Bioactive Based Nanocarriers for the Treatment of Viral Infections and SARS-CoV-2
Abstract
:1. Introduction
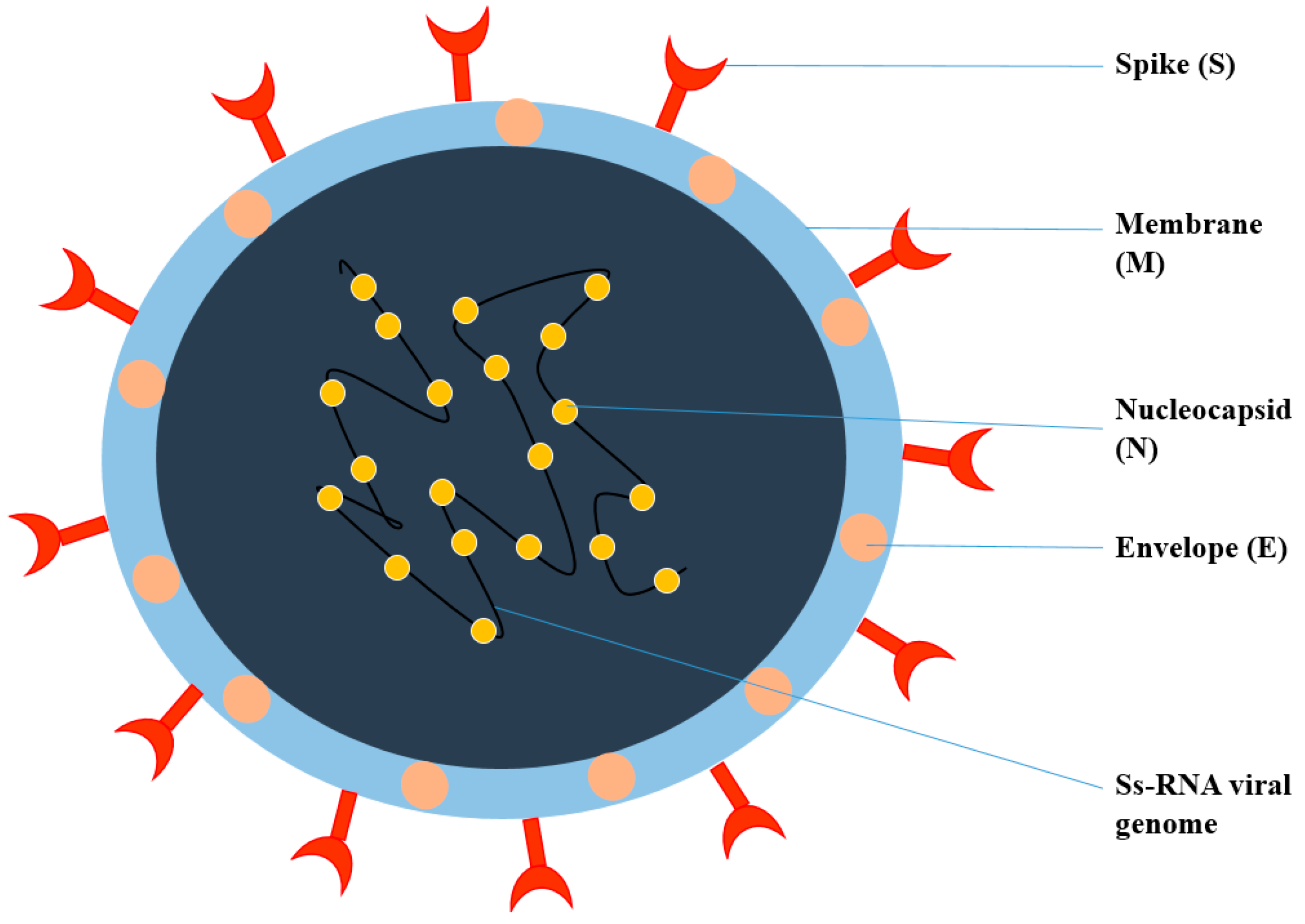
2. General Structure of Viruses
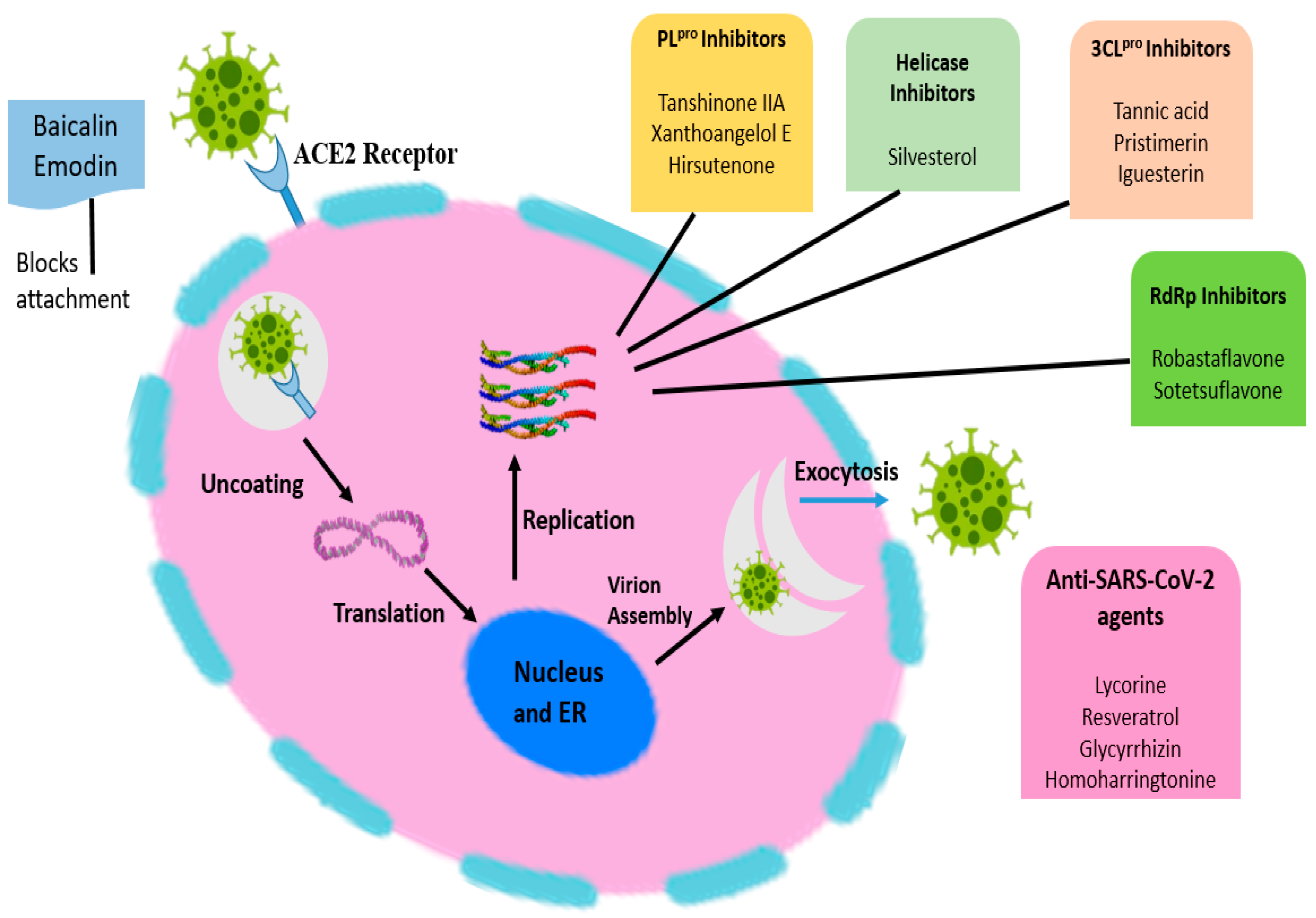
3. Pathophysiology of COVID-19
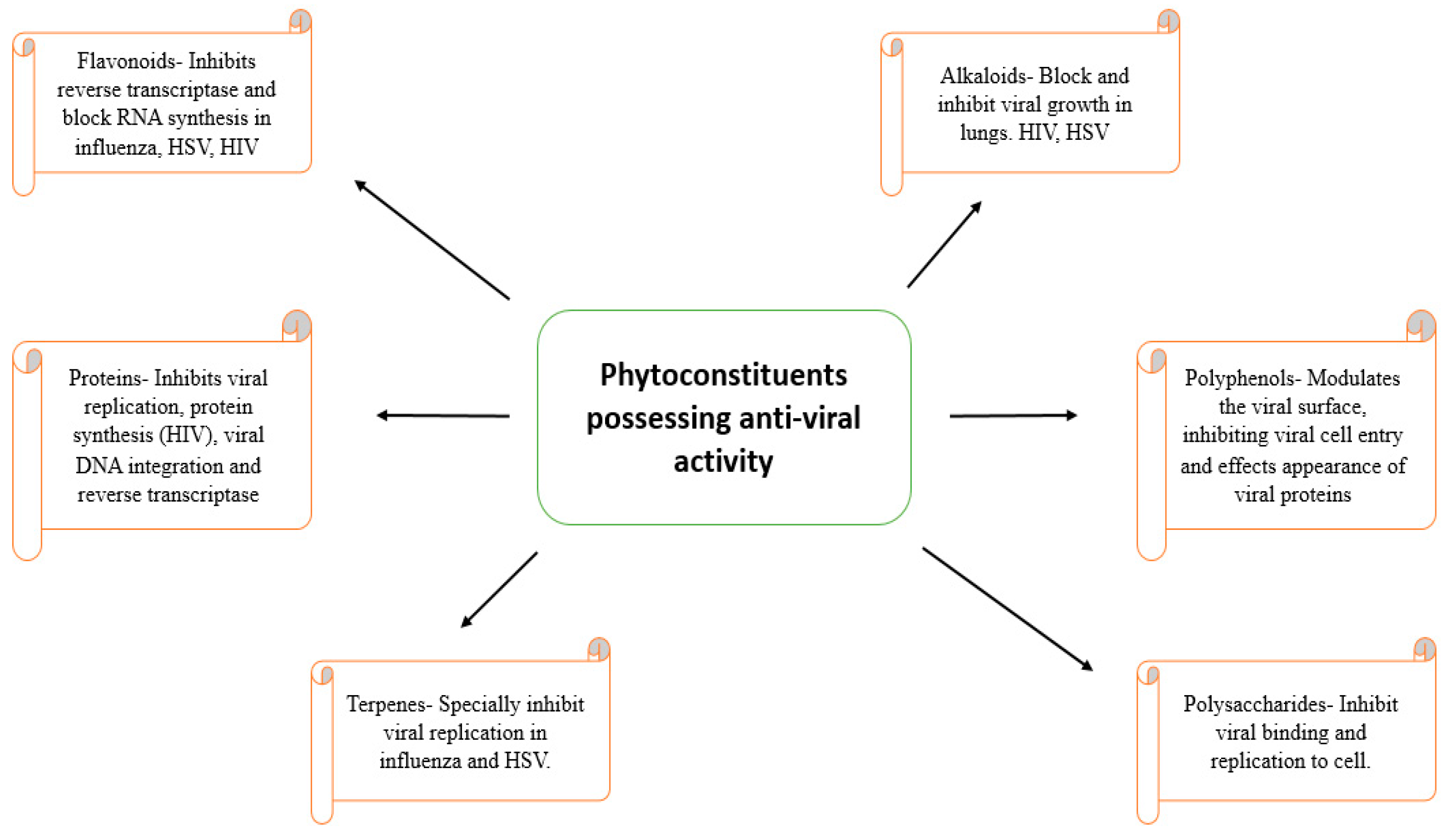
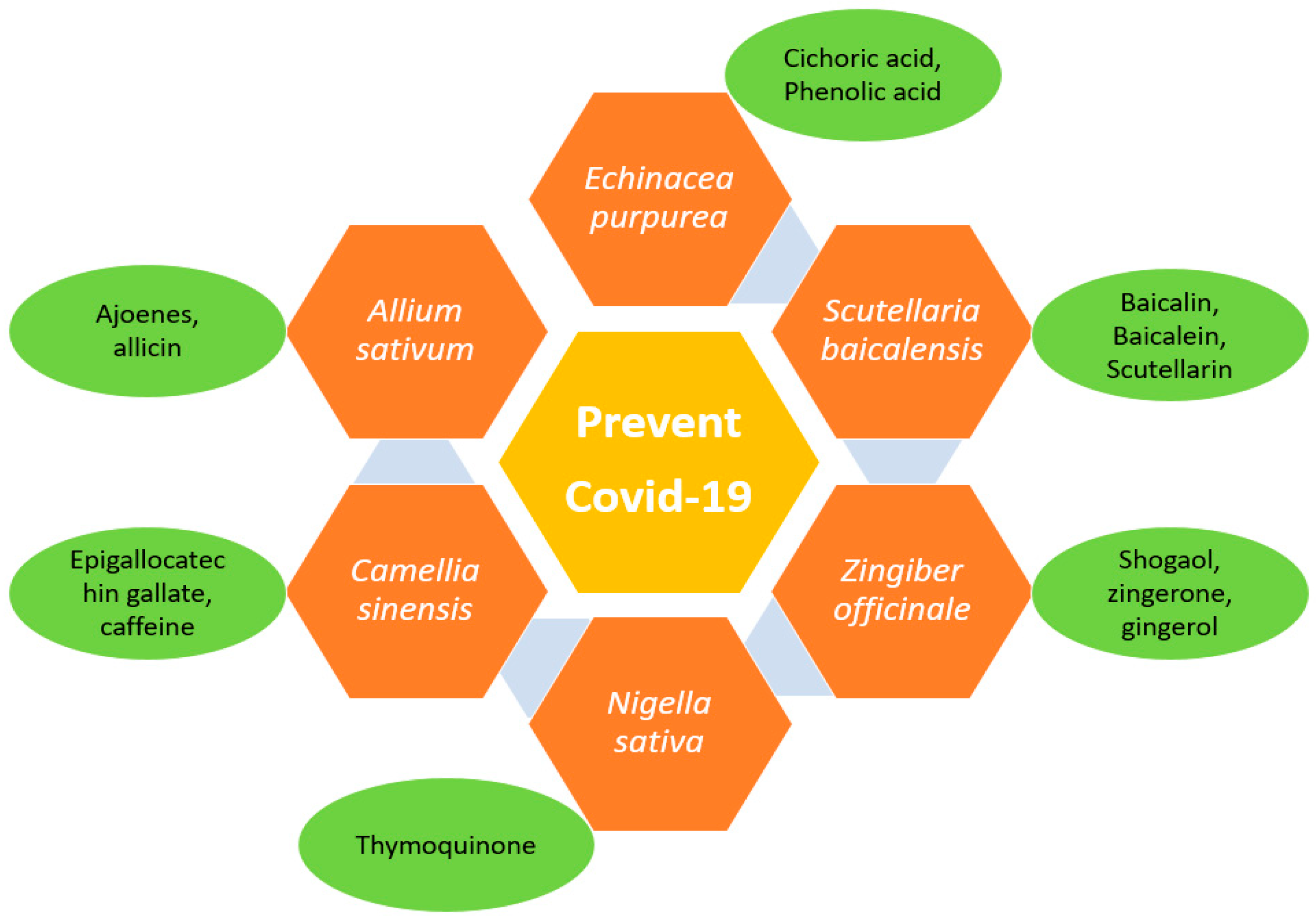
4. Natural Anti-Viral Plants and Phytochemicals
4.1. Scutellaria baicalensis
4.2. Tanacetum vulgare
4.3. Ruta Angustifolia
4.4. Liriope platyphylla
4.5. Citrus reticulate
4.6. Tinospora cardifolia
4.7. Andrographis paniculata
4.8. Saururus chinensis
5. Mechanism of Action of Bioactive Molecules in Various Viral Diseases
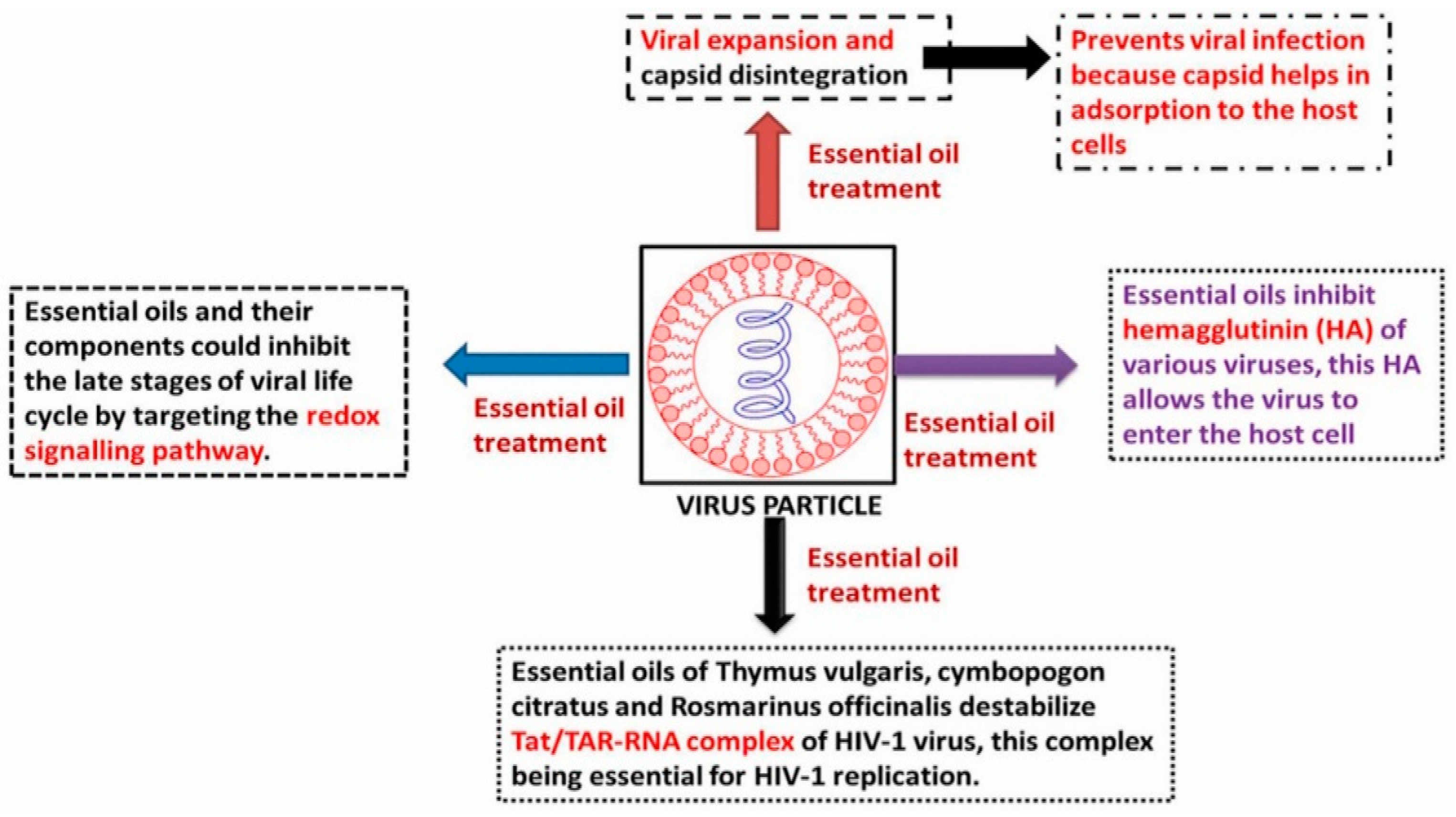
6. Role of Essential Oils in Combating Viral Infection
7. Herbal Strategies to Combat COVID-19
8. Anti-Viral Bioactive-Based Nanocarrier Systems
9. Future Prospective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| WHO | World Health Organization |
| TCM | Traditional Chinese medicine |
| NHP | National Health Portal |
| HSV | Herpes simplex virus |
| SARS | Severe acute respiratory syndrome |
| MERS | Middle East respiratory yndrome |
| TM2 | Transmembrane domain 2 |
| RdRp | RNA-dependent RNA polymerase |
| ORF | Open reading frame |
| ACE2 | Angiotensin-converting enzyme 2 |
| ICD | Disseminated intravascular coagulation |
| ARDS | Acute respiratory distress syndrome |
| IL | Interleukin |
| H3N2 | Influenza A virus |
| PVY | Potato virus Y |
| CMV | Cucumber mosaic virus |
| HCV | Hepatitis C virus |
| LPS | Lipopolysaccharide |
| JNK | c-Jun N-terminal kinase |
| NF-kB | Nuclear factor kappa B |
| RSV | Respiratory syncytial virus |
| EBV | Epstein–Barr virus |
| HIV | Human immunodeficiency virus |
| TPMRSS2 | Transmembrane serine protease 2 |
| PAK1 | P21 (RAC1) activated kinase 1 |
| SNEDDS | Self-nanoemulsifying drug delivery systems |
| EGCG | Epigallocatechin gallate |
| RBC | Red blood cell |
| PLCA | Poly lactide glycolic acid |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si-Yuan, P.; Shu-Feng, Z.; Si-Hua, G.; Zhi-Ling, Y.; Shuo-Feng, Z.; Min-Ke, T.; Jian-Ning, S.; Dik-Lung, M.; Yi-Fan, H.; Wang-Fun, F.; et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid. Based Complementary Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [Green Version]
- Drexler, M. Institute of Medicine (US); What You Need to Know about Infectious Disease; National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Neiderud, C.-J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F.S. How viruses invade cells. Biophys. J. 2016, 110, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Tapparel, C.; Soboa, S.; Constantb, S.; Huangb, S.; Bellea, S.V.; Kaiser, L. Growth and characterization of different human rhinovirus C types in three-dimensional human airway epithelia reconstituted in vitro. Virology 2013, 446, 1–8. [Google Scholar] [CrossRef]
- Akram, M.; Tahir, I.M.; Shah, S.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Irwin, K.K.; Renzette, N.; Kowalik, T.F.; Jensen, J.D. Antiviral drug resistance as an adaptive process. Virus Evol. 2016, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cavalu, S.; Damian, G. Rotational correlation times of 3-carbamoyl-2,2,5,5-tetramethyl-3-pyrrolin-1-yloxy spin label with respect to heme and nonheme proteins. Biomacromolecules 2003, 4, 1630–1635. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, A.; Kaur, J.; Kumari, S.; Garg, M.; Sindhu, R.K.; Rahman, M.H.; Akhtar, M.F.; Tagde, P.; Najda, A.; et al. Bioactive-Based Cosmeceuticals: An Update on Emerging Trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Yang, Y.; Barlow, R.; Trapido, R.H.; Lu, Y. In vitro evaluation of marine-microorganism extracts for antiviral activity. Virol. J. 2010, 7, 182. [Google Scholar] [CrossRef] [Green Version]
