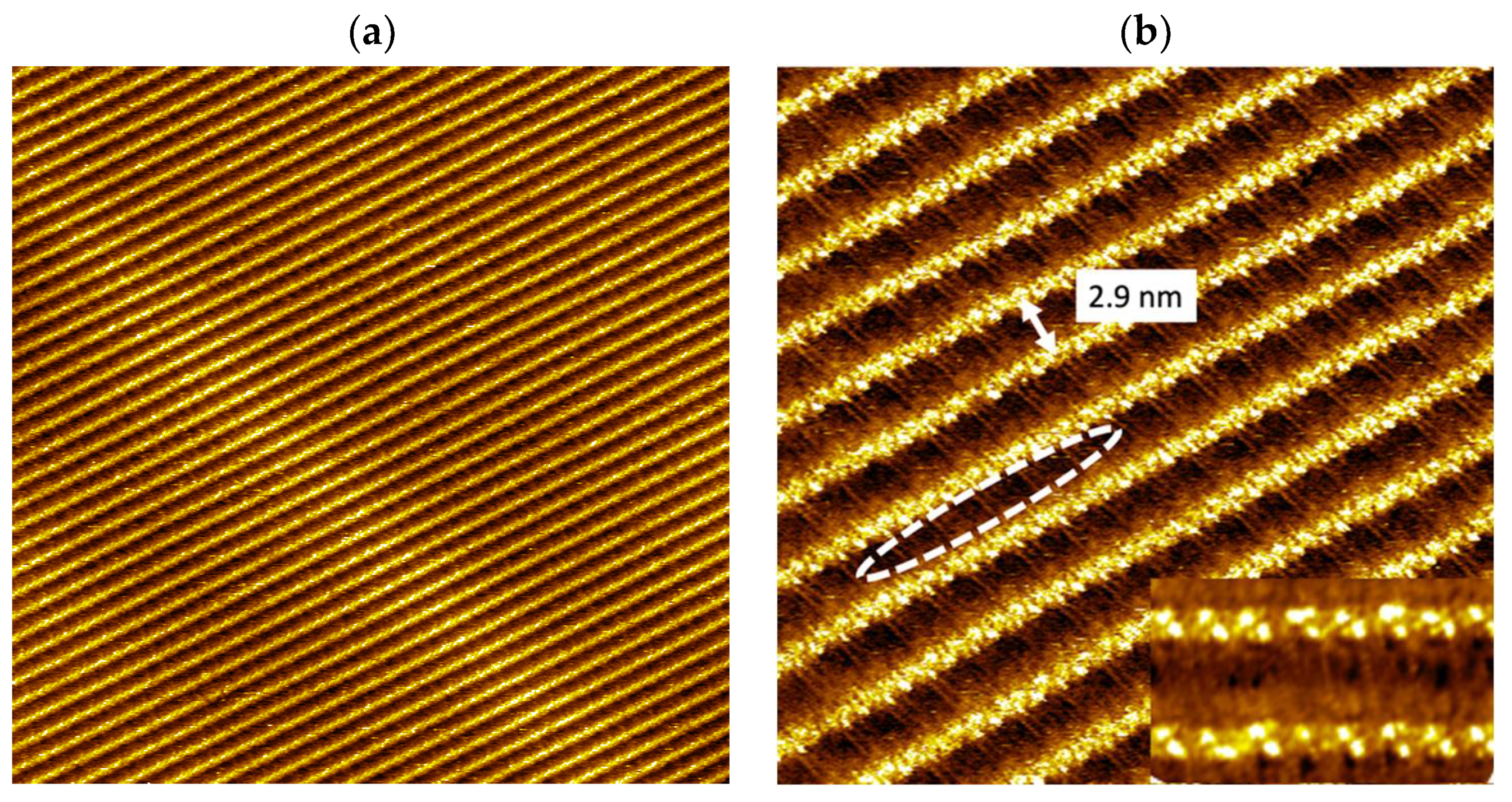
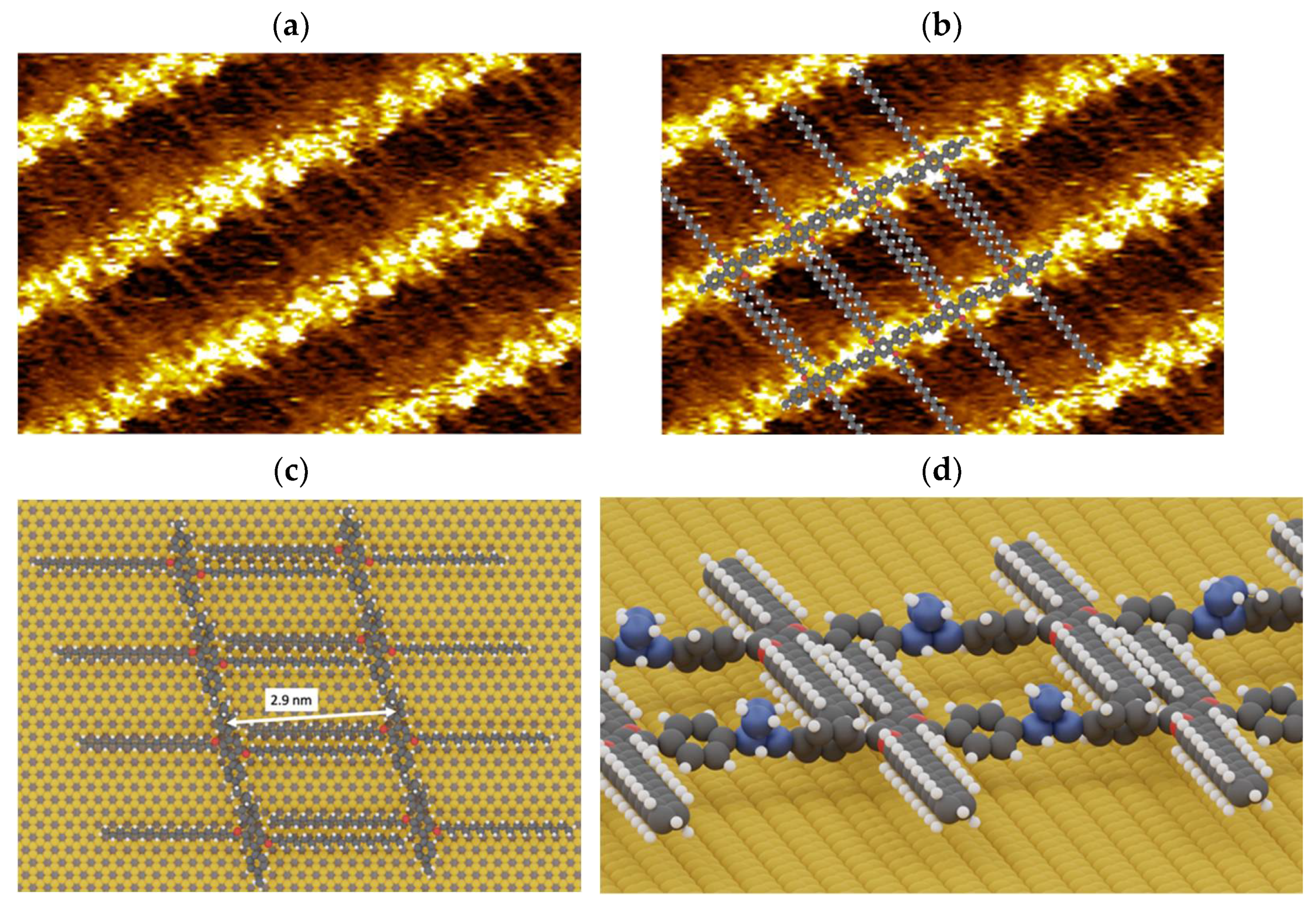
3.1. Supramolecular Self-Assembly on HOPG Surface
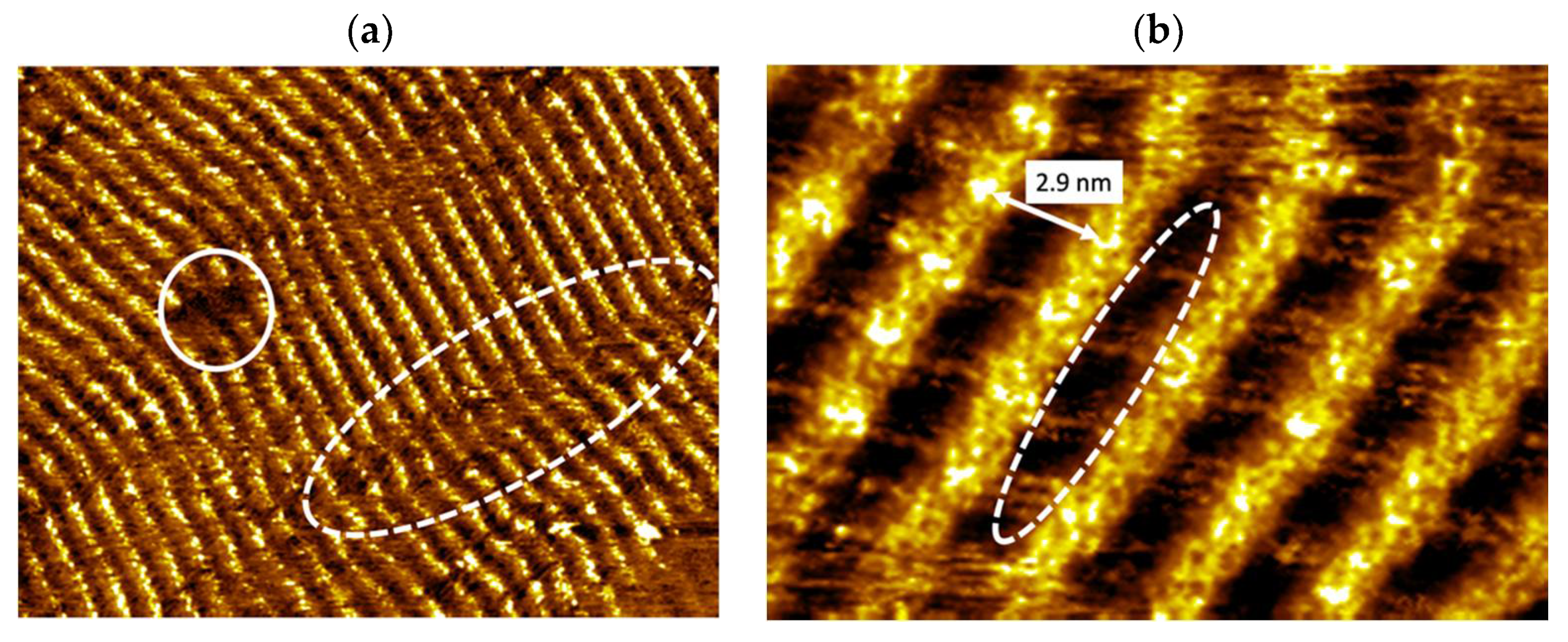
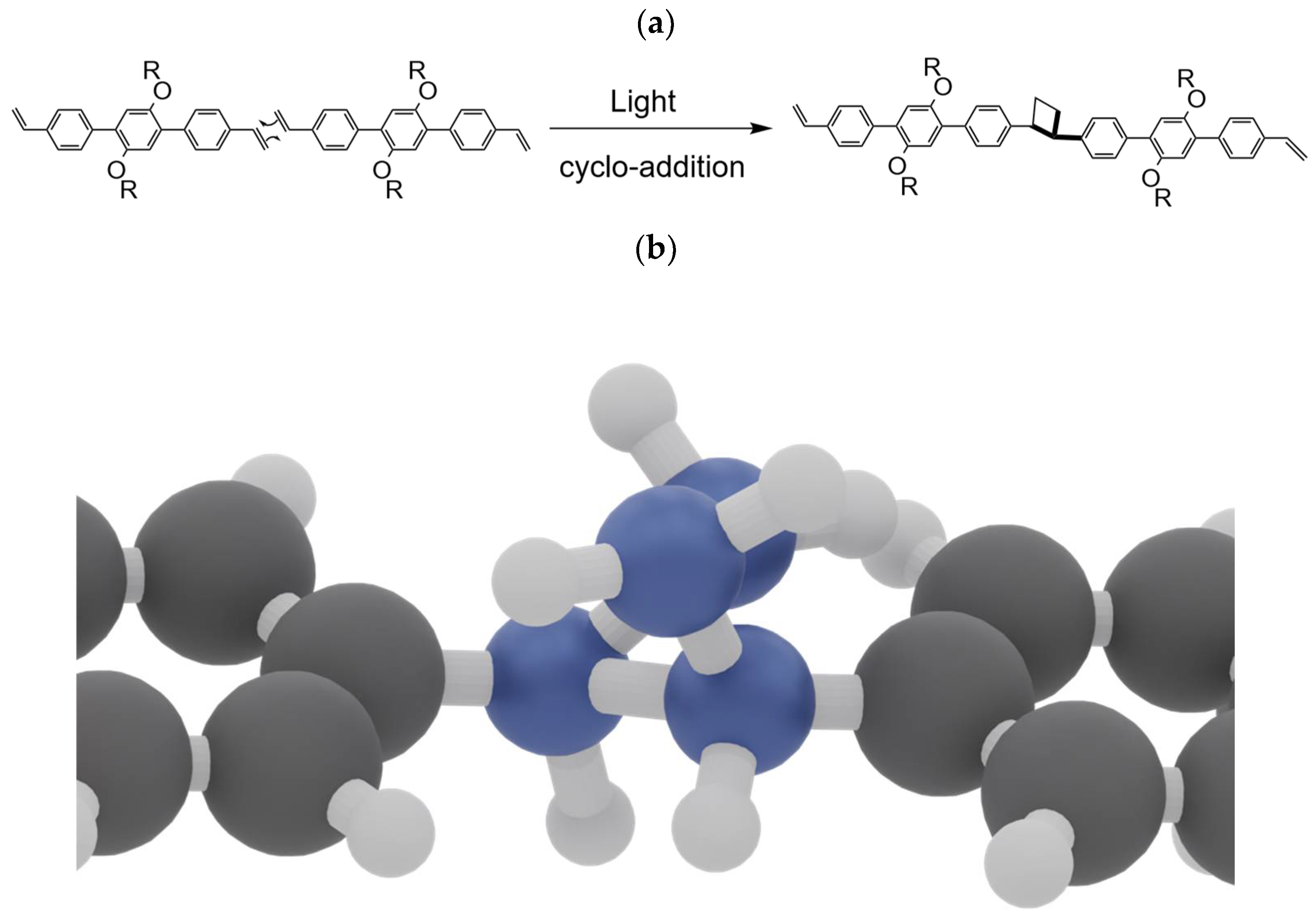
3.2. Illumination of Vinyl-OC18 Supramolecular Network on HOPG Surface by UV-Light
3.3. Annealing of Vinyl-OC18 Supramolecular Network on HOPG Surface
4. Discussion
- (1)
- The presence of HOPG surface alters the lifetimes and quantum yields of the photoexcited states of the UV-light cycloaddition reactions [47]
- (2)
- The thermal energy promotes the sliding of vinyl-OC18 molecules.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xing, L.; Peng, Z.; Li, W.; Wu, K. On Controllability and Applicability of Surface Molecular Self-Assemblies. Acc. Chem. Res. 2019, 52, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering Atomic and Molecular Nanostructures at Surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kudernac, T.; Lei, S.; Elemans, J.A.; De Feyter, S. Two-Dimensional Supramolecular Self-assembly: Nano-Porous Networks on Surfaces. Chem. Soc. Rev. 2009, 38, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Vargas, L.; Kim, E.; Attias, A.-J. Beyond “Decorative” 2D Supramolecular Self-Assembly: Strategies Towards Functional Surfaces for Nanotechnology. Mater. Horiz. 2017, 4, 570–583. [Google Scholar] [CrossRef]
