Encapsulation of Volatile Monoterpene Fragrances in Mesoporous Organosilica Nanoparticles and Potential Application in Fruit Preservation
Abstract
1. Introduction
2. Experiments
2.1. Materials and Methods
2.2. Synthesis of 1,4-Bis(triethoxysilyl)benzene
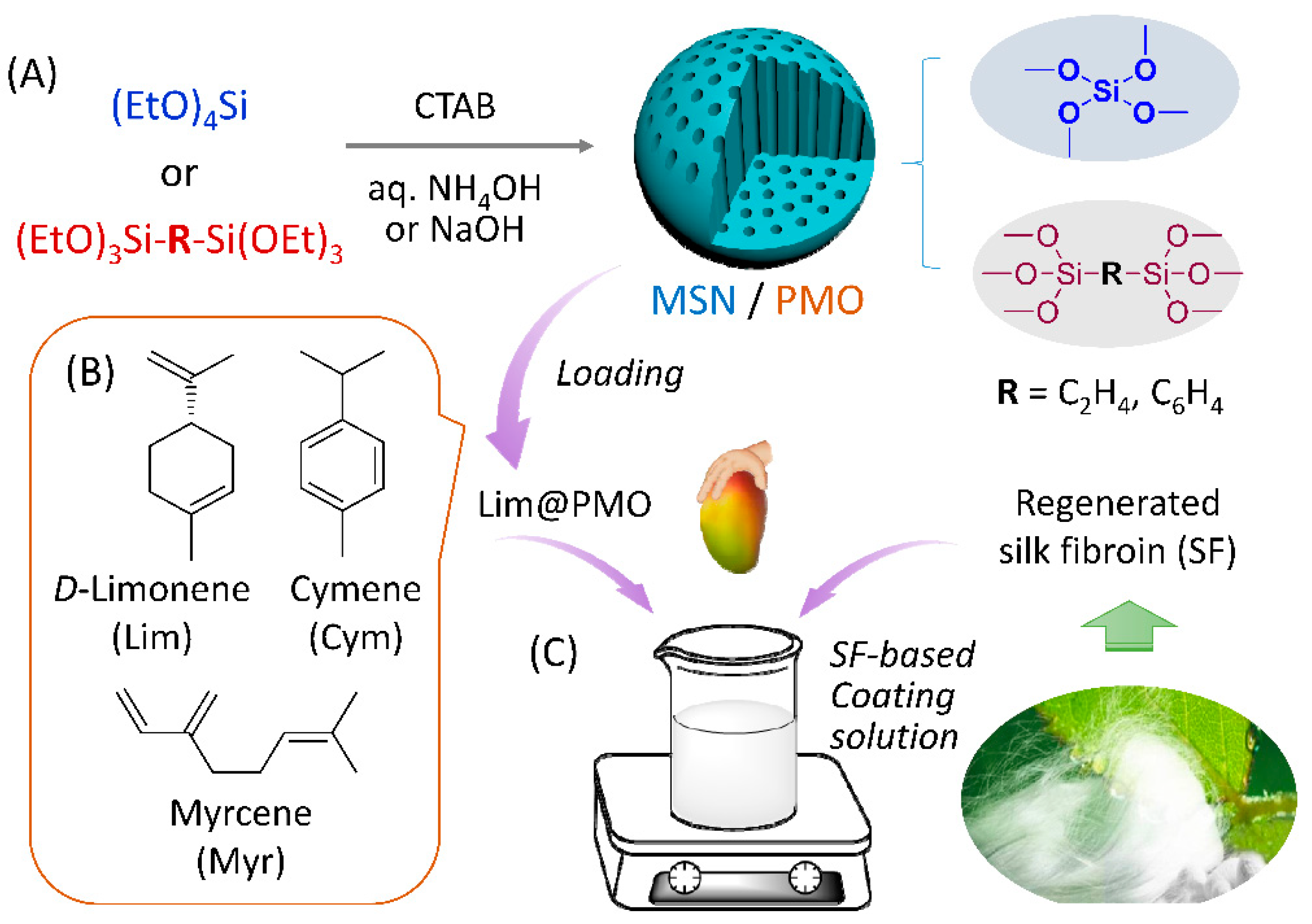
2.3. Synthesis of Mesoporous Silica and Organosilica Nanomaterials
2.4. Encapsulation of Model Fragrances in NPs
2.5. Fragrance Release Experiments Using Gravimetrical Analysis
2.6. Computational Methodology
2.7. Preparation of SF-Based Nanocomposite Coating and Its Preservation Function
3. Results and Discussion
3.1. Fabrication and Characterization of Nanocarrier Materials
3.2. Encapsulation of Fragrances in the Mesoporous Matrices
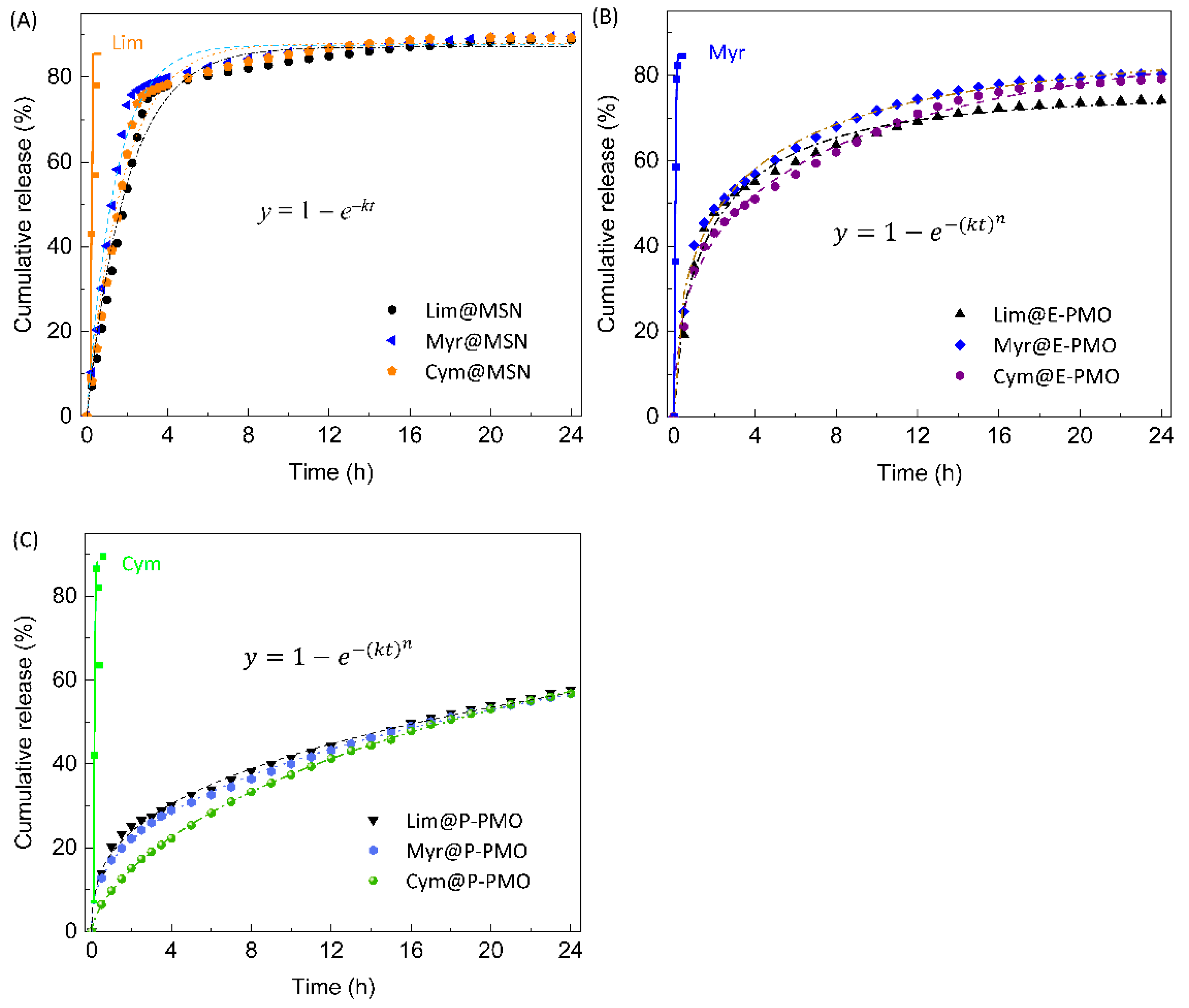
3.3. Kinetics of Fragrance Release
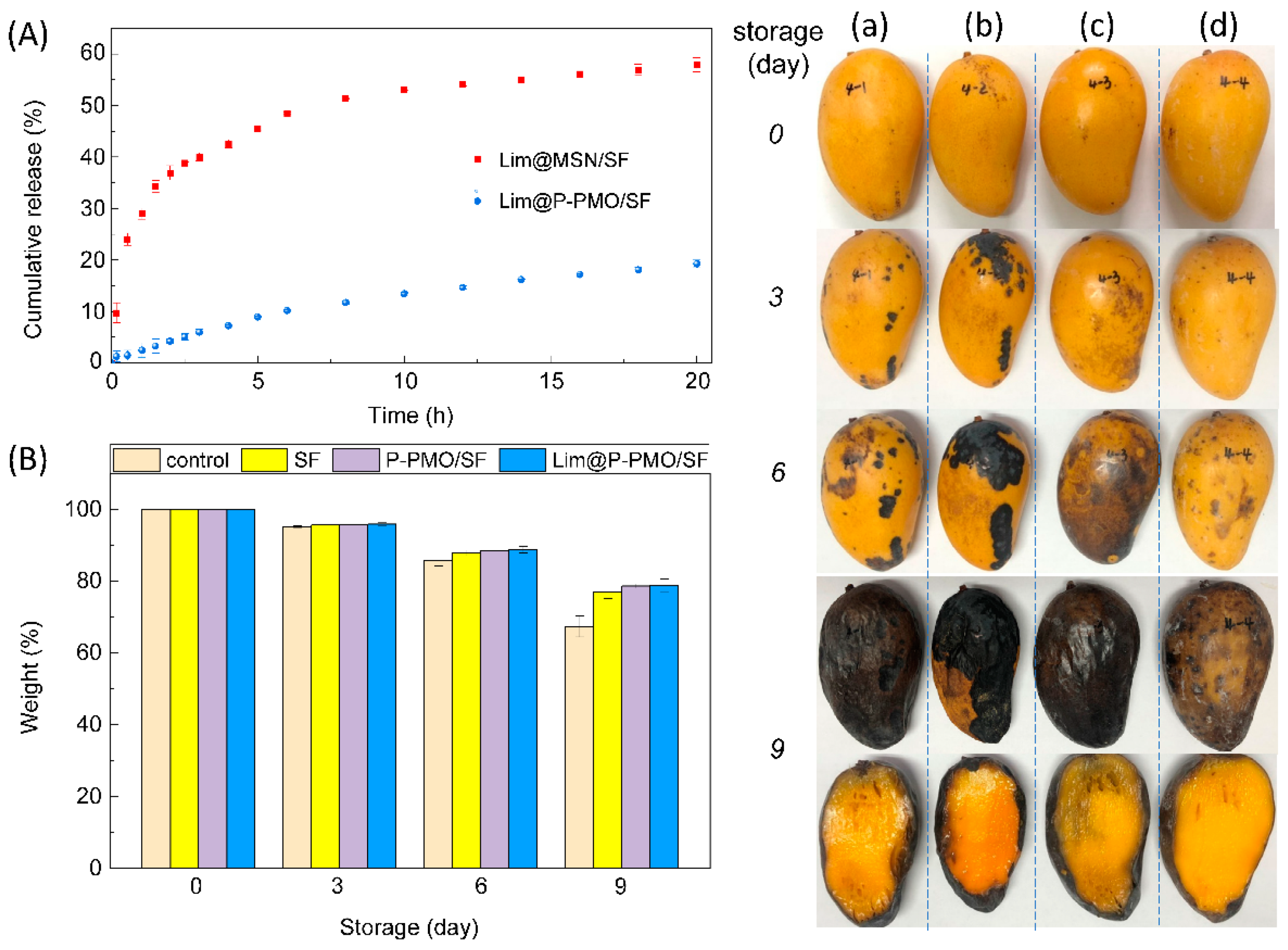
3.4. The Preservation Property of Lim@P-PMO/SF Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, A.J.; Beserra, F.P.; Souza, M.; Totti, B.; Rozza, A. Limonene: Aroma of innovation in health and disease. Chem.-Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety evaluation and risk assessment of d-limonene. J. Toxicol. Environ. Health Part B 2013, 16, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of limonene as an antimicrobial agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Umagiliyage, A.L.; Becerra-Mora, N.; Kohli, P.; Fisher, D.J.; Choudhary, R. Antimicrobial efficacy of liposomes containing d-limonene and its effect on the storage life of blueberries. Postharvest Biol. Technol. 2017, 128, 130–137. [Google Scholar] [CrossRef]
- Luo, S.M.; Chen, J.D.; He, J.; Li, H.S.; Jia, Q.; Hossen, M.A.; Dai, J.W.; Qin, W.; Liu, Y.W. Preparation of corn starch/rock bean protein edible film loaded with d-limonene particles and their application in glutinous rice cake preservation. Int. J. Biol. Macromol. 2022, 206, 313–324. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated limonene: A pleasant lemon-like aroma with promising application in the agri-food industry. A Review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Sol-gel microencapsulation of odorants and favors: Opening the route to sustainable fragrances and aromas. Chem. Soc. Rev. 2013, 42, 9243–9250. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hu, J.; Deng, W. Preparation and application of flavor and fragrance capsules. Polym. Chem. 2019, 9, 4926–4946. [Google Scholar] [CrossRef]
- Elabbadi, A.; Jerri, H.A.; Ouali, L.; Erni, P. Sustainable delivery systems: Retention of model volatile oils trapped on hybrid calcium carbonate microparticles. ACS Sustain. Chem. Eng. 2015, 3, 2178–2186. [Google Scholar] [CrossRef]
- Akhavan-Mahdavi, S.; Sadeghi, R.; Esfanjani, A.F.; Hedayati, S.; Shaddel, R.; Dima, C.; Malekjani, N.; Boostani, S.; Jafari, S.M. Nanodelivery systems for d-limonene; techniques and applications. Food Chem. 2022, 384, 132479. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Nanoencapsulation of d-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocoll. 2018, 77, 152–162. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, J.; Jiang, L.; Zhu, W.; Zhang, X.; Xiao, Z.; Shen, Y. Nanotechnology in fragrances: Current status and future prospects. Sci. Sin. Chim. 2019, 49, 575–580. [Google Scholar] [CrossRef]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M. Nanoencapsulation of lemon essential oil in Chitosan-Hicap system. Part 1: Study on its physical and structural characteristics. Int. J. Biol. Macromol. 2018, 115, 143–151. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M.; Tabarestani, H.S. Production of d-limonene-loaded Pickering emulsions stabilized by chitosan nanoparticles. Food Chem. 2021, 354, 129591. [Google Scholar] [CrossRef]
