Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
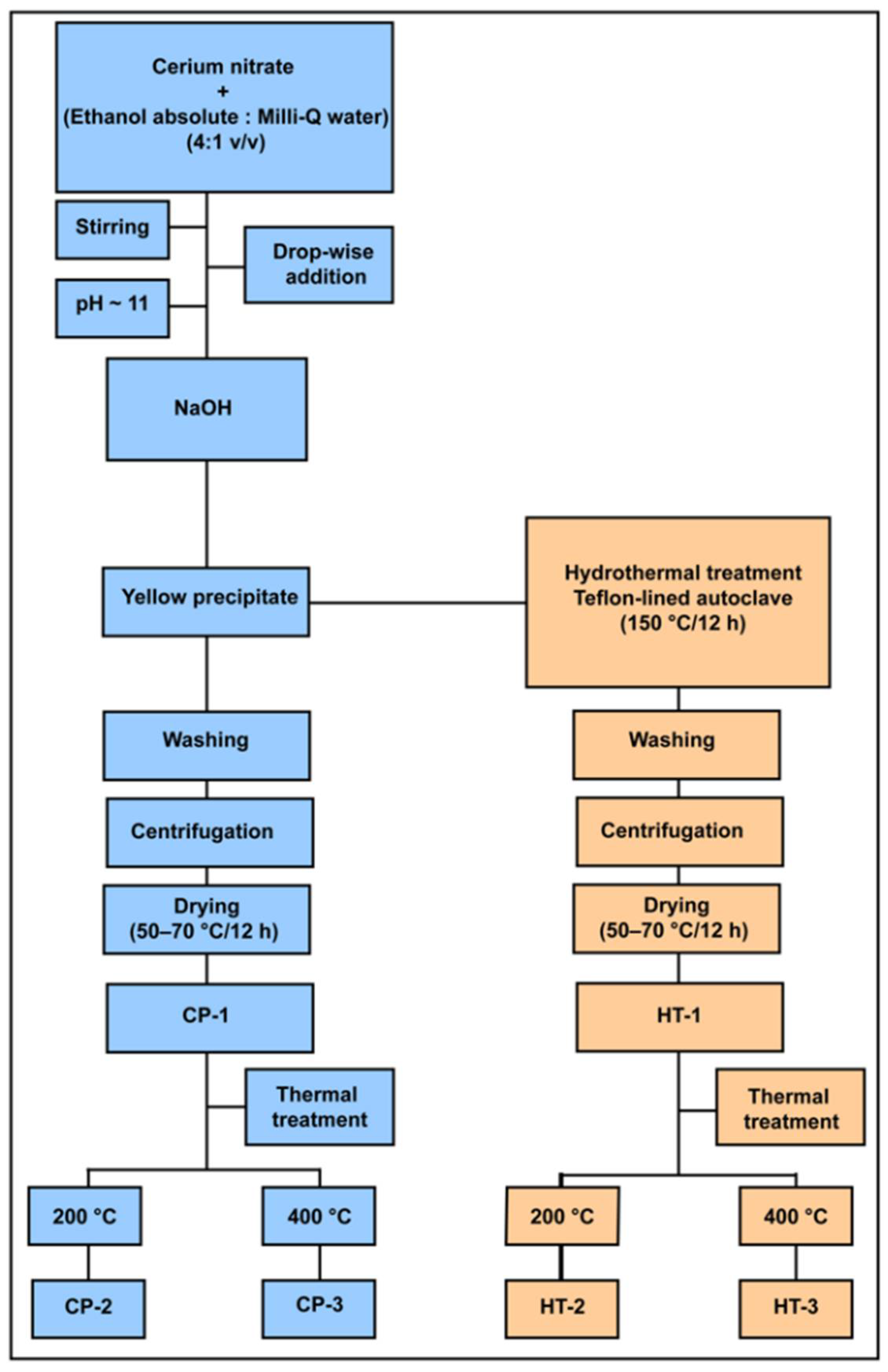
2.3. Synthesis of Cerium Oxides Nanoparticles
2.3.1. Wet Chemical Precipitation Method
2.3.2. Hydrothermal Modification Method
2.4. Batch Distribution Studies
2.4.1. Effect of Solution pH
2.4.2. Sorption Kinetics
2.4.3. Equilibrium Sorption Isotherms
2.4.4. Temperature Effects
3. Results and Discussion
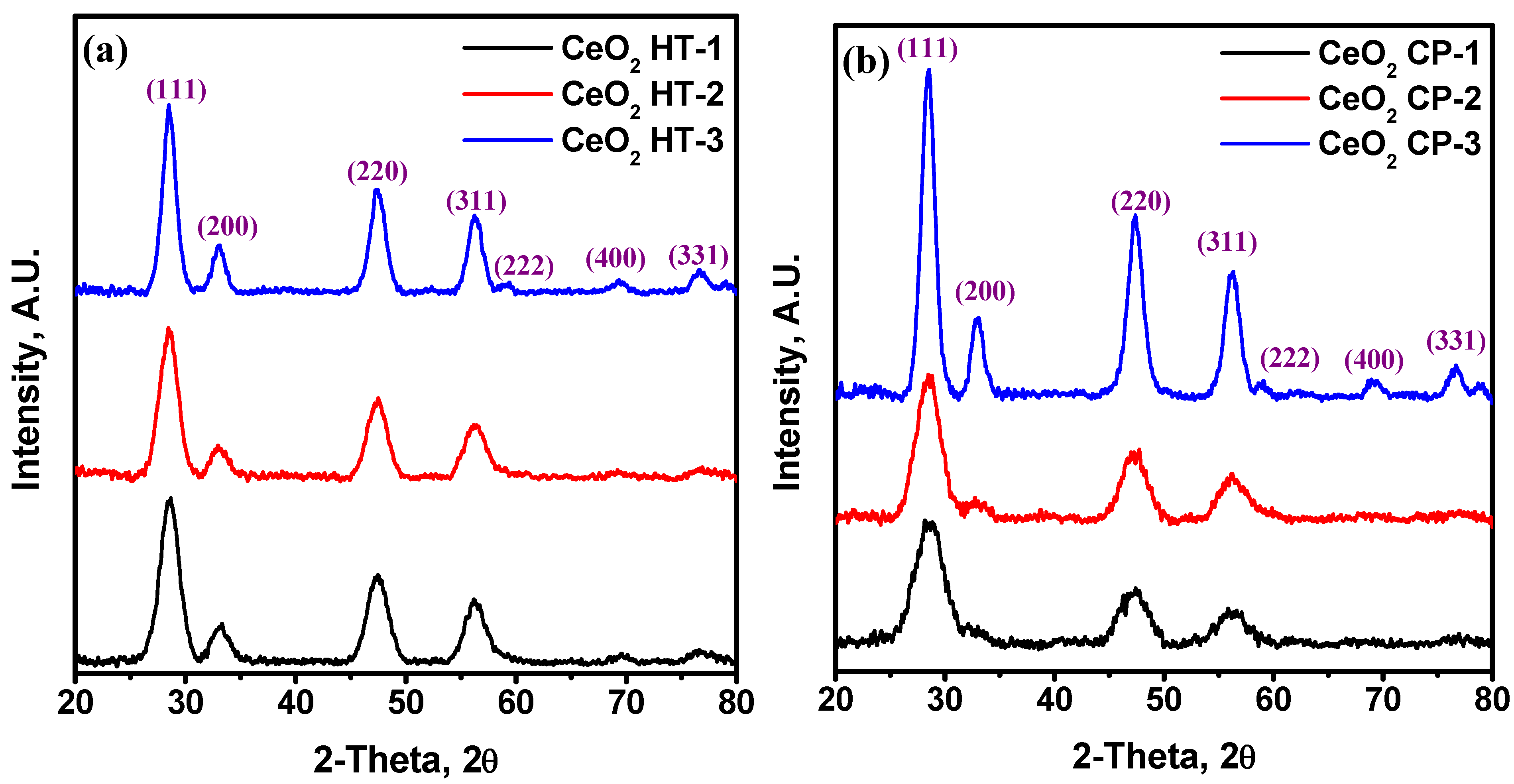
3.1. Structural Characterization of the Prepared CeO2 NPs
3.2. Effect of Solution pH
3.3. Effect of Contact Time
3.3.1. Kinetic Studies
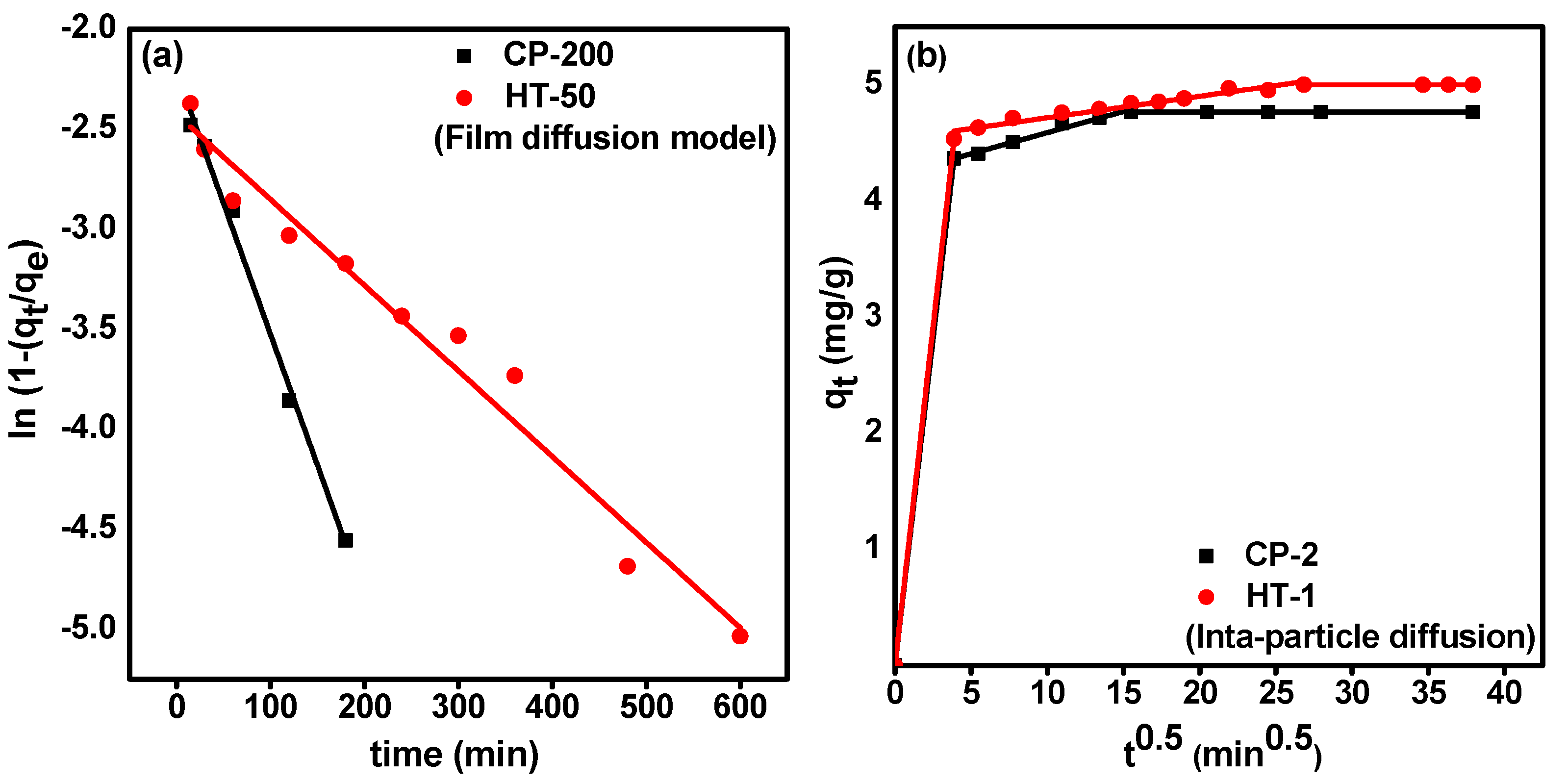
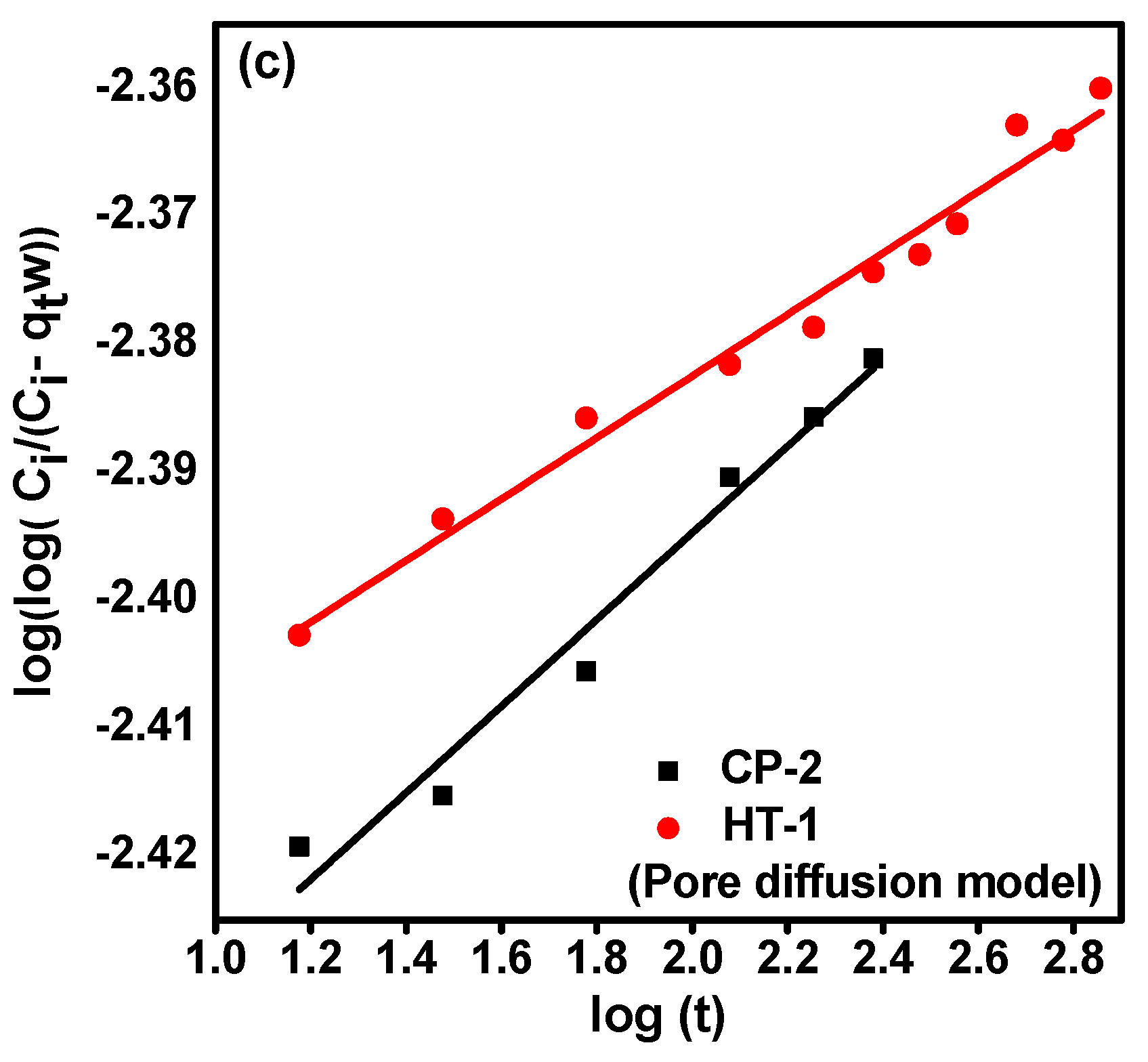
3.3.2. Diffusion Studies
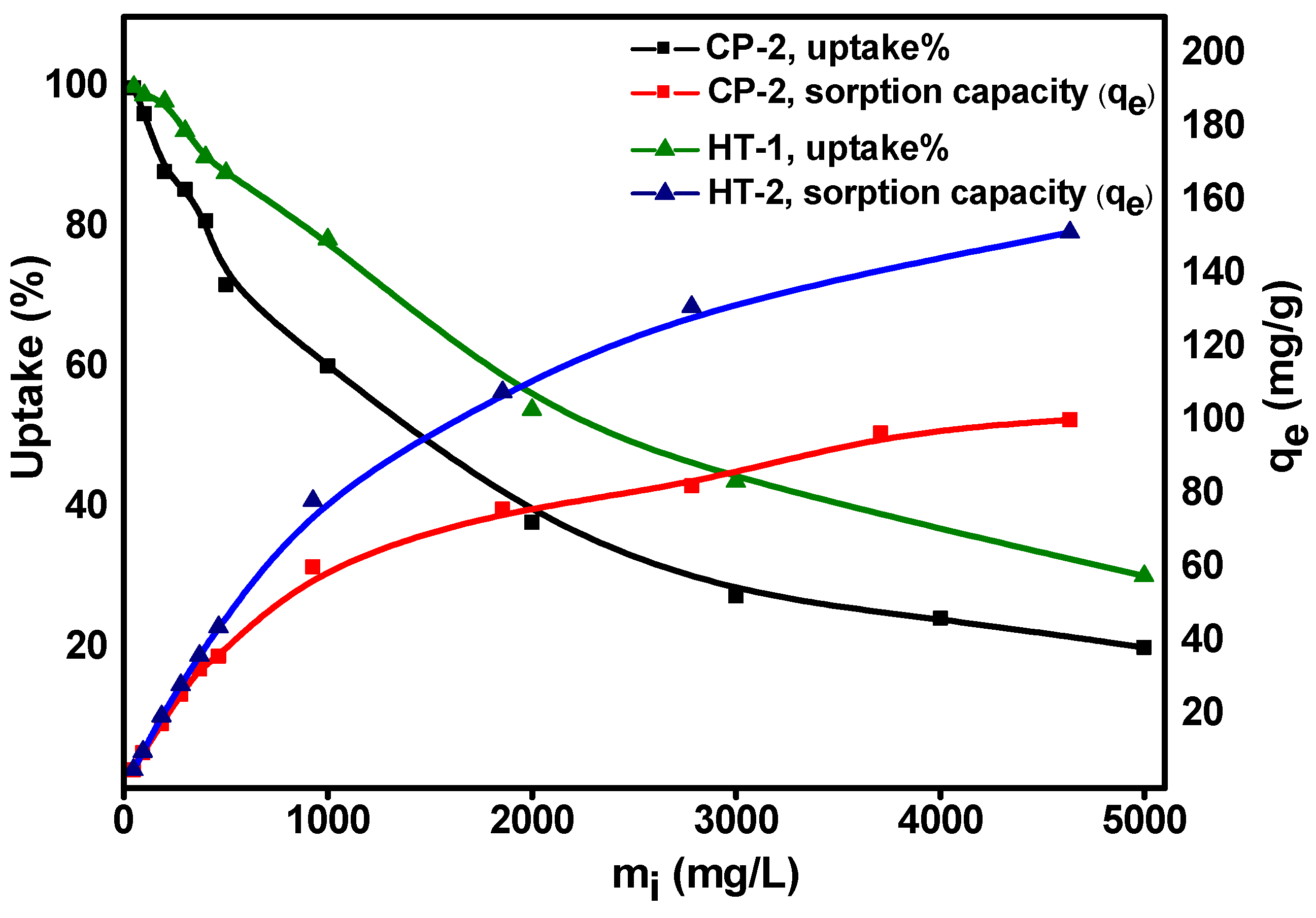
3.4. Equilibrium Isotherm Studies
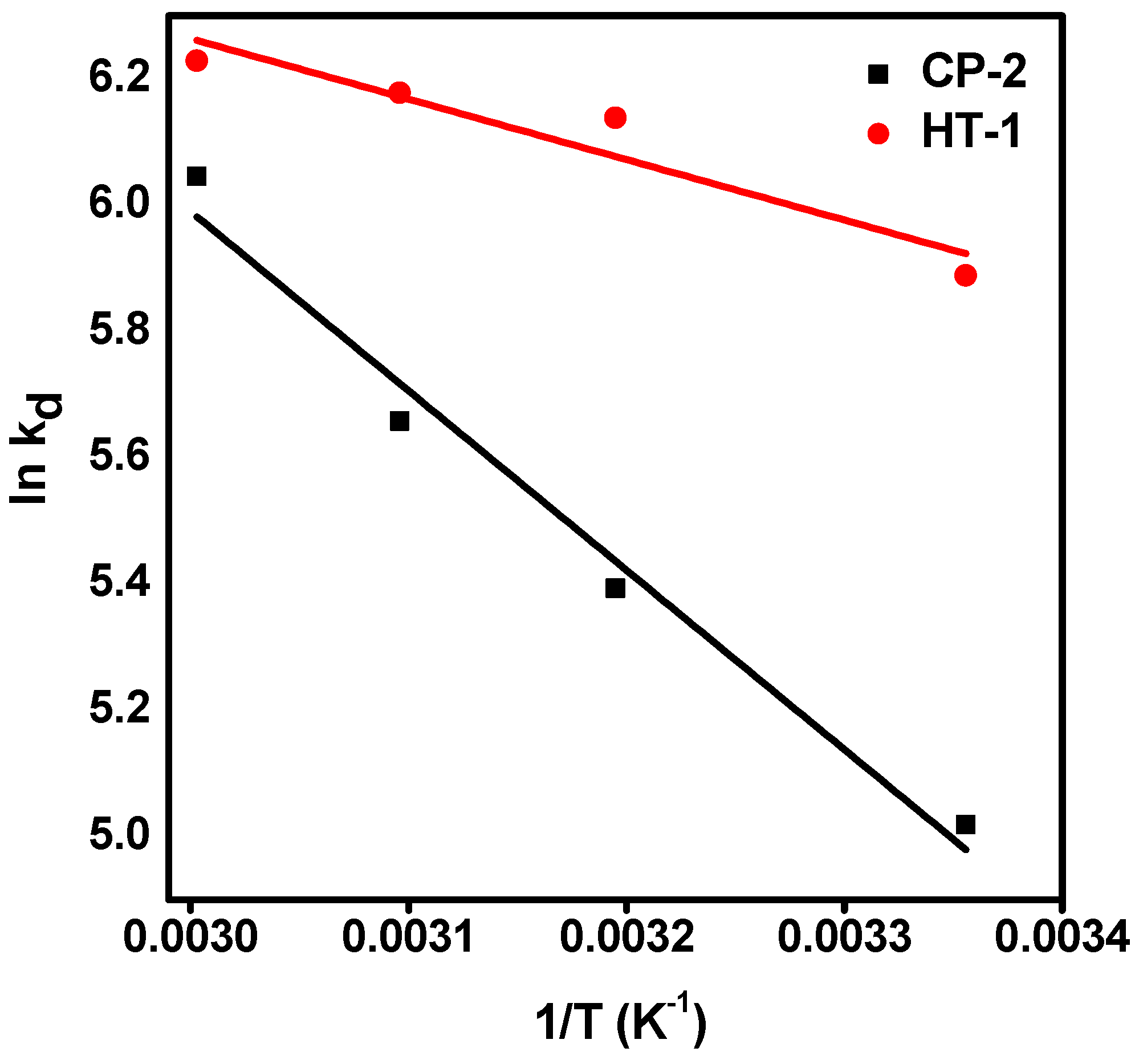
3.5. Thermodynamic Studies
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, Q.; Gan, Q.; Zhang, X.; Fang, S.; Zhang, J. Preparation and Bioevaluation of Novel 99mTc-Labeled Complexes with a 2-Nitroimidazole HYNIC Derivative for Imaging Tumor Hypoxia. Pharmaceuticals 2021, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Gumiela, M. Cyclotron production of 99mTc: Comparison of known separation technologies for isolation of 99mTc from molybdenum targets. Nucl. Med. Biol. 2018, 58, 33–41. [Google Scholar] [CrossRef]
- Martini, P.; Boschi, A.; Cicoria, G.; Zagni, F.; Corazza, A.; Uccelli, L.; Pasquali, M.; Pupillo, G.; Marengo, M.; Loriggiola, M.; et al. In-house cyclotron production of high-purity Tc-99m and Tc-99m radiopharmaceuticals. Appl. Radiat. Isot. 2018, 139, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Urbano, N.; Scimeca, M.; Tancredi, V.; Bonanno, E.; Schillaci, O. 99mTc-sestamibi breast imaging: Current status, new ideas and future perspectives. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Dash, A.; Venkatesh, M. A novel electrochemical technique for the production of clinical grade 99mTc using (n,γ)99Mo. Nucl. Med. Biol. 2010, 37, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H. Types of bulk liquid membrane and its membrane resistance in heavy metal removal and recovery from wastewater. Desalin. Water Treat. 2016, 57, 19785–19793. [Google Scholar] [CrossRef]
- I.A.E.A. Radiotracer Generators for Industrial Applications; International Atomic Energy Agency: Vienna, Austria, 2013. [Google Scholar]
- Molinski, V.J. A review of 99mTc generator technology. Int. J. Appl. Rad. Isot. 1982, 33, 811–819. [Google Scholar] [CrossRef]
- I.A.E.A. Cyclotron Based Production of Technetium-99m; International Atomic Energy Agency: Vienna, Austria, 2017. [Google Scholar]
- Nawar, M.F.; Türler, A. Development of New Generation of 99Mo/99mTc Radioisotope Generators to Meet the Continuing Clinical Demands. In Proceedings of the 2nd International Conference on Radioanalytical and Nuclear Chemistry (RANC 2019), Budapest, Hungary, 5–10 May 2019. [Google Scholar]
- Knapp, F., Jr.; Baum, R. Radionuclide generators-a new renaissance in the development of technologies to provide diagnostic and therapeutic radioisotopes for clinical applications. Curr. Radiopharm. 2012, 5, 175–177. [Google Scholar] [CrossRef]
- Osso, J.A., Jr.; Catanoso, M.F.; Barrio, G.; Brambilla, T.P.; Teodoro, R.; Dias, C.R.B.R.; Suzuki, K.N. Technetium-99m: New Production and Processing Strategies to Provide Adequate Levels for SPECT Imaging. Curr. Radiopharm. 2012, 5, 178–186. [Google Scholar] [CrossRef]
- N.A.P. Molybdenum-99 for Medical Imaging; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef] [Green Version]