- Makoudi, Y.; Jeannoutot, J.; Palmino, F.; Chérioux, F.; Copie, G.; Krzeminski, C.; Cléri, F.; Grandidier, B. Supramolecular self-assembly on the B-Si(111)-(√3x√3)R30° surface: From Single Molecules to Multicomponent Networks. Surf. Sci. Report 2017, 72, 316–349. [Google Scholar] [CrossRef]
- Koepf, M.; Chérioux, F.; Wytko, J.A.; Weiss, J. 1D and 3D Surface-Assisted Self-Organization. Coord. Chem. Rev. 2012, 256, 2872–2892. [Google Scholar] [CrossRef]
- Sun, X.; Yao, X.; Lafolet, F.; Lemercier, G.; Maurel, F.; Lacroix, J.-C. Molecular Isomerization and Multiscale Phase Transitions of a Ditopic Ligand on a Surface. J. Phys. Chem. C 2017, 121, 20925–20930. [Google Scholar] [CrossRef]
- Sun, X.; Frath, D.; Lafolet, F.; Lacroix, J.-C. Supramolecular Networks and Wires Dominated by Intermolecular BiEDOT Interactions. J. Phys. Chem. C 2018, 122, 22760–22766. [Google Scholar] [CrossRef]
- Hnid, I.; Sun, X.; Frath, D.; Lafolet, F.; Lacroix, J.-C. Multi-functional Switches of Ditopic lignads with Azobenzene Central Bridges at a Molecular Scale. Nanoscale 2019, 11, 23042–23048. [Google Scholar] [CrossRef]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.V.; Hecht, S. Nano-Architectures by Covalent Assembly of Molecular Building Blocks. Nat. Nanotechnol. 2007, 2, 687–691. [Google Scholar] [CrossRef]
