An Effective Strategy for Template-Free Electrodeposition of Aluminum Nanowires with Highly Controllable Irregular Morphologies
Abstract
:1. Introduction
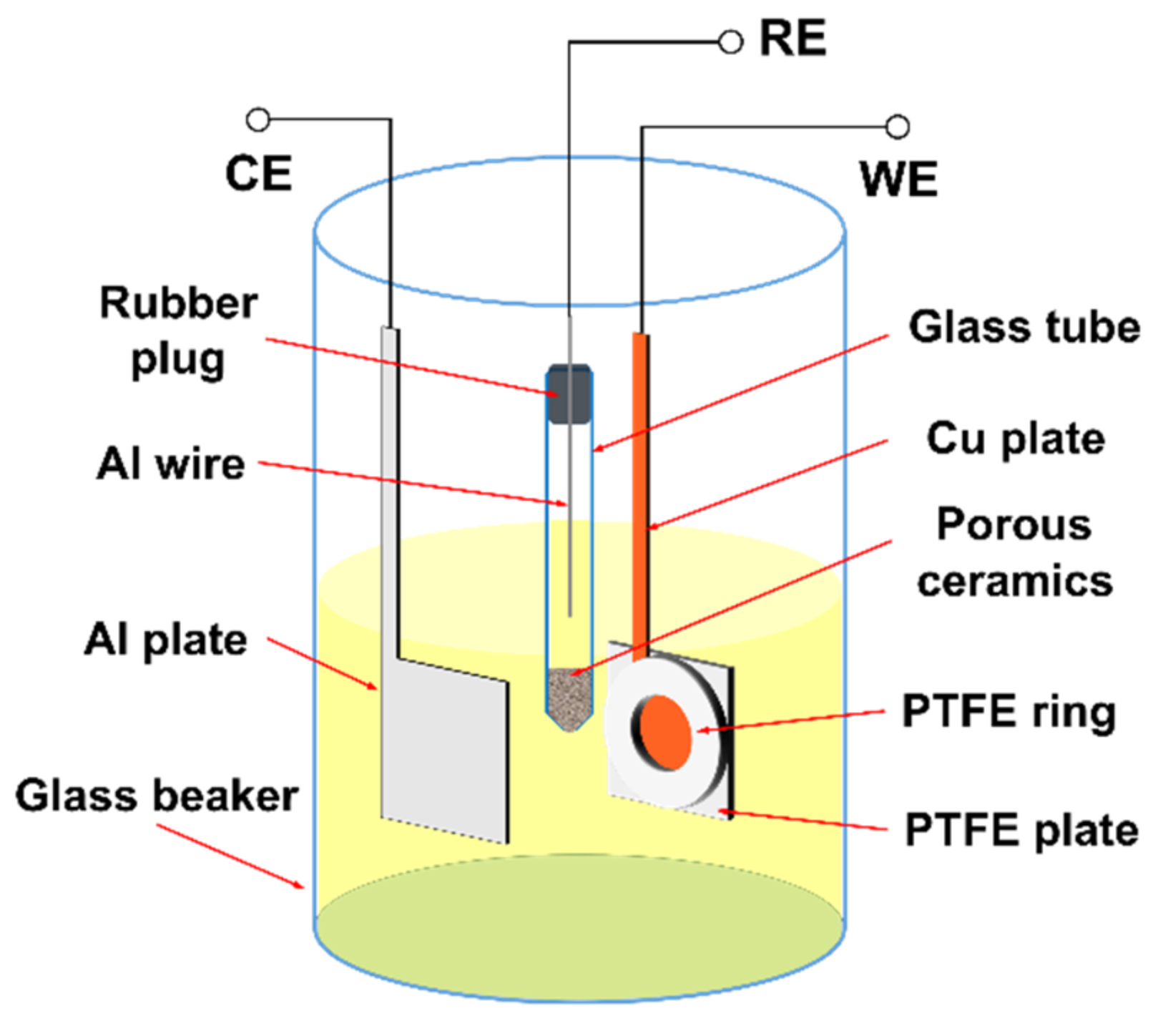
2. Materials and Methods
2.1. Materials and Synthesis of Al Nanowires
2.2. Characterization Techniques
3. Results and Discussion
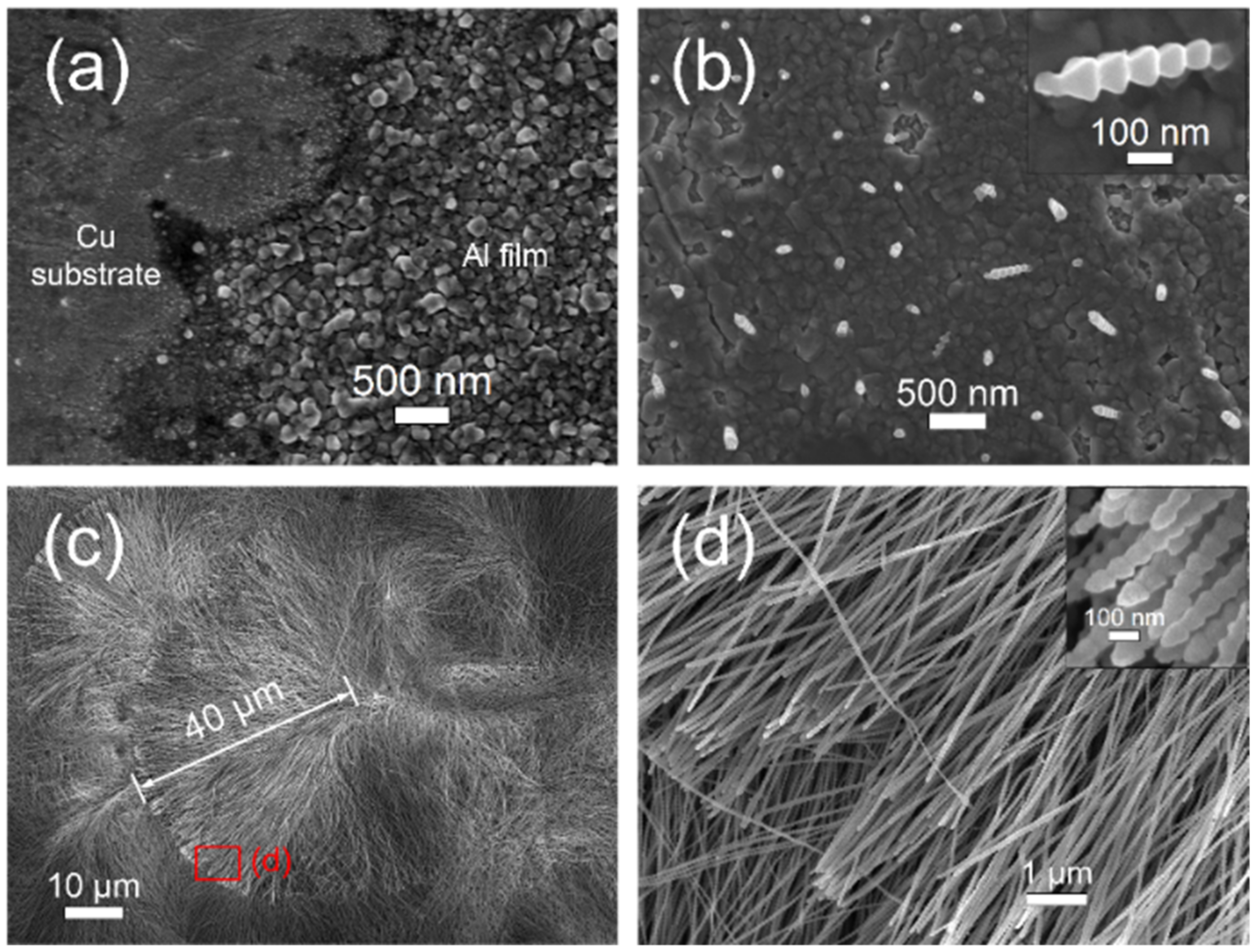
3.1. Electrodeposition in the Unrestricted Diffusion Condition
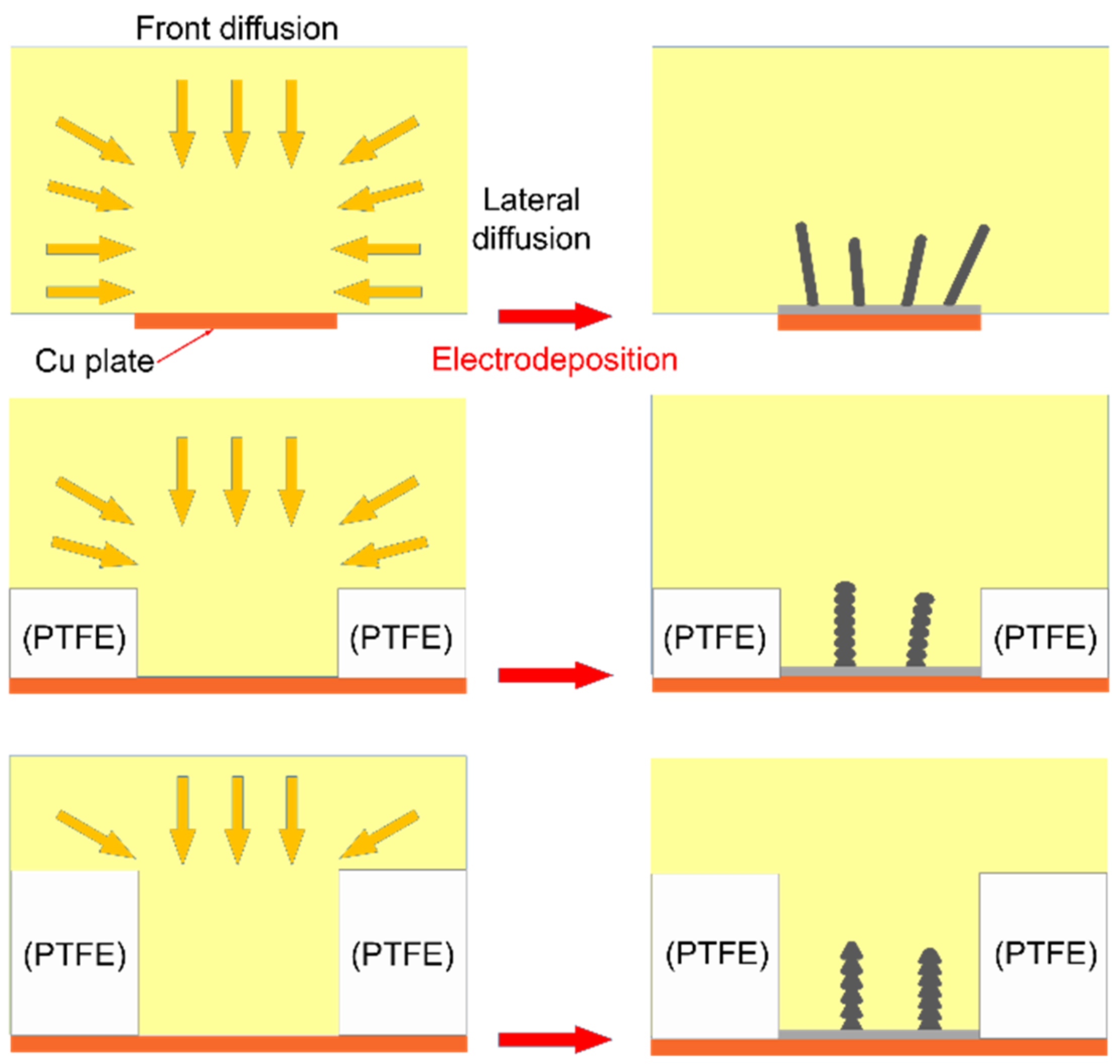
3.2. Electrodeposition in the Restricted Diffusion Condition
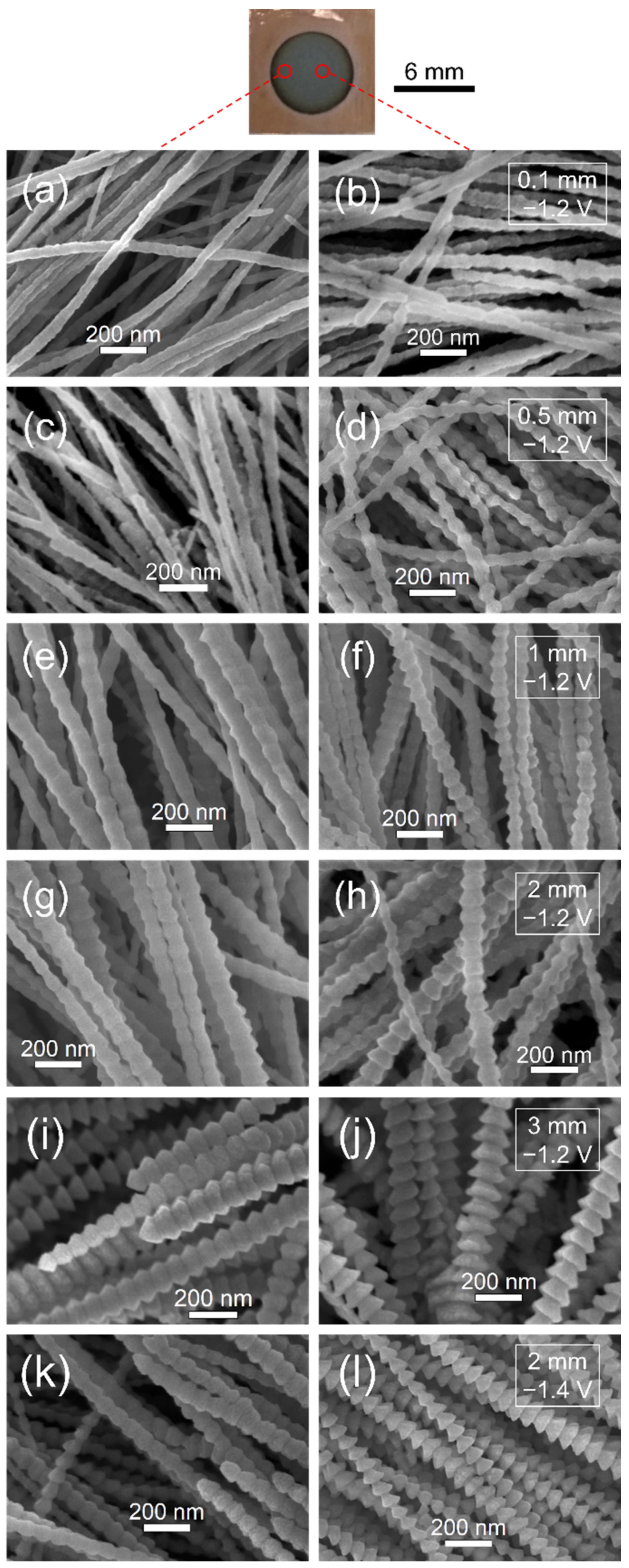
3.3. Effect of Insulation Ring Size
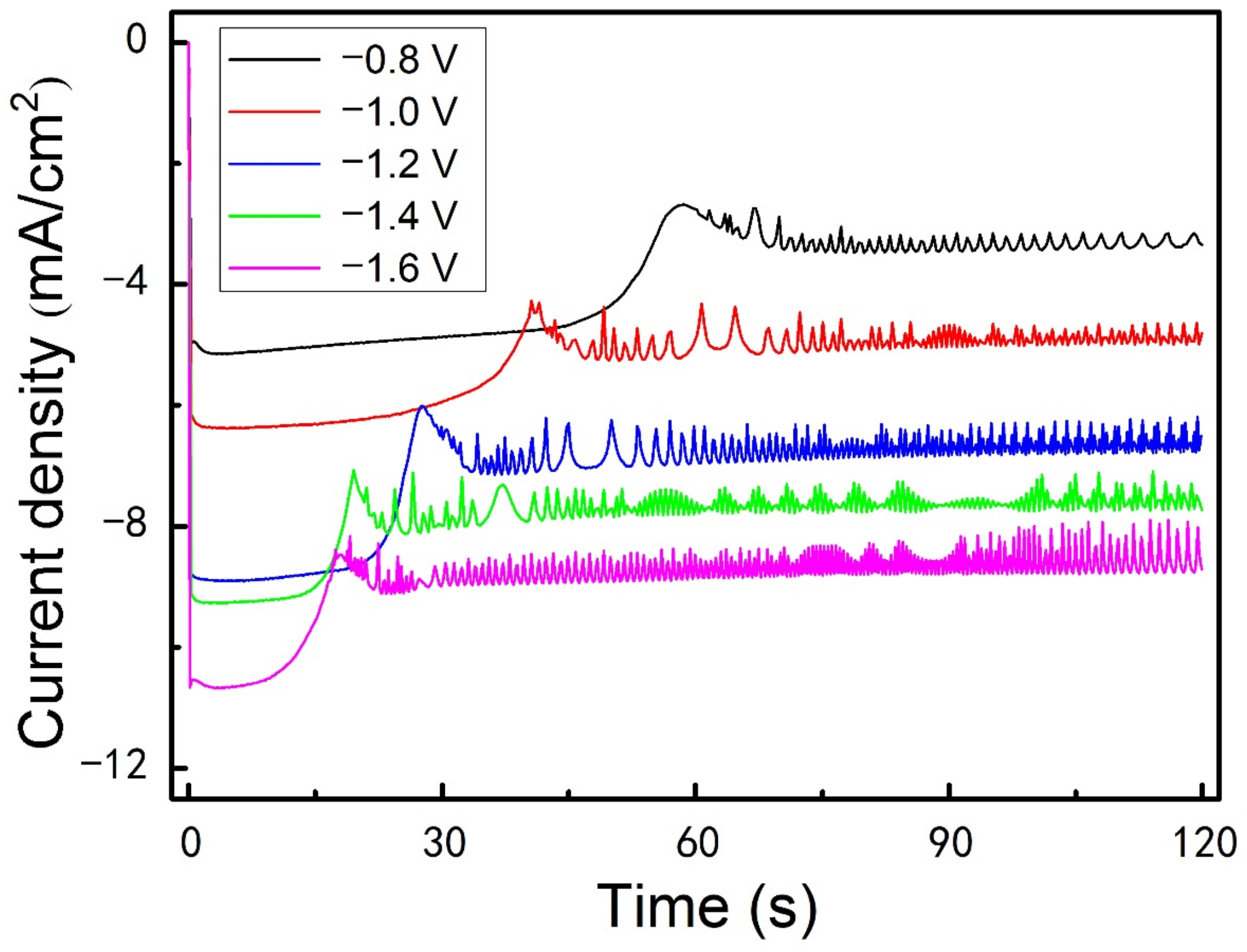
3.4. Effect of Overpotential
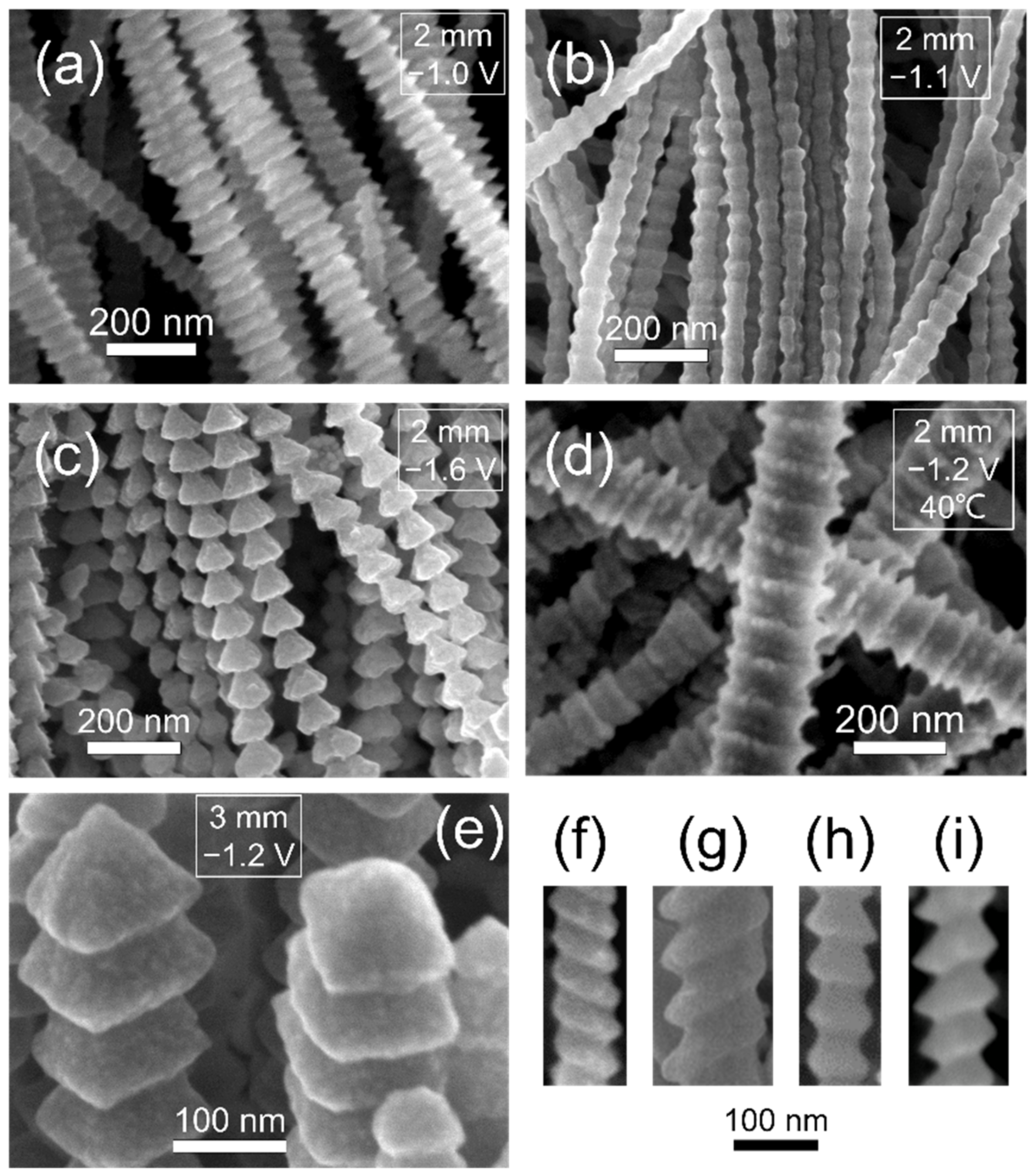
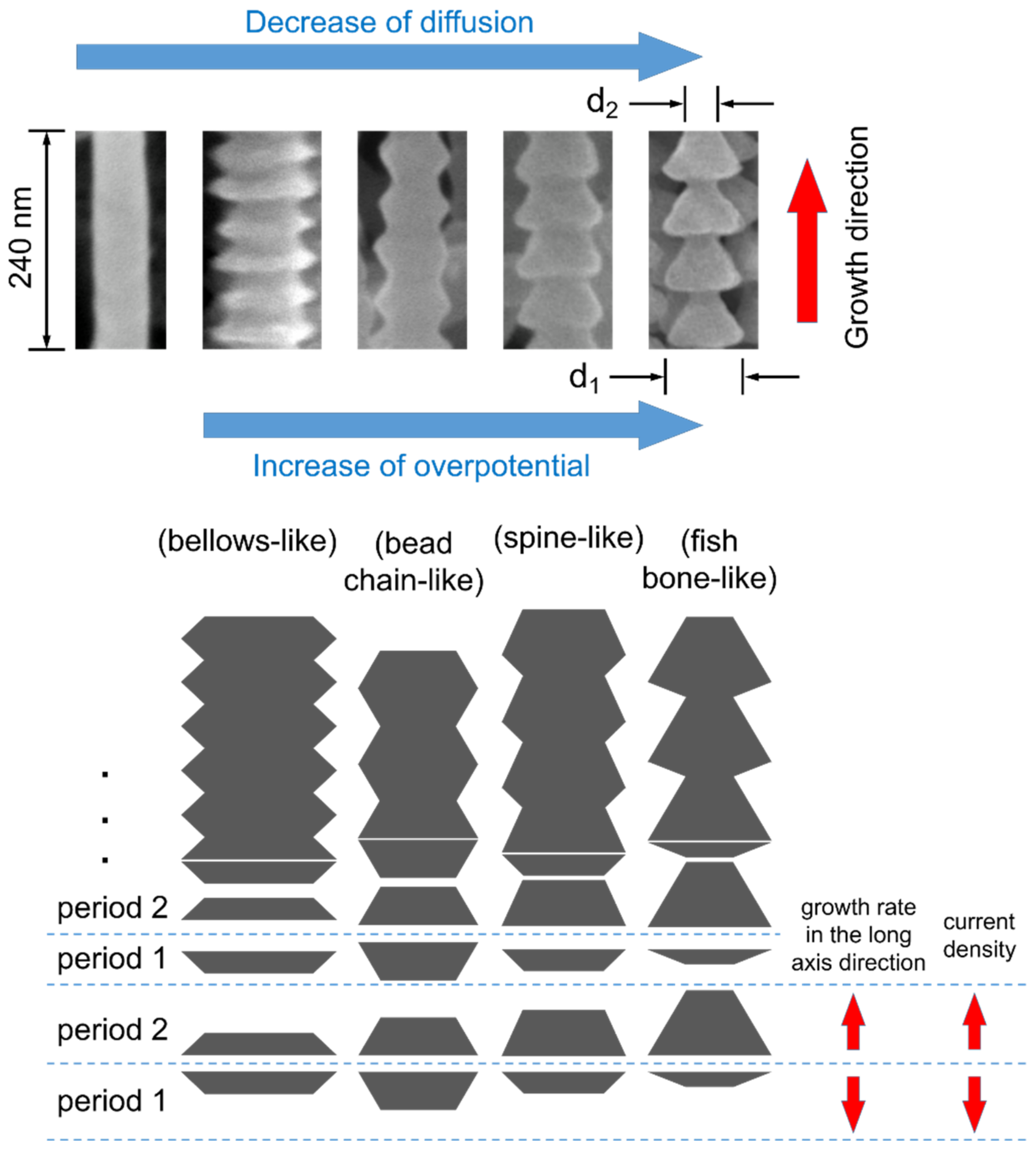
3.5. Typical Morphologies
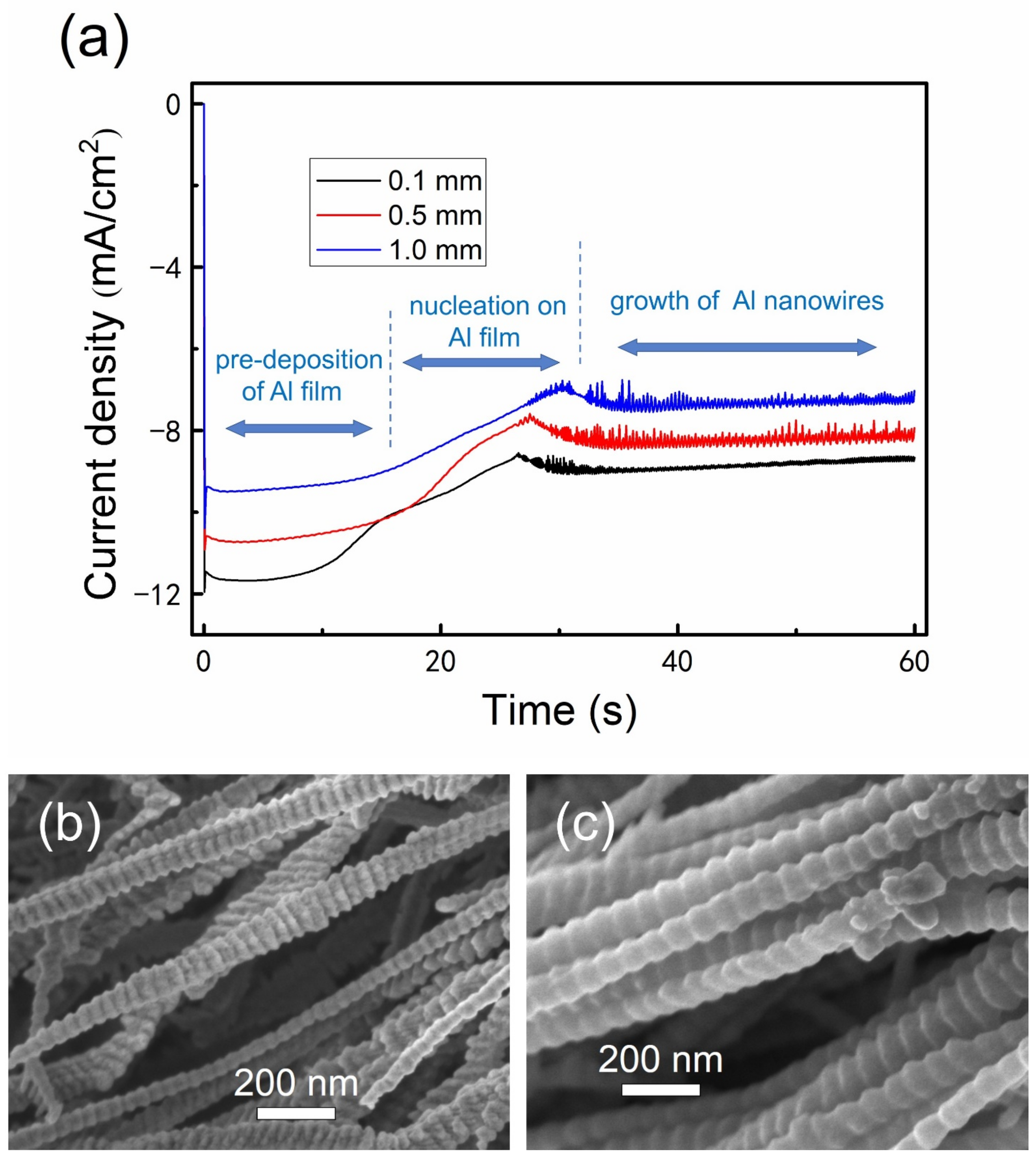
3.6. Growth Process
3.7. Growth Orientation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Lv, F.; Lei, B.; Wan, S.; Luo, M.; Guo, S. Tuning Nanowires and Nanotubes for Efficient Fuel-Cell Electrocatalysis. Adv. Mater. 2016, 28, 10117–10141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, L.; Chang, R.; Du, L.; Zhu, C.; Geng, D.; Yang, D. Palladium-Cobalt Nanowires Decorated with Jagged Appearance for Efficient Methanol Electro-Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 29965–29971. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Jiang, G.; Cano, Z.P.; Ma, Z.; Chen, Z. Spontaneous weaving: 3D porous PtCu networks with ultrathin jagged nanowires for highly efficient oxygen reduction reaction. Appl. Catal. B-Environ. 2018, 236, 359–367. [Google Scholar] [CrossRef]
- Chao, T.; Zhang, Y.; Hu, Y.; Zheng, X.; Qu, Y.; Xu, Q.; Hong, X. Atomically Dispersed Pt on Screw-like Pd/Au Core-shell Nanowires for Enhanced Electrocatalysis. Chem.-A Eur. J. 2019, 26, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, C.; Wu, Z.; Han, W.; Wang, Y.; Fu, W.; Li, X.; Xie, E. Toward efficient photoelectrochemical water-splitting by using screw-like SnO2 nanostructures as photoanode after being decorated with CdS quantum dots. Nano Energy 2016, 19, 318–327. [Google Scholar] [CrossRef]