- Ganje, M.; Jafari, S.M.; Tamadon, A.M.; Niakosari, M.; Maghsoudlou, Y. Mathematical and fuzzy modeling of limonene release from amylose nanostructures and evaluation of its release kinetics. Food Hydrocoll. 2019, 95, 186–194. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, M.; Yao, X.; Wu, K.; Zhang, K.; He, Y.; Nishinari, K.; Phillips, G.O.; Yao, X.; Jiang, F. Effect of zein-based microencapsules on the release and oxidation of loaded limonene. Food Hydrocoll. 2018, 84, 330–336. [Google Scholar] [CrossRef]
- Dede, S.; Lokumcu Altay, F. Nanofibre encapsulation of limonene and modelling its release mechanisms. Acta Aliment. 2019, 48, 56–64. [Google Scholar] [CrossRef]
- Humblet-Hua, K.; Scheltens, G.; Van Der Linden, E.; Sagis, L. Encapsulation systems based on ovalbumin fibrils and high methoxyl pectin. Food Hydrocoll. 2011, 25, 569–576. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, Y.; Yang, X.; Chen, A. Prolonged-release performance of perfume encapsulated by tailoring mesoporous silica spheres. Flavour Fragr. J. 2008, 23, 29–34. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, T.; Shen, J.; Xiao, Z.; Hu, J.; Niu, Y.; Yu, D.; Chen, L.; Zhang, X. Effects of fragrance-loaded mesoporous silica nanocolumns on the central nervous system. J. Biomed. Nanotechnol. 2018, 14, 1578–1589. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, J.P.; Liu, Z.C.; Huang, Y.J.; Xiong, X.P. Solid particles surface-modified with beta-cyclodextrin for sustained release of flavor. Mater. Today Commun. 2022, 33, 104905. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Ye, L.; Lv, Y.; Zhou, Z.; Shen, Y.; He, Y.; Jiang, L. Encapsulation of highly volatile fragrances in zeolites Y for sustained release: Experimental and theoretical studies. ACS Omega 2020, 5, 31925–31935. [Google Scholar] [CrossRef]