- Qaim, S.M. The present and future of medical radionuclide production. Radiochim. Acta 2012, 100, 635–651. [Google Scholar] [CrossRef]
- I.A.E.A. Non-HEU Production Technologies for Molybdenum-99 and Technetium-99m; International Atomic Energy Agency: Vienna, Austria, 2013. [Google Scholar]
- I.A.E.A. Feasibility of Producing Molybdenum-99 on a Small Scale Using Fission of Low Enriched Uranium or Neutron Activation of Natural Molybdenum; International Atomic Energy Agency: Vienna, Austria, 2015. [Google Scholar]
- Evans, J.V.; Moore, P.W.; Shying, M.E.; Sodeau, J.M. Zirconium molybdate gel as a generator for technetium-99m—I. The concept and its evaluation. Int. J. Rad. Appl. Instrum. A 1987, 38, 19–23. [Google Scholar] [CrossRef]
- Maoliang, L. Production of gel-type Tc-99m generator for nuclear medicine. In Proceedings of the 12th KAIF/KNS Annual Conference, Seoul, Korea, 3–4 April 1997. [Google Scholar]
- Mostafa, M.; Saber, H.M.; El-Sadek, A.A.; Nassar, M.Y. Preparation and performance of 99Mo/99mTc chromatographic column generator based on zirconium molybdosilicate. Radiochim. Acta 2016, 104, 257–265. [Google Scholar] [CrossRef]
- Sekimoto, S.; Tatenuma, K.; Suzuki, Y.; Tsuguchi, A.; Tanaka, A.; Tadokoro, T.; Kani, Y.; Morikawa, Y.; Yamamoto, A.; Ohtsuki, T. Separation and purification of 99mTc from 99Mo produced by electron linear accelerator. J. Radioanal. Nucl. Chem. 2017, 311, 1361–1366. [Google Scholar] [CrossRef]
- Sebastian, V.; Arruebo, M.; Santamaria, J. Reaction Engineering Strategies for the Production of Inorganic Nanomaterials. Small 2014, 10, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A. Wet Chemical Methods for Nanop article Synthesis. In Chemical Methods