- Van Duy, N.; Hoa, N.D.; Van Hieu, N. Effective hydrogen gas nanosensor based on bead-like nanowires of platinum-decorated tin oxide. Sens. Actuators B Chem. 2012, 173, 211–217. [Google Scholar] [CrossRef]
- Jia, C.; Chen, H.S.; Yang, P. Selective growth of TiO2 beads on Ag nanowires and their photocatalytic performance. CrystEngComm 2015, 17, 4895–4902. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.; Wang, E. Facile synthesis of highly active PdAu nanowire networks as self-supported electrocatalyst for ethanol electrooxidation. ACS Appl. Mater. Interfaces 2014, 6, 9481–9487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Shen, W.J.; Kuang, W.T.; Guo, S.; Li, Y.J.; Wang, Z.H. Serrated Au/Pd Core/Shell Nanowires with Jagged Edges for Boosting Liquid Fuel Electrooxidation. ChemSusChem 2017, 10, 2375–2379. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Li, L.; Jia, Q.; Yang, Y.; Cui, H. Facile synthesis of jagged Au/Ir nanochains with superior electrocatalytic activity for oxygen evolution reaction. Appl. Surf. Sci. 2019, 463, 58–65. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Su, Y.; Hu, J.; Yang, Z.; Zhang, Y. Synthesis of CuInS2 nanowire arrays via solution transformation of Cu2S self-template for enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2017, 203, 715–724. [Google Scholar] [CrossRef]
- Kim, B.Y.; Yu, S.H.; Kim, H.S.; Lee, D.C.; Shim, I.B.; Derosa, S.E.; Sung, Y.E.; Pyun, J. Morphological conversion of dipolar core-shell Au-Co nanoparticles into beaded Au-Co3O4 nanowires. J. Mater. Chem. 2011, 21, 14163–14166. [Google Scholar] [CrossRef]
- Qian, S.; Yu, H.; Cheng, X.; Zheng, R.; Zhu, H.; Liu, T.; Shui, M.; Xie, Y.; Shu, J. Rapid and durable electrochemical storage behavior enabled by V4Nb18O55 beaded nanofibers: A joint theoretical and experimental study. J. Mater. Chem. A 2018, 6, 17389–17400. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Wang, J.; Che, Q.; Wang, G.; Yang, P. Hierarchical WO3/ZnWO4 1D fibrous heterostructures with tunable in-situ growth of WO3 nanoparticles on surface for efficient low concentration HCHO detection. Sens. Actuators B Chem. 2019, 286, 564–574. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, N.; Deng, S.; Zhang, Y.; Liu, F.; Chen, J. A Mo nanoscrew formed by crystalline Mo grains with high conductivity and excellent field emission properties. Nanoscale 2014, 6, 4659–4668. [Google Scholar] [CrossRef]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef]
- Mukherjee, B.; Varghese, B.; Zheng, M.; Karthik, K.R.G.; Mathews, N.; Mhaisalkar, S.G.; Tok, E.S.; Sow, C.H. Synthesis, characterization and electrical properties of hybrid Zn2GeO4-ZnO beaded nanowire arrays. J. Cryst. Growth 2012, 346, 32–39. [Google Scholar] [CrossRef]
- Filippo, E.; Micocci, G.; Tepore, A.; Siciliano, T. Fabrication of α-TeO2 smooth and beaded microwires by thermal evaporation method. J. Cryst. Growth 2011, 336, 101–105. [Google Scholar] [CrossRef]
- Hafeez, M.; Zhai, T.; Bhatti, A.S.; Bando, Y.; Golberg, D. Oxygen Vacancy Driven Modulations in In2O3 Pyramidal Beaded Nanowires. Cryst. Growth Des. 2012, 12, 4935–4943. [Google Scholar] [CrossRef]
- Koh, T.L.; Ohara, E.C.; Gordon, M.J. Microplasma-based synthesis of vertically aligned metal oxide nanostructures. Nanotechnology 2012, 23, 425603. [Google Scholar] [CrossRef]
- Kolathodi, M.S.; Hanumantha Rao, S.N.; Natarajan, T.S.; Singh, G. Beaded manganese oxide (Mn2O3) nanofibers: Preparation and application for capacitive energy storage. J. Mater. Chem. A 2016, 4, 7883–7891. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Ma, Q.; Wang, J.; Che, Q.; Wang, G.; Tan, Y.; Yang, P. The effect of ZIF-8 on the phase structure and morphology of bead-like CuMn2O4/ZnO photocatalystic electrospun nanofibers. Mater. Lett. 2018, 216, 199–202. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, J.; Li, B.; Li, G.; Zheng, H.; Men, J.; Hou, X. Tunable fabrication and photoluminescence property of SiC nanowires with different microstructures. Appl. Surf. Sci. 2020, 506, 144979. [Google Scholar] [CrossRef]