- Farrar, J.; Focks, D.; Gubler, D.; Barrera, R.; Guzman, M.G.; Simmons, C.; Kalayanarooj, S.; Lum, L.; Mc Call, P.J.; Lloyd, L.; et al. Towards a global dengue research agenda. Trop. Med. Int. Health 2007, 12, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.; Chávez, J.H.; Reginatto, F.H.; Zucolotto, S.M.; Niero, R.; Navarro, D.; Yunes, R.A.; Schenkel, E.P.; Barardi, C.R.; Zanetti, C.R.; et al. Evaluation of antiviral activity of South American plant extracts against herpes simplex virus type 1 and rabies virus. Phytother. Res. 2007, 21, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, A.-I.; Miere, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the Combined Effects of Ocimum basilicum and Trifolium pratense Extracts in Terms of Phytochemical Profile and Pharmacological Effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.A.; Newman, D.J. Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 2005, 77, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Guasch, L.; Iwaszkiewicz, J.; Mulero, M.; Salvado, M.J.; Bladé, C.; Ceballos, M.; Valls, C.; Zoete, V.; Grosdidier, A.; et al. Identification of human IKK-2 inhibitors of natural origin (Part II): In silico prediction of IKK-2 inhibitors in natural extracts with known anti-inflammatory activity. Eur. J. Med. Chem. 2011, 46, 6098–6103. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.T.; Lu, H.T.; Fan, K.W.; Cheng, V.C.C.; Tsui, W.H.W.; Hung, I.F.N.; Lee, T.S.W.; et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef]
- Ma, Y.; Tao, Y.; Qu, H.; Wang, C.; Yan, F.; Gao, X.; Zhang, M. Exploration of plant-derived natural polyphenols toward COVID-19 main protease inhibitors: DFT, molecular docking approach, and molecular dynamics simulations. RSC Adv. 2022, 12, 5357–5368. [Google Scholar] [CrossRef]
- Miere, F.; Vicas, S.I.; Timar, A.V.; Ganea, M.; Zdrinca, M.; Cavalu, S.; Fritea, L.; Vicas, L.; Muresan, M.; Pallag, A.; et al. Preparation and Characterization of Two Different Liposomal Formulations with Bioactive Natural Extract for Multiple Applications. Processes 2021, 9, 432. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef]
- Wu, K.; Werner, A.P.; Moliva, J.I.; Koch, M.; Choi, A.; Stewart-Jones, G.B.E.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; Graham, B.S.; et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. BioRxiv 2021. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halaji, M.; Farahani, A.; Ranjbar, R.; Heiat, M.; Dehkordi, F.S. Emerging coronaviruses: First SARS, second MERS and third SARS-CoV-2, epidemiological updates of COVID-19. Le Infez. Med. 2020, 28, 6–17. [Google Scholar]