for Processing Nanomaterials, 1st ed.; Singh, V.N., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 49–58. [Google Scholar]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Priya, S.D.; Nesaraj, A.S.; Selvakumar, A.I. Facile wet-chemical synthesis and evaluation of physico-chemical characteristics of novel nanocrystalline NdCoO3-based perovskite oxide as cathode for LT-SOFC applications. Bull. Mater. Sci. 2021, 44, 115. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Kigozi, M.; Ezealigo, B.N.; Onwualu, A.P.; Dzade, N.Y. Hydrothermal Synthesis of Metal Oxide Composite Cathode Materials for High Energy Application. In Chemically Deposited Nanocrystalline Metal Oxide Thin Films: Synthesis, Characterizations, and Applications, 1st ed.; Ezema, F.I., Lokhande, C.D., Rajan, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 489–508. [Google Scholar] [CrossRef]
- Sakr, T.M.; Nawar, M.F.; Fasih, T.; El-Bayoumy, S.; Abd El-Rehim, H.A. Nano-technology contributions towards the development of high performance radioisotope generators: The future promise to meet the continuing clinical demand. Appl. Radiat. Isot. 2017, 129, 67–75. [Google Scholar] [CrossRef]
- Madkour, M.; Allam, O.G.; Abdel Nazeer, A.; Amin, M.O.; Al-Hetlani, E. CeO2-based nanoheterostructures with p–n and n–n heterojunction arrangements for enhancing the solar-driven photodegradation of rhodamine 6G dye. J. Mater. Sci. Mater. Electron. 2019, 30, 10857–10866. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Wang, Z.; Liu, W.; Wanga, L. Catalytic oxidation of CO over mesoporous copper-doped ceria catalysts via a facile CTAB-assisted synthesis. RSC Adv. 2018, 8, 14888–14897. [Google Scholar] [CrossRef] [Green Version]