- Singh, H.; Laibinis, P.E.; Hatton, T.A. Synthesis of flexible magnetic nanowires of permanently linked core-shell magnetic beads tethered to a glass surface patterned by microcontact printing. Nano Lett. 2005, 5, 2149–2154. [Google Scholar] [CrossRef]
- Tan, R.L.S.; Chong, W.H.; Feng, Y.; Song, X.; Tham, C.L.; Wei, J.; Lin, M.; Chen, H. Nanoscrews: Asymmetrical Etching of Silver Nanowires. J. Am. Chem. Soc. 2016, 138, 10770–10773. [Google Scholar] [CrossRef]
- Bu, L.; Ding, J.; Guo, S.; Zhang, X.; Su, D.; Zhu, X.; Yao, J.; Guo, J.; Lu, G.; Huang, X. A General Method for Multimetallic Platinum Alloy Nanowires as Highly Active and Stable Oxygen Reduction Catalysts. Adv. Mater. 2015, 27, 7204–7212. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Bu, L.; Guo, S.; Guo, J.; Huang, X. Screw Thread-Like Platinum-Copper Nanowires Bounded with High-Index Facets for Efficient Electrocatalysis. Nano Lett. 2016, 16, 5037–5043. [Google Scholar] [CrossRef]
- Jiang, K.; Shao, Q.; Zhao, D.; Bu, L.; Guo, J.; Huang, X. Phase and Composition Tuning of 1D Platinum-Nickel Nanostructures for Highly Efficient Electrocatalysis. Adv. Funct. Mater. 2017, 27, 1700830. [Google Scholar] [CrossRef]
- Luo, M.; Sun, Y.; Zhang, X.; Qin, Y.; Li, M.; Li, Y.; Li, C.; Yang, Y.; Wang, L.; Gao, P.; et al. Stable High-Index Faceted Pt Skin on Zigzag-Like PtFe Nanowires Enhances Oxygen Reduction Catalysis. Adv. Mater. 2018, 30, 1705515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, T.; Goddard, W.A., III. Atomistic Explanation of the Dramatically Improved Oxygen Reduction Reaction of Jagged Platinum Nanowires, 50 Times Better than Pt. J. Am. Chem. Soc. 2020, 142, 8625–8632. [Google Scholar] [CrossRef] [PubMed]
- Tishkevich, D.; Vorobjova, A.; Shimanovich, D.; Kaniukov, E.; Kozlovskiy, A.; Zdorovets, M.; Vinnik, D.; Turutin, A.; Kubasov, I.; Kislyuk, A.; et al. Magnetic properties of the densely packed ultra-long Ni nanowires encapsulated in alumina membrane. Nanomaterials 2021, 11, 1175. [Google Scholar] [CrossRef]