- Cai, L.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; Mullen, K.; et al. Atomically Precise Bottom-Up Fabrication of Graphene Nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Matena, M.; Riehm, T.; Stöhr, M.; Jung, T.A.; Gade, L.H. Transforming Surface Coordination Polymers into Covalent Surface Polymers: Linked Polycondensed Aromatics through Oligomerization of N-Heterocyclic Carbene Intermediates. Angew. Chem. Int. Ed. 2008, 47, 2414–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grill, L.; Hecht, S. Covalent on-Surface Polymerization. Nat. Chem. 2020, 12, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Clair, S.; de Oteyza, D.G. Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis. Chem. Rev. 2019, 119, 4717–4776. [Google Scholar] [CrossRef] [PubMed]
- Held, P.A.; Fuchs, H.; Studer, A. Covalent-Bond Formation via On-Surface Chemistry. Chem. Eur. J. 2017, 23, 5874–5892. [Google Scholar] [CrossRef] [PubMed]
- Bartels, L. Tailoring Molecular Layers at Metal Surfaces. Nat. Chem. 2010, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lackinger, M. On-Surface Polymerization—A Versatile Synthetic Route to Two-Dimensional Polymers: On-surface Polymerization. Polym. Inter. 2015, 64, 1073–1078. [Google Scholar] [CrossRef]