- World Health Organization. Modes of Transmission of Virus Causing COVID-19, Implications for IPC Precaution Recommendations: Scientific Brief; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. Author correction: COVID-19, the vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.K.; Najda, A.; Kaur, P.; Shah, M.; Singh, H.; Kaur, P.; Cavalu, S.; Jaroszuk-Sierocińska, M.; Rahman, M.H. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials 2021, 14, 5965. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, 46–47. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Chawla, S.M.; Chatterjee, T.; Dey, R.; Bag, P.; Chakraborti, S.; Khan, M.T. Recent advancements for the evaluation of antiviral activities of natural products. New Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Vicas, S.I.; Cavalu, S.; Laslo, V.; Tocai, M.; Costea, T.O.; Moldovan, L. Growth, Photosynthetic Pigments, Phenolic, Glucosinolates Content and Antioxidant Capacity of Broccoli Sprouts in Response to Nanoselenium Particles Supply. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 821–828. [Google Scholar] [CrossRef]
- Tai, D.Y. Pharmacologic treatment of SARS: Current knowledge and recommendations. Ann. Acad.Med. 2007, 36, 438–443. [Google Scholar]
- Li, B.Q.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Y.; Chen, F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B 2004, 812, 277–290. [Google Scholar] [CrossRef]
- Ma, S.C.; Du, J.; But, P.P.; Deng, X.L.; Zhang, Y.W.; Ooi, V.E.; Xu, H.X.; Lee, S.H.; Lee, S.F. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 2002, 79, 205–211. [Google Scholar] [CrossRef]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar]
- Stevovic, S.; Surinski, V.; Calic-Dragosavac, D. Environmental adaptabil¬ity of tansy (Tanacetum vulgare L.). Afr. J. Biotechnol. 2009, 8, 6290–6294. [Google Scholar]
- Chandler, R.F.; Hooper, S.N.; Hoopper, D.L.; Jamieson, W.D.; Lewis, E. Herbal remedies of the maritime Indians: Sterols and triterpenes of Tanacetum vulgare L. (Tansy). Lipids 1982, 17, 102–106. [Google Scholar] [CrossRef]
- Onozato, T.; Nakamura, C.V.; Cortez, D.A.; Dias Filho, B.P.; Ueda-Nakamura, T. Tanacetum vulgare: An tiherpes virus activity of crude extract and the purified compound parthenolide. Phytother. Res. 2009, 23, 791–796. [Google Scholar] [CrossRef]
- Álvarez, Á.L.; Habtemariam, S.; Juan-Badaturuge, M.; Jackson, C.; Parra, F. In vitro anti HSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action. Phytother. Res. 2011, 25, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrov, N.; Stoyanova, M.; Valkova, M. Antiviral activity of plant extract from Tanacetum vulgare against Cucumber Mosaic Virus and Potato Virus Y. J. Bio. Sci. Biotechnol. 2016, 5, 189–194. [Google Scholar]
- Pollio, A.; De Natale, A.; Appetiti, E.; Aliotta, G.; Touwaide, A. Continuity and change in the Mediterranean medical tradition: Ruta spp. (rutaceae) in Hippocratic medicine and present practices. J. Ethnopharmacol. 2008, 116, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, E.M.K.; Bandara, B.M.R.; Gunatilaka, A.A.L.; Tezuka, Y.; Kikuchi, T. Chemical constituents of three Rutaceae species from Sri Lanka. J. Nat. Prod. 1992, 55, 1261–1269. [Google Scholar] [CrossRef]
- Han, Y.; Jung, H.W.; Lee, D.H.; Kwon, S.Y.; Son, K.H.; Park, Y.K. Anti-inflammatory effects of prosapogenin III from the dried roots of Liriope platyphylla in LPS-stimulated RAW264.7 cells. J. Asian Nat. Prod. Res. 2013, 15, 1038–1049. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the Remedial Potential of Nano Delivery-Based Flavonoids for Breast Cancer Treatment. Molecules 2021, 26, 5163. [Google Scholar] [CrossRef]