- Thomas, S.; Mallet, J.; Bahuleyan, B.K.; Molinari, M. Growth of homogeneous luminescent silicon-terbium nanowires by one-step electrodeposition in ionic liquids. Nanomaterials 2020, 10, 2390. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D.; Brzózka, A.; Liu, L. Fabrication of diameter-modulated and ultrathin porous nanowires in anodic aluminum oxide templates. Electrochim. Acta 2011, 56, 4972–4979. [Google Scholar] [CrossRef]
- Rodríguez, L.A.; Bran, C.; Reyes, D.; Berganza, E.; Vázquez, M.; Gatel, C.; Snoeck, E.; Asenjo, A. Quantitative Nanoscale Magnetic Study of Isolated Diameter-Modulated FeCoCu Nanowires. ACS Nano 2016, 10, 9669–9678. [Google Scholar] [CrossRef]
- Oázkale, B.; Shamsudhin, N.; Chatzipirpiridis, G.; Hoop, M.; Gramm, F.; Chen, X.; Martí, X.; Sort, J.; Pellicer, E.; Pané, S. Multisegmented FeCo/Cu nanowires: Electrosynthesis, characterization, and magnetic control of biomolecule desorption. ACS Appl. Mater. Interfaces 2015, 7, 7389–7396. [Google Scholar] [CrossRef] [Green Version]
- Reyes, D.; Biziere, N.; Warot-Fonrose, B.; Wade, T.; Gatel, C. Magnetic Configurations in Co/Cu Multilayered Nanowires: Evidence of Structural and Magnetic Interplay. Nano Lett. 2016, 16, 1230–1236. [Google Scholar] [CrossRef]
- Palmero, E.M.; Méndez, M.; González, S.; Bran, C.; Vega, V.; Vázquez, M.; Prida, V.M. Stepwise magnetization reversal of geometrically tuned in diameter Ni and FeCo bi-segmented nanowire arrays. Nano Res. 2019, 12, 1547–1553. [Google Scholar] [CrossRef]
- Chi, C.; Hao, J.; Liu, X.; Ma, X.; Yang, Y.; Liu, X.; Endres, F.; Zhao, J.; Li, Y. UV-assisted, template-free electrodeposition of germanium nanowire cluster arrays from an ionic liquid for anodes in lithium-ion batteries. New J. Chem. 2017, 41, 15210–15215. [Google Scholar] [CrossRef]
- Al-Salman, R.; Sommer, H.; Brezesinski, T.; Janek, J. Template-free electrochemical synthesis of high aspect ratio sn nanowires in ionic liquids: A general route to large-area metal and semimetal nanowire arrays? Chem. Mater. 2015, 27, 3830–3837. [Google Scholar] [CrossRef]