- Geagea, E.; Jeannoutot, J.; Morgenthaler, L.; Lamare, S.; Rochefort, A.; Palmino, F.; Chérioux, F. Unravelling the Growth Mechanism of (3,1) Graphene Nanoribbons on a Cu(111) Surface. Chem. Commun. 2021, 57, 6043–6045. [Google Scholar] [CrossRef]
- Geagea, E.; Jeannoutot, J.; Féron, M.; Palmino, F.; Thomas, C.M.; Rochefort, A.; Chérioux, F. Collective Radical Oligomerisation Induced by STM Tip on a Silicon Surface. Nanoscale 2021, 13, 349–351. [Google Scholar] [CrossRef]
- Hla, S.-W.; Bartels, L.; Meyer, G.; Rieder, K.-H. Inducing All Steps of a Chemical Reaction with the Scanning Tunneling Microscope Tip: Towards Single Molecule Engineering. Phys. Rev. Lett. 2000, 85, 2777. [Google Scholar] [CrossRef]
- Okawa, Y.; Aono, M. Nanoscale Control of Chain Polymerization. Nature 2001, 409, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, D.; Elemans, J.A.A.W. Triggered Chemical Reactions by Scanning Tunneling Microscopy: From Atoms to Polymers. Eur. J. Polym. 2016, 83, 390–406. [Google Scholar] [CrossRef]
- Para, F.; Bocquet, F.; Nony, L.; Loppacher, C.; Féron, M.; Chérioux, F.; Gao, D.Z.; Canova, F.F.; Watkins, M.B. Micrometre-Long Covalent Organic Fibres by Photoinitiated Chain-Growth Radical Polymerization on an Alkali-Halide Surface. Nat. Chem. 2018, 10, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; King, B.T.; Reichlmaier, S.; Hartmann, N.; Rosen, J.; Heckl, W.M.; Bjork, J.; Lackinger, M. On-Surface Photopolymerization of Two-dimensional Polymers Ordered on the Mesoscale. Nat. Chem. 2021, 13, 730–736. [Google Scholar] [CrossRef]
- Palmino, F.; Loppacher, C.; Chérioux, F. Photochemistry Highlights on On-Surface Synthesis. ChemPhysChem 2019, 20, 2271–2280. [Google Scholar] [CrossRef]
- Basagni, A.; Ferrighi, L.; Cattelan, M.; Nicolas, L.; Handrup, K.; Vaghi, L.; Papagni, A.; Sedona, F.; Di Valentin, C.; Agnoli, S.; et al. On-surface photo-dissociation of C–Br bonds: Towards room temperature Ullmann coupling. Chem. Commun. 2015, 51, 12593–12596. [Google Scholar] [CrossRef]
- Deshpande, A.; Sham, C.H.; Alaboson, J.M.P.; Mullin, J.M.; Schatz, G.C.; Hersam, M.C. Self-Assembly and Photopolymerization of Sub-2 nm One-Dimensional Organic Nanostructures on Graphene. J. Am. Chem. Soc. 2012, 123, 16759–16764. [Google Scholar] [CrossRef]
- Miura, A.A.; De Feyter, S.; Abdel-Mottaleb, M.M.S.; Gesquière, A.; Grim, P.C.M.; Moessner, G.; Sieffert, M.; Klapper, M.; Müllen, K.; De Schryver, F.C. Light- and STM-Tip-Induced Formation of One-Dimensional and Two-Dimensional Organic Nanostructures. Langmuir 2003, 19, 6474–6482. [Google Scholar] [CrossRef]