- Ivanets, A.; Kitikova, N.; Shashkova, I.; Radkevich, A.; Stepanchuk, T.; Maslova, M.; Mudruk, N. One-Stage Adsorption Treatment of Liquid Radioactive Wastes with Complex Radionuclide Composition. Water Air Soil Pollut. 2020, 231, 144. [Google Scholar] [CrossRef]
- Metwally, S.S.; Attallah, M.F. Impact of surface modification of chabazite on the sorption of iodine and molybdenum radioisotopes from liquid phase. J. Mol. Liq. 2019, 290, 111237. [Google Scholar] [CrossRef]
- Hashem, A.; Sanousy, M.A.; Mohamed, L.A.; Okoye, P.U.; Hameed, B.H. Natural and Low-Cost P. turgidum for Efficient Adsorption of Hg(II) Ions from Contaminated Solution: Isotherms and Kinetics Studies. J. Polym. Environ. 2021, 29, 304–312. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, R.; Falyouna, O.; Bensaida, K.; Sugihara, Y.; Eljamal, O. Insights into kinetics, isotherms and thermodynamics of phosphorus sorption onto nanoscale zero-valent iron. J. Mol. Liq. 2021, 328, 115402. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Hashem, A.; Badawy, S.M.; Farag, S.; Mohamed, L.A.; Fletcher, A.J.; Taha, G.M. Non-linear adsorption characteristics of modified pine wood sawdust optimised for adsorption of Cd(II) from aqueous systems. J. Environ. Chem. Eng. 2020, 8, 103966. [Google Scholar] [CrossRef]
- Chatterjee, R.; Majumder, C. Modelling of adsorption process in industrial wastewater treatment—A review. J. Indian Chem. Soc. 2019, 96, 499–506. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- McKay, G.; Poots, V.J.P. Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. J. Chem. Technol. Biotechnol. 1980, 30, 279–292. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elmaghraby, E.K. Retention behavior of cesium radioisotope on poly (acrylamido-sulfonic acid) synthesized by chain polymerization. Appl. Radiat. Isot. 2019, 146, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, S.; Toyohara, Y.; Saha, G.C.; Awual, M.R.; Kuba, T. Development of synthetic zeolites from bio-slag for cesium adsorption: Kinetic, isotherm and thermodynamic studies. J. Water Process. Eng. 2020, 33, 101055. [Google Scholar] [CrossRef]
- Benmessaoud, A.; Nibou, D.; Mekatel, E.H.; Amokrane, S. A Comparative Study of the Linear and Non-Linear Methods for Determination of the Optimum Equilibrium Isotherm for Adsorption of Pb2+ Ions onto Algerian Treated Clay. Iran. J. Chem. Chem. Eng. 2020, 39, 153–171. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Saad, E.A.; El-Khatib, A.M.; Soliman, M.A.; Allam, E.A. Adsorptive removal of radioactive isotopes of cobalt and zinc from water and radioactive wastewater using TiO2/Ag2O nanoadsorbents. Prog. Nucl. Energy 2018, 106, 51–63. [Google Scholar] [CrossRef]
- Karimzadeh, L.; Lippold, H.; Stockmann, M.; Fischer, C. Effect of DTPA on europium sorption onto quartz—Batch sorption experiments and surface complexation modeling. Chemosphere 2020, 239, 124771. [Google Scholar] [CrossRef]
- Luo, W.; Chen, J.; Lin, H.; Ye, X. Biomass base membrane with phenol hydroxy-amino group for ultraselective adsorption of radioactive Co(II) in trace concentration. Sep. Purif. Technol. 2021, 272, 118878. [Google Scholar] [CrossRef]
- Awual, M.R.; Khraisheh, M.; Alharthi, N.H.; Luqman, M.; Islam, A.; Karim, M.R.; Rahman, M.M.; Khaleque, M.A. Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem. Eng. J. 2018, 343, 118–127. [Google Scholar] [CrossRef]
- Zsigmondy, R. Über amikroskopische Goldkeime. I. Z. Für Phys. Chem. 1906, 56U, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physiochim. URSS 1940, 12, 217–225. [Google Scholar]