- Al-Salman, R.; Sommer, H.; Brezesinski, T.; Janek, J. Template-Free Electrodeposition of Uniform and Highly Crystalline Tin Nanowires from Organic Solvents Using Unconventional Additives. Electrochim. Acta 2017, 246, 1016–1022. [Google Scholar] [CrossRef]
- Yang, J.M.; Hsieh, Y.T.; Chu-Tien, T.T.; Sun, I.W. Electrodeposition of Distinct One-Dimensional Zn Biaxial Microbelt from the Zinc Chloride-1-Ethyl-3-methylidazolium Chloride Ionic Liquid. J. Electrochem. Soc. 2011, 158, D235–D239. [Google Scholar] [CrossRef]
- Pulletikurthi, G.; Shapouri Ghazvini, M.; Prowald, A.; Zein El Abedin, S.; Endres, F. Template-Free Electrodeposition of Zinc Nanowires from an Ionic Liquid. ChemElectroChem 2015, 2, 1366–1371. [Google Scholar] [CrossRef]
- Szymczak, J.; Legeai, S.; Diliberto, S.; Migot, S.; Stein, N.; Boulanger, C.; Chatel, G.; Draye, M. Template-free electrodeposition of tellurium nanostructures in a room-temperature ionic liquid. Electrochem. Commun. 2012, 24, 57–60. [Google Scholar] [CrossRef]
- Thiebaud, L.; Legeai, S.; Stein, N. Tuning the morphology of Te one-dimensional nanostructures by template-free electrochemical deposition in an ionic liquid. Electrochim. Acta 2016, 197, 300–306. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Lai, M.C.; Huang, H.L.; Sun, I.W. Speciation of cobalt-chloride-based ionic liquids and electrodeposition of Co wires. Electrochim. Acta 2014, 117, 217–223. [Google Scholar] [CrossRef]
- Al-Salman, R.; Sedlmaier, S.J.; Sommer, H.; Brezesinski, T.; Janek, J. Facile synthesis of micrometer-long antimony nanowires by template-free electrodeposition for next generation Li-ion batteries. J. Mater. Chem. A 2016, 4, 12726–12729. [Google Scholar] [CrossRef]
- Su, C.J.; Hsieh, Y.T.; Chen, C.C.; Sun, I.W. Electrodeposition of aluminum wires from the Lewis acidic AlCl3/trimethylamine hydrochloride ionic liquid without using a template. Electrochem. Commun. 2013, 34, 170–173. [Google Scholar] [CrossRef]