- Huang, T.J.; Tsai, Y.C.; Chiang, S.Y.; Wang, G.J.; Kuo, Y.C.; Chang, Y.C.; Wu, Y.Y.; Wu, Y.C. Anti-viral effect of a compound isolated from Liriope platyphylla against hepatitis B virus in vitro. Virus. Res. 2014, 192, 16–24. [Google Scholar] [CrossRef]
- Kumar, P.; Bhaskar, A. Determination of bioactive components from the ethanolic peel extract of Citrus reticulata by gas chromatography–mass spectrometry. Int. J. Drug Dev. Res. 2012, 4, 166–174. [Google Scholar]
- Kirbaslar, F.G.; Tavman, A.; Dülger, B.; Türker, G. Antimicrobial activity of Turkish Citrus peel oils. Pak. J. Bot. 2009, 41, 3207–3212. [Google Scholar]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef]
- Choi, M.Y.; Chai, C.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Effects of storage period and heat treatment on phenolic compound composition in dried Citrus peels (Chenpi) and discrimination of Chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J. Pharm. Biomed. Anal. 2011, 54, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Wu, X.; Li, M.M.; Li, G.Q.; Yang, Y.T.; Luo, H.J.; Huang, W.H.; Chung, H.Y.; Ye, W.C.; Wang, G.C.; et al. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Savi, L.A.; Caon, T.; de Oliveira, A.P.; Sobottka, A.M.; Werner, W.; Reginatto, F.H.; Schenkel, E.P.; Barardi, C.R.; Simões, C.M. Evaluation of antirotavirus activity of flavonoids. Fitoterapia 2010, 81, 1142–1146. [Google Scholar] [CrossRef]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Marraro, G.A.; Zeng, Y. From SARS and MERS to COVID-19, a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Krupanidhi, S.; Abraham Peele, K.; Venkateswarulu, T.C.; Ayyagari, V.S.; Nazneen Bobby, M.; John Babu, D.; Venkata Narayana, A.; Aishwarya, G. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2, an in silico study. J. Biomol. Struct. Dyn. 2021, 39, 5799–5803. [Google Scholar] [CrossRef]
- Vellingiri, B.; Jayaramayya, K.; Iyer, M.; Narayanasamy, A.; Govindasamy, V.; Giridharan, B.; Ganesan, S.; Venugopal, A.; Venkatesan, D.; Ganesan, H.; et al. COVID-19, a promising cure for the global panic. Sci. Total Environ. 2020, 725, 138277. [Google Scholar] [CrossRef]
- Dudani, T.; Saraogi, A. Use of herbal medicines on coronavirus. Acta. Sci. Pharm. Sci. 2020, 11, 416–419. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Lee, J.H.; Nam, J.B.; Hong, Y.S.; Lee, J.J. Lignans from Saururus chinensis inhibiting the transcription factor NF-κB. Phytochemistry 2003, 64, 765–771. [Google Scholar] [CrossRef]
- Du, H.; Yang, X.; Li, H.; Han, L.; Li, X.; Dong, X.; Zhu, Q.; Ye, M.; Feng, Q.; Niu, X. Preparation and evaluation of andrographolide- loaded microemulsion. J. Microencapsul. 2012, 29, 657–665. [Google Scholar] [CrossRef]
- Sermkaew, N.; Ketjinda, W.; Boonme, P.; Phadoongsombut, N.; Wiwattanapatapee, R. Liquid and solid self-microemulsifyin drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculata. Eur. J. Pharm. Sci. 2013, 50, 459–466. [Google Scholar] [CrossRef]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) ofandrographolide isolated from Andrographis paniculata Nees: Characterization, in-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 514–520. [Google Scholar] [CrossRef]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Ming, G.; Kim, G.J.; Ha, T.K.Q.; Cho, H.; Sung, S.H. Jubanines F–J, cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochemistry 2015, 43, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Carli, I.; Vecchio, C.D.; Xu, L.; Corona, A.; Grandi, N.; Piano, D.; Maccioni, E.; Distinto, S.; Parolin, C.; et al. Sennoside A, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine 2016, 23, 1383–1391. [Google Scholar] [CrossRef]