- Sun, X.; Yao, X.; Trippé-Allard, G.; Lacroix, J.-C. On-Surface Dimerization and Coordination of 4-(Bis-ethylenedioxythiophene)benzoic Acid. J. Phys. Chem. C 2021, 125, 957–963. [Google Scholar] [CrossRef]
- Sun, X.; Yao, X.; Lafolet, F.; Lemercier, G.; Lacroix, J.-C. One-Dimensional Double Wires and Two-Dimensional Mobile Grids: Cobalt/Bipyridine Coordination Networks at the Solid/Liquid Interface. J. Phys. Chem. Lett. 2019, 10, 4164–4169. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Sun, X.; Lafolet, F.; Audibert, J.-F.; Miomandre, F.; Lemercier, G.; Loiseau, F.; Lacroix, J.-C. Unprecedented Self-Organized Monolayer of a Ru(II) Complex by Diazonium Electroreduction. J. Am. Chem. Soc. 2016, 138, 9381–9384. [Google Scholar] [CrossRef] [PubMed]
- A Cyclo-Addition Reaction Is a “Reaction in Which Two or More Unsaturated Molecules (or Parts of the Same Molecule) Combine with the Formation of a Cyclic Adduct in Which There Is a Net Reduction of the Bond Multiplicity. A Cycloadduct Is a New Chemical Species AB, Each Molecular Entity of Which Is Formed by Direct Combination of Two Separate Molecular Entities A and B in Such a Way That There Is Change in Connectivity, but No Loss, of Atoms within the Moieties A and B.” IUPAC. Available online: https://goldbook.iupac.org/terms/view/C01496 (accessed on 11 April 2022).