- Wang, H.; Li, B. Direct Electrodeposition of Aluminum Nanowires from a Room Temperature Ionic Liquid: An Electrochemical 2D-3D-1D Process. J. Electrochem. Soc. 2018, 165, D641–D646. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Leong, T.-I.; Huang, C.-C.; Yeh, C.-S.; Sun, I.-W. Direct template-free electrochemical growth of hexagonal CuSn tubes from an ionic liquid. Chem. Commun. 2010, 46, 484–486. [Google Scholar] [CrossRef]
- Yang, J.-M.; Gou, S.-P.; Sun, I.-W. Single-step large-scale and template-free electrochemical growth of Ni–Zn alloy filament arrays from a zinc chloride based ionic liquid. Chem. Commun. 2010, 46, 2686–2688. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Hsieh, Y.T.; Zhuang, D.X.; Sun, I.W. Direct electrodeposition of FeCoZn wire arrays from a zinc chloride-based ionic liquid. Electrochem. Commun. 2011, 13, 1178–1181. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Tsai, R.W.; Su, C.J.; Sun, I.W. Electrodeposition of CuZn from chlorozincate ionic liquid: From hollow tubes to segmented nanowires. J. Phys. Chem. C 2014, 118, 22347–22355. [Google Scholar] [CrossRef]
- Elbasiony, A.M.; Olschewski, M.; El Abedin, S.Z.; Endres, F. Template-Free Electrodeposition of SnSi Nanowires from an Ionic Liquid. ChemElectroChem 2015, 2, 1361–1365. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Li, B. Electrodeposition of SmCo alloy nanowires with a large length-diameter ratio from SmCl3-CoCl2-1-ethyl-3-methylimidazolium chloride ionic liquid without template. RSC Adv. 2015, 5, 39620–39624. [Google Scholar] [CrossRef]
- Su, C.-J.; Hsieh, Y.-T.; Fong, J.-D.; Chang, C.-C.; Sun, I.-W. Template free synthesis of beaded aluminium sub- microwires via pulse potential electrodeposition. RSC Adv. 2016, 6, 75054–75057. [Google Scholar] [CrossRef]