- Chen, T.; Wang, Q.; Lyu, J.; Bai, P.; Guo, X. Boron removal and reclamation by magnetic magnetite (Fe3O4) nanoparticle: An adsorption and isotopic separation study. Sep. Purif. Technol. 2020, 231, 115930. [Google Scholar] [CrossRef]
- Marcu, C.; Balla, A.; Balázs, J.Z.S.; Lar, C. Adsorption Isotherms and Thermodynamics for Chromium(VI) Using an Anion Exchange Resin. Anal. Lett. 2021, 54, 1783–1793. [Google Scholar] [CrossRef]





| Sample | SBET, (m2·g−1) | Pore Volume, (cm3·g−1) | Pore Size, (nm) | Crystallite Size, (nm) | pHIEP |
|---|---|---|---|---|---|
| CP-1 | 150.2 | 0.085 | 2.26 | 2.50 | 6.91 |
| CP-2 | 175.3 | 0.057 | 2.38 | 4.62 | 6.92 |
| CP-3 | 97.4 | 0.031 | 2.33 | 5.66 | 6.94 |
| HT-1 | 187.2 | 0.128 | 2.75 | 3.54 | 6.99 |
| HT-2 | 179.8 | 0.122 | 2.71 | 3.61 | 7.00 |
| HT-3 | 114.7 | 0.078 | 2.71 | 3.50 | 7.02 |
| Kinetic Model | Parameter | CP-2 | HT-1 |
|---|---|---|---|
| Lageregen-first-order | qe,1 (mg·g−1) | 4.686 | 4.881 |
| K1 (min−1) | 0.171 | 0.170 | |
| R2 | 0.990 | 0.989 | |
| Pseudo-second-order | qe,2 (mg·g−1) | 4.751 | 4.930 |
| K2 (g/mg·min) | 0.128 | 0.127 | |
| R2 | 0.991 | 0.990 | |
| Elovich | α (mg/g·min) | 3.877 × 1016 | |
| β (g·mg−1) | 9.874 | 9.48458 | |
| R2 | 0.999 | 0.999 |
| Diffusion Model | Parameter | CP-2 | HT-1 |
|---|---|---|---|
| McKay (Film diffusion) | (min−1) | 0.013 | 0.004 |
| intercept | −2.218 | −2.425 | |
| R2 | 0.992 | 0.968 | |
| Weber and Morris (Intra-particle diffusion) | (mg/g·min1/2) | 0.036 | 0.019 |
| Intercept (C) * | 4.224 | 4.531 | |
| R2 | 0.977 | 0.946 | |
| Bangham (Pore diffusion) | σ ** | 0.034 | 0.024 |
| intercept | −2.462 | −2.431 | |
| R2 | 0.967 | 0.980 |
| Isotherm Model | Parameter | CP-2 | HT-1 |