- Zhang, C.; Kazuma, E.; Kim, Y. Atomic-Scale Visualization of the Stepwise Metal-Mediated Dehalogenative Cycloaddition Reaction Pathways: Competition between Radicals and Organometallic Intermediates. Angew. Chem. Int. Ed. 2019, 58, 17736–17744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xia, B.; Xu, H.; Lin, N. Identifying Multinuclear Organometallic Intermediates in On-Surface [2+2] Cycloaddition Reactions. Angew. Chem. Int. Ed. 2019, 58, 16485–16489. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Y.; Qiu, X.; Li, S.-W.; Ren, Y.-T.; Zhu, Y.-C.; Shu, C.-H.; Hou, X.-Y.; Liu, M.; Shi, X.-Q.; Qiu, X.; et al. Ladder Phenylenes Synthesized on Au(111) Surface via Selective [2+2] Cycloaddition. J. Am. Chem. Soc. 2021, 143, 12955–12960. [Google Scholar] [CrossRef]
- Hoffmann, R.; Woodward, R.B. Selection Rules for Concerted Cycloaddition Recations. J. Am. Chem. Soc. 1965, 87, 2046–2048. [Google Scholar] [CrossRef]
- Garcia, A.L.L.; Carpes, M.J.S.; De Oca, A.C.B.M.; Dos Santos, A.A.G.; Santan, C.C.; Correira, C.R.D. Synthesis of 4-Aryl-2-pyrrolidones and β-Aryl-γ-amino-butyric Acid (GABA) Analogues by Heck Arylation of 3-Pyrrolines with Arenediazonium Tetrafluoroborates. Synthesis of (±)-Rolipram on a Multigram Scale and Chromatographic Resolution by Semipreparative Chiral Simulated Moving Bed Chromatography. J. Org. Chem. 2005, 70, 10383–10394. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Silly, F. A robust method for processing scanning probe microscopy images and determining nanoobject position and dimensions. J. Micros 2009, 236, 211–218. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W.; Lei, S.; De Feyter, S. Molecular and Supramolecular Networks on Surfaces: From Two-Dimensional Crystal Engineering to Reactivity. Angew. Chem. Int. Ed. 2009, 48, 1050–1053. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, L.; Ding, Y.; Xie, L.; Zhang, C.; Tan, Q.; Xu, W. Dehydrogenative Homocoupling of Terminal Alkenes on Copper Surfaces: A Route to Dienes. Angew. Chem. Int. Ed. 2015, 54, 4549–4552. [Google Scholar] [CrossRef]