- Vaughn, J.; Wu, H.; Efremovska, B.; Olson, D.H.; Mattai, J.; Ortiz, C.; Puchalski, A.; Li, J.; Pan, L. Encapsulated recyclable porous materials: An effective moisture-triggered fragrance release system. Chem. Commun. 2013, 49, 5724–5726. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Y.X.; Huang, J.X.; Zhou, Z.X.; Zhao, D.; Jiang, L.M.; Shen, Y.Q. Encapsulation and controlled release of fragrances from functionalized porous metal-organic frameworks. AIChE J. 2019, 65, 491–499. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Zhou, B.; Mei, L.; Wang, Q.; Zhang, B. Porous metal-organic framework (MOF) carrier for incorporation of volatile antimicrobial essential oil. Food Control. 2019, 98, 174–178. [Google Scholar] [CrossRef]
- Monteagudo-Olivan, R.; Cocero, M.J.; Coronas, J.; Rodriguez-Rojo, S. Supercritical CO2 encapsulation of bioactive molecules in carboxylate based MOFs. J. CO2 Util. 2019, 30, 38–47. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.X.; Liu, K.; Zhou, Z.X.; Jiang, L.M.; Shen, Y.Q.; Zhao, D. Biocompatible cyclodextrin-based metal−organic frameworks for long-term sustained release of fragrances. Ind. Eng. Chem. Res. 2019, 58, 19767–19777. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, H.; Hu, Q.; Jiang, L.; Shen, Y.; Zhao, D. CelluMOFs: Green, facile, and flexible metal-organic frameworks for versatile applications. Adv. Funct. Mater. 2021, 31, 2105395. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, H.; Jiang, L.; Shen, Y.; Zhao, D.; Zhou, Z. A breathing A4 paper by in situ growth of green metal–organic frameworks for air freshening and cleaning. Chin. J. Chem. Engi. 2022, 52, 95–102. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoen, X.; Durand, J.-O.; Wong Chi Man, M.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lofgreen, J.E.; Ozin, G.A. Why PMO? Towards functionality and utility of periodic mesoporous organosilicas. Small 2016, 6, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Ganji, N.; Pourshiani, O.; Thiel, W.R. Periodic mesoporous organosilicas (PMOs): From synthesis strategies to applications. Prog. Mater. Sci. 2022, 125, 100896. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A.; Webster, O. Arylsilsesquioxane gels and related materials. New hybrids of organic and inorganic networks. J. Am. Chem. Soc. 1992, 114, 6700–6710. [Google Scholar] [CrossRef]