- Wu, S.F.; Lin, C.K.; Chuang, Y.S.; Chang, F.R.; Tseng, C.K.; Wu, Y.C.; Lee, J.C. Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J. Viral Hepat. 2012, 19, 364–370. [Google Scholar] [CrossRef]
- Bachmetov, L.; Tanamy, M.G.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Han, Q.; Chen, J.; Lv, Y. Xanthohumol enhances antiviral effect of interferon a-2b against bovine viral diarrhea virus, a surrogate of hepatitis C virus. Phytomedicine 2010, 17, 310–316. [Google Scholar] [CrossRef]
- Dao, T.T.; Nguyen, P.H.; Lee, H.S.; Kim, E.; Park, J.; Lim, S.I.; Oh, W.K. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflate. Bioorg. Med. Chem. Lett. 2011, 21, 294–298. [Google Scholar] [CrossRef]
- Biedenkopf, N.; Lange-Grünweller, K.; Schulte, F.W.; Weiber, A.; Muller, C.; Becker, D.; Becker, S.; Hartmann, R.K.; Grünweller, A. The natural compound Silvestrolis a potent inhibitor of Ebola virus replication. Antivir. Res. 2016, 137, 76–81. [Google Scholar] [CrossRef]
- Jin, Y.H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomedicine Int. J. Phytother Phytopharm. 2021, 86, 153440. [Google Scholar] [CrossRef]
- Hogue, B.G.; Machamer, C.E. Coronavirus structural proteins and virus assembly. Nidoviruses 2014, 28, 569. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhou, Y.; Lu, L.; Li, F.; Jiang, S. MERS-CoV spike protein: A key target for antivirals. Expert Opin. Ther. Targets 2017, 21, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, K.; Nakane, H. Mechanisms of inhibition of various cellular DNA and RNA polymerases by several flavonoids. J. Biochem. 1990, 108, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Li, S.Y.; Huang, R.L.; Wu, M.D.; Huang, H.C.; Lee, K.H. Schizarin B, C, D, and E, four new lignans from Kadsura matsudai and their antihepatitis activities. J. Nat. Prod. 2010, 64, 487–490. [Google Scholar] [CrossRef]
- Hostettmann-Kaldas, M.; Nakanishi, K. Moronic acid, a simple triterpenoid keto acid with antimicrobial activity isolated from Ozoroa mucronata. Planta Med. 1979, 37, 358–360. [Google Scholar] [CrossRef]
- Xu, H.X.; Zeng, F.Q.; Wan, M.; Sim, K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef]
- Sydiskis, R.J.; Owen, D.G.; Lohr, J.L.; Rosler, K.H.; Blomster, R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chem. 1991, 35, 2463–2466. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.; Pisani, S.; Pietropaolo, V.; Seganti, L.; Nicoletti, R.; Degener, A.; Orsi, N. Antiviral effect of a polysaccharide from Sclerotium glucanicum towards herpes simplex virus type 1 infection. Planta Med. 1996, 62, 303–307. [Google Scholar] [CrossRef]
- Fischer, W.B.; Thiel, G.; Fink, R.H.A. Viral membrane proteins. Eur. Biophys. J. 2010, 39, 1041–1042. [Google Scholar] [CrossRef] [Green Version]
- Schnitzler, P.; Schon, K.; Reichling, J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharm. 2001, 56, 343–347. [Google Scholar]