- Sicignano, M.; Rodriguez, R.I.; Aleman, J. Recent Visible Light and Metal Free Strategies in [2+2] and [4+2] Photocycloadditions. Eur. J. Org. Chem. 2021, 22, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, R.J.; Rawn, J.D. Organic Chemistry—Structure, Mechanism, Synthesis, 2nd ed.; Academic Press: San Diego, CA, USA, 2018; pp. 87–133. [Google Scholar]
- Brückner, R. Advanced Organic Chemistry; Academic Press: San Diego, CA, USA, 2002; pp. 477–486. [Google Scholar]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. Exploiting [2+2] cycloaddition chemistry: Achievements with allenes. Chem. Soc. Rev. 2010, 39, 783–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchatcher, R.J.; Newby, T.E.; Price, P.; Loyns, C.; Chechik, V. A new intermediate in spontaneous styrene polymerization inhibited by 2-nitrophenol. RSC Adv. 2014, 4, 35131–35136. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
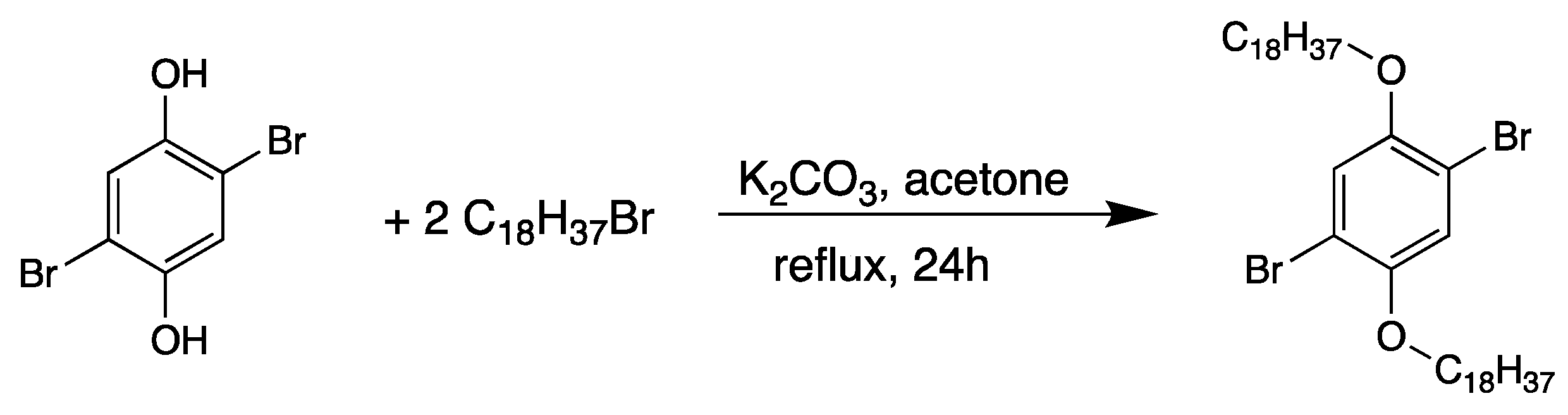




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, L.; Palmino, F.; Lacroix, J.-C.; Chérioux, F.; Sun, X. [2+2] Cyclo-Addition Reactions for Efficient Polymerization on a HOPG Surface at Ambient Conditions. Nanomaterials 2022, 12, 1334. https://doi.org/10.3390/nano12081334
Guan L, Palmino F, Lacroix J-C, Chérioux F, Sun X. [2+2] Cyclo-Addition Reactions for Efficient Polymerization on a HOPG Surface at Ambient Conditions. Nanomaterials. 2022; 12(8):1334. https://doi.org/10.3390/nano12081334
Chicago/Turabian StyleGuan, Lihao, Frank Palmino, Jean-Christophe Lacroix, Frédéric Chérioux, and Xiaonan Sun. 2022. "[2+2] Cyclo-Addition Reactions for Efficient Polymerization on a HOPG Surface at Ambient Conditions" Nanomaterials 12, no. 8: 1334. https://doi.org/10.3390/nano12081334
APA StyleGuan, L., Palmino, F., Lacroix, J.-C., Chérioux, F., & Sun, X. (2022). [2+2] Cyclo-Addition Reactions for Efficient Polymerization on a HOPG Surface at Ambient Conditions. Nanomaterials, 12(8), 1334. https://doi.org/10.3390/nano12081334