- Park, C.; Lee, K.; Kim, C. Photoresponsive cyclodextrin-covered nanocontainers and their sol-gel transition induced by molecular recognition. Angew. Chem. Int. Ed. 2009, 48, 1275–1278. [Google Scholar] [CrossRef]
- Guan, B.Y.; Cui, Y.; Ren, Z.Y.; Qiao, Z.A.; Wang, L.; Liu, Y.L.; Huo, Q.S. Highly ordered periodic mesoporous organosilica nanoparticles with controllable pore structures. Nanoscale 2012, 4, 6588–6596. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.X.; et al. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ye, L.; Li, Z.; Niu, R.; Zhou, Z.; Shen, Y.; Jiang, L. All-aqueous direct deposition of fragrance-loaded nanoparticles onto fabric surfaces by electrospraying. ACS Appl. Polym. Mater. 2019, 1, 2590–2596. [Google Scholar] [CrossRef]
- Knežević, N.; Ilić, N.; Đokić, V.; Petrović, R.; Janaćković, Đ. Mesoporous silica and organosilica nanomaterials as UV-blocking agents. ACS Appl. Mater. Interfaces 2018, 10, 20231–20236. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Kennedy, G.J.; Kresge, C.T.; Roth, W.J.; Schramm, S.E. Molecular or supramolecular templating: Defining the role of surfactant chemistry in the formation of microporous and mesoporous molecular sieves. Chem. Mater. 1994, 6, 1816–1821. [Google Scholar] [CrossRef]
- Liang, Y.C.; Hanzlikd, M.; Anwander, R. Ethylene-bridged periodic mesoporous organosilicas with Fm3m symmetry. J. Mater. Chem. 2015, 15, 3919–3928. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems–−with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. Mesoporous silica molecular sieves prepared by ionic and neutral surfactant templating: A comparison of physical properties. Chem. Mater. 