- Benencia, F.; Courreges, M.C. Antiviral activity of sandalwood oil against herpes simplex viruses-1 and -2. Phytomedicine 1999, 6, 119–123. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Wang, S.M.; Huang, K.J.; Amber, I.; Lee, R.; Chiang, C.C.; Wang, C.T. Self-assembly of severe acute respiratory syndrome coronavirus membrane protein. J. Biol. Chem. 2010, 285, 12862–12872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnasamy, N.; Harishankar, N.; Kumar, P.U.; Rukmini, C. Toxicological studies on debitterized neem oil (Azadirachta indica). Food Chem. Toxicol. 1993, 31, 297–301. [Google Scholar] [CrossRef]
- Greig, A.; Bouillant, A. Binding effects of concanavalin A on a coronavirus. Can. J. Comp. Med. 1977, 41, 122. [Google Scholar]
- Li, S.Y.; Chen, C.; Zhang, H.Q.; Guo, H.Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.N.; Yu, J.; Xiao, P.G.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.Z.; Hesse-Fong, J.; Lin, J.G.; Yuan, C.S. Application of Chinese medicine in acute and critical medical conditions. Am. J. Chin. Med. 2019, 47, 1223–1235. [Google Scholar] [CrossRef]
- Ho, T.Y.; Wu, S.L.; Chen, J.C.; Li, C.C.; Hsiang, C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007, 74, 92–101. [Google Scholar] [CrossRef]
- Yang, R.; Liu, H.; Bai, C.; Wang, Y.; Zhang, X.; Guo, R.; Wu, S.; Wang, J.; Leung, E.; Chang, H.; et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol. Res. 2020, 157, 104820. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, X.; Hu, N.; Zhang, X.; Sun, T.; Xu, J.; Bian, X. Baicalin attenuates angiotensin II-induced endothelial dysfunction. Biochem. Biophys. Res. Commun. 2015, 465, 101–107. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Leung, E.L.; Liu, X.; Liu, K.; Wang, Q.; Lan, Y.; Li, X.; Yu, H.; Cui, L.; et al. A review of therapeutic agents and Chinese herbal medicines against SARS-COV-2 (COVID-19). Pharmacol. Res. 2020, 158, 104929. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Guo, L.; Li, J.; Zhong, D.; Zhang, Y.; Clarke, M.; Jin, R. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: Protocol for a systematic review and meta-analysis. Syst. Rev. 2020, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, Q.; Liu, S.; Yang, X.; Zhang, Y.; Huang, F. Sini decoction alleviates E. coli induced acute lung injury in mice via equilibrating ACE-AngII-AT1R and ACE2-Ang-(1-7)-Mas axis. Life Sci. 2018, 208, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schulte, F.W.; Lange-Grünweller, K.; Obermann, W.; Madhugiri, R.; Pleschka, S.; Grünweller, A. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona-and picornaviruses. Antivir. Res. 2018, 150, 123–129. [Google Scholar] [CrossRef]
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Jiang, P. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. Virol. J. 2004, 78, 11334–11339. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.J.; Michaelis, M.; Hsu, H.K.; Tsai, C.C.; Yang, K.D.; Wu, Y.C.; Doerr, H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Qiu, H.; Gong, J.; Liu, Q.; Xiao, H.; Chen, X.W.; Sun, B.L.; Yang, R.G. (-)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch. Virol. 2012, 157, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dhanjal, J.K.; Bhargava, P.; Kaul, A.; Wang, J.; Zhang, H.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Jin, D.Q.; Mok, H.; Oh, S.J.; Lee, J.U.; Hwang, J.K.; Ha, I.; Han, J.S. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J. Neurosci. Res. 2005, 82, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.Q.; Lim, C.S.; Hwang, J.K.; Ha, I.; Han, J.S. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem. Biophys. Res. Commun. 2005, 331, 1264–1269. [Google Scholar] [CrossRef]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2017, 23, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur J Pharm. Sci. 2010, 9, 219–225. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Gupta, R.; Wadhera, G.; Kumar, P. Modern Herbal Nanogels: Formulation, Delivery Methods, and Applications. Gels 2022, 8, 97. [Google Scholar] [CrossRef]
- Sims, K.R.; He, B.; Koo, H.; Benoit, D.S.W. Electrostatic Interactions Enable Nanoparticle Delivery of the Flavonoid Myricetin. ACS Omega 2020, 28, 12649–12659. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: A green approach. PLoS ONE 2012, 7, e41230. [Google Scholar] [CrossRef] [Green Version]
- Fiorani, M.; Accorsi, A.; Cantoni, O. Human Red Blood Cell as a Natural Flavonoid Reservoir. Free Radic. Res. 2003, 37, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, X.; Zhao, L.; Jiao, Y.; Liu, J.; Zhai, G. In vitro and in vivo study of Baicalin-loaded mixed micelles for oral delivery. Drug Deliv. 2016, 23, 1933–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)--challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, R.; Zhang, Z.; Li, Z.; Huang, G. Preparation and in vitro evaluation of etoposide-loaded PLGA microspheres for pulmonary drug delivery. DrugDeliv. 2014, 21, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Yue, P.F.; Yuan, H.L.; Xie, H.; Xiao, X.H.; Yang, M.; Liao, M.X.; Zhu, W.F.; Cai, P.L. Preparation, characterization, and bioavailability of ursodeoxycholic acid-phospholipid complex in vivo. Drug Dev. Ind. Pharm. 2008, 34, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, M.M.; Abelyan, N.; Abdelgawad, M.A.; Musa, A.; Ghoneim, M.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Alnusaire, T.S.; Abdelwahab, S.F.; et al. Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-CoV-2 assisted by in silico modeling and metabolic profiling. Antibiotics 2021, 10, 824. [Google Scholar] [CrossRef]
- Kurniawan, D.W.; Ikhsanudin, A. Potential of Jamun in nanotechnology perspective as an alternative treatment for COVID-19. J. Pharm. Sci. 2020, 7, 1. [Google Scholar]
- Yang, R.; Huang, X.; Dou, J.; Zhai, G.; Su, L. Self-microemulsifying drug delivery system for improved oral bioavailability of oleanolic acid: Design and evaluation. Int. J. Nanomed. 2013, 8, 2917–2926. [Google Scholar]
- Jiang, Y.; Wang, F.; Xu, H.; Liu, H.; Meng, Q.; Liu, W. Development of andrographolide loaded PLGA microspheres: Optimization, characterization and in vitro-in vivo correlation. Int. J. Pharm. 2014, 475, 475–484. [Google Scholar] [CrossRef]
- Yue, P.F.; Yuan, H.L.; Li, X.Y.; Yang, M.; Zhu, W.F. Process optimization, characterization and evaluation in vivo of oxymatrine-phospholipid complex. Int. J. Pharm. 2010, 387, 139–146. [Google Scholar] [CrossRef]
- Fadda, A.M.; Sinico, C.; Lai, F.; Logu, A.D. Liposomal incorporation of artimisia arborescenceL. Essential oil and in vitro antiviral activity. Eur. J. Pharma. Pharm. 2005, 59, 161–168. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Gu, F. Characterization of anticancer hypocrellin A encapsulated with silica nanoparticles. J. Therm. Anal. Calorim. 2010, 102, 69–74. [Google Scholar] [CrossRef]