|---|---|---|---|
| Langmuir | Qmax (mg·g−1) | 96.083 | 109.114 |
| KL (L·mg−1) | 0.005 | 0.012 | |
| R2 | 0.958 | 0.983 | |
| X2 | 41.989 | 138.843 | |
| APE | 28.75 | 36.032 | |
| RMSE | 7.190 | 11.185 | |
| Freundlich | KF (mg1-n·Ln·g−1) | 8.668 | 15.078 |
| n | 3.350 | 3.981 | |
| R2 | 0.986 | 0.989 | |
| X2 | 3.888 | 4.678 | |
| APE | 11.442 | 15.304 | |
| RMSE | 4.060 | 5.524 | |
| Temkin | bt | 0.260 | 0.266 |
| At (L·g−1) * | 1.535 | 8.199 | |
| R2 | 0.794 | 0.756 | |
| X2 | 32.407 | 70.355 | |
| APE | 81.781 | 137.723 | |
| RMSE | 15.917 | 28.175 |
| CeO2 NPs | Temperature (K) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (kJ·mol−1·K−1) | R2 |
|---|---|---|---|---|---|
| CP-2 | 298 | −12.338 | 23.620 | 0.121 | 0.970 |
| 313 | −14.149 | ||||
| 323 | −15.355 | ||||
| 333 | −16.562 | ||||
| HT-1 | 298 | −14.676 | 7.959 | 0.076 | 0.867 |
| 313 | −15.816 | ||||
| 323 | −16.575 | ||||
| 333 | −17.335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawar, M.F.; El-Daoushy, A.F.; Madkour, M.; Türler, A. Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies. Nanomaterials 2022, 12, 1587. https://doi.org/10.3390/nano12091587
Nawar MF, El-Daoushy AF, Madkour M, Türler A. Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies. Nanomaterials. 2022; 12(9):1587. https://doi.org/10.3390/nano12091587
Chicago/Turabian StyleNawar, Mohamed F., Alaa F. El-Daoushy, Metwally Madkour, and Andreas Türler. 2022. "Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies" Nanomaterials 12, no. 9: 1587. https://doi.org/10.3390/nano12091587
APA StyleNawar, M. F., El-Daoushy, A. F., Madkour, M., & Türler, A. (2022). Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies. Nanomaterials, 12(9), 1587. https://doi.org/10.3390/nano12091587