1996, 8, 2068–2079. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Argon adsorption at 77 K as a useful tool for the elucidation of pore connectivity in ordered materials with large cagelike mesopores. Chem. Mater. 2003, 15, 2942–2949. [Google Scholar] [CrossRef]
- Hao, N.; Yang, Y.; Wang, H.; Webley, P.A.; Zhao, D. Synthesis of large-pore phenyl-bridged mesoporous organosilica with thick walls by evaporation-induced self-assembly for efficient benzene adsorption. J. Colloid Interface Sci. 2010, 346, 429–435. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of D-limonene loaded polymeric nanoparticles with enhanced antimicrobial properties for potential application in food packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef]
- Khoshakhlagh, K.; Mohebbi, M.; Koocheki, A.; Allafchian, A. Encapsulation of D-limonene in Alyssum homolocarpum seed gum nanocapsules by emulsion electrospraying: Morphology characterization and stability assessment. Bioact. Carbohydr. Diet. Fibre 2018, 16, 43–52. [Google Scholar] [CrossRef]
- Royal Society of Chemistry. ChemSpider, a Free Chemical Structure Database. Available online: http://www.chemspider.com (accessed on 5 September 2022).
- Kurczewskaa, J.; Cegłowskia, M.; Messyaszb, B.; Schroedera, G. Dendrimer-functionalized halloysite nanotubes for effective drug delivery. Appl. Clay Sci. 2018, 153, 134–143. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bergera, D.; Nastasea, S.; Mitrana, R.A.; Petrescua, M.; Vasileb, E.; Mateia, C.; Negreanu-Pirjolc, T. Mesostructured silica and aluminosilicate carriers for oxytetracycline delivery systems. Int. J. Pharm. 2016, 510, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate metal-organic frameworks for dialing-in the binding and programming the release of drug molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Brenckle, M.A.; Kaplan, D.L.; Omenetto, F.G. Silk fibroin as edible coating for perishable food preservation. Sci. Rep. 2016, 6, 25263. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Gamage, M.V.; Dumée, L.F.; Kong, L.; Zhao, S. Edible films and coatings for shelf life extension of mango: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2432–2459. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.D.; Hollingsworth, R.G.; Leroux, E.; Salmieri, S.; Lacroix, M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res. Int. 2011, 44, 198–203. [Google Scholar] [CrossRef]
- Dhitala, R.; Morab, N.B.; Watsona, D.G.; Kohlib, P.; Choudharya, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT–Food Sci. Technol. 2018, 97, 124–134. [Google Scholar] [CrossRef]
- El-Ghaouth, A.; Arul, J.; Ponnampalam, R.; Boulet, M. Use of chitosan coating to reduce water loss and maintain quality of cucumber and bell pepper fruits. J. Food Process. Preserv. 1991, 15, 359–368. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Xu, J.; Macqueen, L.A.; Attac, Z.; Peters, M.M.; Zimmerman, J.F.; Xu, T.; Demokritou, P.; Parker, K.K. High-throughput coating with biodegradable antimicrobial pullulan fibres extends shelf life and reduces weight loss in an avocado model. Nat. Food 2022, 3, 428–436. [Google Scholar] [CrossRef]
- Kim, U.J.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775. [Google Scholar] [CrossRef] [PubMed]

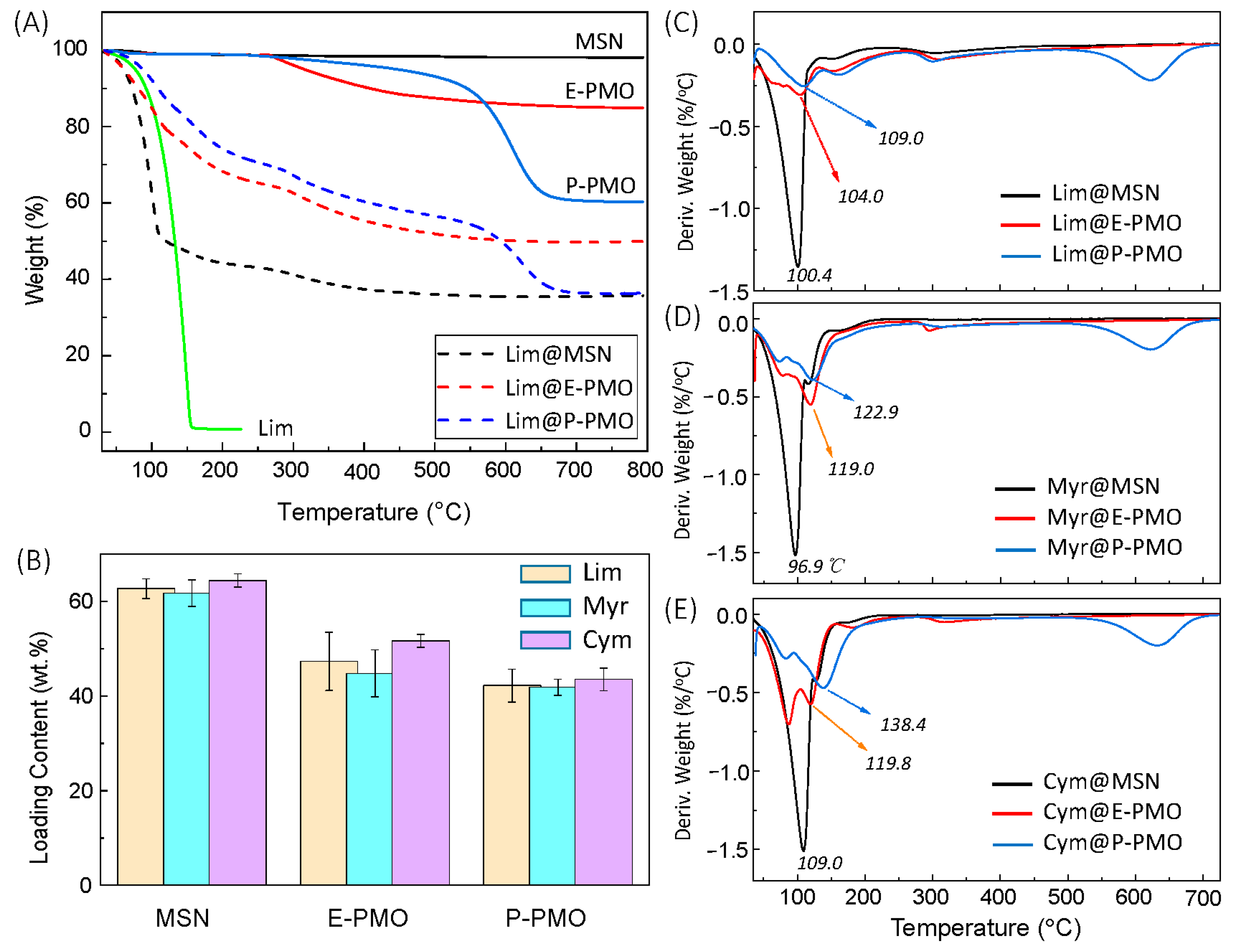
| Entry | Sample | T/°C; t/h | dTEMb/nm | SBETc/m2 g−1 | Vpd/cm3 g−1 | dpe/nm | dp,peakf/nm |
|---|---|---|---|---|---|---|---|
| 1 | MSN | 80; 2 | 81.9 ± 7.9 | 882.5 | 2.04 | 6.42 | 2.53 |
| 2 | E-PMO | 50; 6 | 104.4 ± 14.4 | 1082.3 | 1.45 | 3.71 | 2.82 |
| 3 | E-PMO | 80; 2 | 120.2 ± 19.8 | 904.3 | 0.73 | 2.58 | 2.53 |
| 4 | P-PMO | 50; 6 | 120.1 ± 22.6 | 1300.0 | 1.46 | 3.28 | 2.00 |
| 5 | P-PMO | 80; 2 | 105.4 ± 4.9 | 1654.0 | 1.34 | 2.52 | 2.26 |