- Sun, S.W.; Yeh, P.C. Analysis of rhubarb anthraquinones and bianthrones by microemulsion electrokinetic chromatography. J. Pharm. Biomed. Anal. 2005, 36, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, V.; Madhan, B.; Sehgal, P. Formulation and evalution of quercetin polycaprolactone microsphere for the treatment of Rheumatoid arthritis. J. Pharm. Sophora Alopecuroides Sci. 2010, 100, 195–205. [Google Scholar] [CrossRef]
- Casettari, L.; Gennari, L.; Angelino, D.; Ninfali, P.; Castagnino, E. ORAC of chitosan and its derivatives. Food Hydrocoll. 2012, 28, 243–247. [Google Scholar] [CrossRef]
- Novakova, L.; Pavlik, J.; Chrenkova, L.; Martinec, O.; Cerveny, L. Current antiviral drugs and their analysis in biological materials—part II: Antivirals against hepatitis and HIV viruses. J. Pharm. Biomed. Anal. 2018, 147, 378–399. [Google Scholar] [CrossRef]
| Plant | Part | Phytochemical | Class | Active against Virus | Reference |
|---|---|---|---|---|---|
| Ziziphus jujuba | Roots | Jubanines | Alkaloids | PEDV | [65] |
| Rheum palmatum | Roots | Sennoside A | Glycoside | HIV-1 | [66] |
| Swietenia macrophylla | Stem | Limonoids | Lignin | HSV | [67] |
| Embelia ribes | Seeds | Quercetin | Flavonoid | HSV | [68] |
| Humulus lupulus | Whole plant | Xanthohumol | Chalcone | BVDV | [69] |
| Glycyrrhiza inflate | Roots | Chalcones | Ketone | Influenza A | [70] |
| Active Phytoconstituent | Formulation | Applications | References |
|---|---|---|---|
| Oxymatrine | Phytosome | Enhanced bioavailability | [121] |
| Artemisia arborescens | Liposomal | Raised anti-viral activity and improved stability | [122] |
| Hypocrellins | Nanoparticulate | Enhanced hydrophilicity and stability | [123] |
| Matrine | Emulsion | Enhanced sustained released activity | [124] |
| Quercetin | Microsphere | Enhanced bioavailability and sustained release formulation | [125] |
| Curcumin | Nanostructured solid lipid carrier systems | Improved mucoadhesion and mucus penetration | [126] |
| Honokiol | Cyclodextrin inclusion complexes | Enhanced solubility and bioavailability | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, R.; Bala, R.; Sindhu, R.K.; Zehravi, M.; Madaan, R.; Ramproshad, S.; Mondal, B.; Dey, A.; Rahman, M.H.; Cavalu, S. Bioactive Based Nanocarriers for the Treatment of Viral Infections and SARS-CoV-2. Nanomaterials 2022, 12, 1530. https://doi.org/10.3390/nano12091530
Goyal R, Bala R, Sindhu RK, Zehravi M, Madaan R, Ramproshad S, Mondal B, Dey A, Rahman MH, Cavalu S. Bioactive Based Nanocarriers for the Treatment of Viral Infections and SARS-CoV-2. Nanomaterials. 2022; 12(9):1530. https://doi.org/10.3390/nano12091530
Chicago/Turabian StyleGoyal, Ravi, Rajni Bala, Rakesh K. Sindhu, Mehrukh Zehravi, Reecha Madaan, Sarker Ramproshad, Banani Mondal, Abhijit Dey, Md. Habibur Rahman, and Simona Cavalu. 2022. "Bioactive Based Nanocarriers for the Treatment of Viral Infections and SARS-CoV-2" Nanomaterials 12, no. 9: 1530. https://doi.org/10.3390/nano12091530
APA StyleGoyal, R., Bala, R., Sindhu, R. K., Zehravi, M., Madaan, R., Ramproshad, S., Mondal, B., Dey, A., Rahman, M. H., & Cavalu, S. (2022). Bioactive Based Nanocarriers for the Treatment of Viral Infections and SARS-CoV-2. Nanomaterials, 12(9), 1530. https://doi.org/10.3390/nano12091530