- Chang, C.-C.; Sun, I.-W. Template-Free Fabrication of Diameter-Modulated Co-Zn/Oxide Wires from a Chlorozincate Ionic Liquid by Using Pulse Potential Electrodeposition. J. Electrochem. Soc. 2017, 164, D425–D428. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.X.; Chen, Q.S.; You, L.X.; Liao, H.G.; Sun, S.G.; Zhou, S.G.; Xu, Z.N.; Chen, Y.M.; Guo, G.C. Screw-like PdPt nanowires as highly efficient electrocatalysts for methanol and ethylene glycol oxidation. J. Mater. Chem. A 2018, 6, 2327–2336. [Google Scholar] [CrossRef]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0471648515. [Google Scholar]
- Fannin, A.A.; Floreani, D.A.; King, L.A.; Landers, J.S.; Piersma, B.J.; Stech, D.J.; Vaughn, R.L.; Wilkes, J.S.; Williams, J.L. Properties of 1,3-dialkylimidazolium chloride-aluminum chloride ionic liquids. 2. Phase transitions, densities, electrical conductivities, and viscosities. J. Phys. Chem. 1984, 88, 2614–2621. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Lu, X.; Zhang, S. Electrodeposition of Al from chloroaluminate ionic liquids with different cations. Ionics 2017, 23, 2449–2455. [Google Scholar] [CrossRef]



| Thickness of Insulation Ring (mm) | 0 (without Insulation Ring) | 0.1 | 0.5 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|
| Average current density of plateaus (mA/cm2) | −21.43 | −11.84 | −10.80 | −9.23 | −6.79 | −5.06 |
| Decrease of diffusion flux (%) | 0 | 44.75 | 49.6 | 56.93 | 68.32 | 76.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, G.-M.; Li, B.; You, J.-L. An Effective Strategy for Template-Free Electrodeposition of Aluminum Nanowires with Highly Controllable Irregular Morphologies. Nanomaterials 2022, 12, 1390. https://doi.org/10.3390/nano12091390
Wang H, Li G-M, Li B, You J-L. An Effective Strategy for Template-Free Electrodeposition of Aluminum Nanowires with Highly Controllable Irregular Morphologies. Nanomaterials. 2022; 12(9):1390. https://doi.org/10.3390/nano12091390
Chicago/Turabian StyleWang, Heng, Guo-Min Li, Bing Li, and Jing-Lin You. 2022. "An Effective Strategy for Template-Free Electrodeposition of Aluminum Nanowires with Highly Controllable Irregular Morphologies" Nanomaterials 12, no. 9: 1390. https://doi.org/10.3390/nano12091390