| Entry | Samples | Kinetic Model b | R2 | k (h−1) | t50% (h) | n |
|---|---|---|---|---|---|---|
| 1 | Lim@MSN | first-order | 0.9802 | 0.4981 | 1.86 | – |
| 2 | Myr@MSN | 0.9835 | 0.7125 | 1.26 | – | |
| 3 | Cym@MSN | 0.9820 | 0.5631 | 1.61 | – | |
| 4 | Lim@E-PMO | Weibull | 0.9900 | 0.4287 | 2.43 | 0.5895 |
| 5 | Myr@E-PMO | 0.9958 | 0.2930 | 2.23 | 0.4810 | |
| 6 | Cym@E-PMO | 0.9947 | 0.1377 | 3.67 | 0.4624 | |
| 7 | Lim@P-PMO | 0.9966 | 0.0203 | 16.20 | 0.3590 | |
| 8 | Myr@P-PMO | 0.9991 | 0.0064 | 16.92 | 0.3970 | |
| 9 | Cym@P-PMO | 0.9998 | 0.0309 | 17.56 | 0.6576 |
| Fragrances | MSN | E-PMO | P-PMO |
|---|---|---|---|
| Lim | −11.99 | −15.04 | −19.98 |
| Cym | −12.41 | −13.84 | −22.23 |
| Myr | −9.91 | −13.91 | −21.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Bai, T.; Liu, Y.; Lv, Y.; Zhou, Z.; Shen, Y.; Jiang, L. Encapsulation of Volatile Monoterpene Fragrances in Mesoporous Organosilica Nanoparticles and Potential Application in Fruit Preservation. Nanomaterials 2023, 13, 104. https://doi.org/10.3390/nano13010104
Zhao Y, Bai T, Liu Y, Lv Y, Zhou Z, Shen Y, Jiang L. Encapsulation of Volatile Monoterpene Fragrances in Mesoporous Organosilica Nanoparticles and Potential Application in Fruit Preservation. Nanomaterials. 2023; 13(1):104. https://doi.org/10.3390/nano13010104
Chicago/Turabian StyleZhao, Yuanjiang, Tianwen Bai, Yuhang Liu, Yichao Lv, Zhuxian Zhou, Youqing Shen, and Liming Jiang. 2023. "Encapsulation of Volatile Monoterpene Fragrances in Mesoporous Organosilica Nanoparticles and Potential Application in Fruit Preservation" Nanomaterials 13, no. 1: 104. https://doi.org/10.3390/nano13010104
APA StyleZhao, Y., Bai, T., Liu, Y., Lv, Y., Zhou, Z., Shen, Y., & Jiang, L. (2023). Encapsulation of Volatile Monoterpene Fragrances in Mesoporous Organosilica Nanoparticles and Potential Application in Fruit Preservation. Nanomaterials, 13(1), 104. https://doi.org/10.3390/nano13010104







