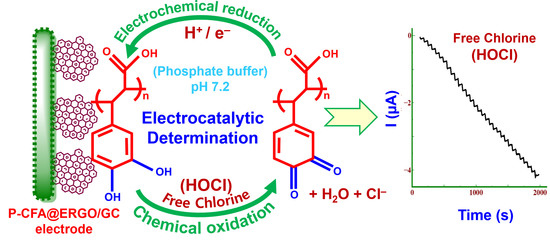
Poly(caffeic acid) Redox Couple Decorated on Electrochemically Reduced Graphene Oxide for Electrocatalytic Sensing Free Chlorine in Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Fabrication of p-CFA@ERGO/GC
3. Results and Discussion
3.1. GO Electrochemical Reduction and p-CFA Formation Mechanism on the ERGO Surface
3.2. p-CFA@ERGO/GC Electrochemical Study
3.3. Electron Microscopic Study of the Modified Electrode
3.4. X-ray Photoelectron and Raman Spectra Studies
3.5. Electrochemical Impedance Spectroscopy (EIS)
3.6. Electrocatalytic Free Chlorine Determination
3.7. Chronoamperometric Detection of Free Chlorine
3.8. Selectivity, Storage Stability, and Reproducibility Test
3.9. Realistic Drinking and Tap Water Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- White, G.C. Handbook of Chlorination, 2nd ed.; Van Nostrand Reinhold Co., Inc.: New York, NY, USA, 1986. [Google Scholar]
- Sobsey, M.D.; Handzel, T.; Venczel, L. Chlorination and safe storage of household drinking water in developing countries to reduce waterborne disease. Water Sci. Technol. 2003, 47, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.K.; Anupam, K.; Swaroop, V.; Lal, P.S.; Bist, V. Pilot scale soda-anthraquinone pulping of palm oil empty fruit bunches and elemental chlorine free bleaching of resulting pulp. J. Clean. Prod. 2015, 106, 422–429. [Google Scholar] [CrossRef]
- Ordeig, O.; Mas, R.; Gonzalo, J.; Del Campo, F.J.; Muñoz, F.J.; de Haro, C. Continuous Detection of Hypochlorous Acid/Hypochlorite for Water Quality Monitoring and Control. Electroanalysis 2005, 17, 1641–1648. [Google Scholar] [CrossRef]
- Skarzewski, J.; Siedlecka, R. Synthetic Oxidations with Hypochlorites. A review. Org. Prep. Proced. Int. 1992, 24, 623–647. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997, 10, 597–610. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality—Recommendations, 2nd ed.; WHO: Geneva, Switzerland, 1993; Volume 1. [Google Scholar]
- Pattison, D.I.; Davies, M.J. Evidence for Rapid Inter- and Intramolecular Chlorine Transfer Reactions of Histamine and Carnosine Chloramines: Implications for the Prevention of Hypochlorous-Acid-Mediated Damage. Biochemistry 2006, 45, 8152–8162. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency U.S. Environmental Protection Agency. Available online: https://www.epa.gov/dwreginfo/drinking-water-regulations (accessed on 20 September 2022).
- Soldatkin, A.P.; Gorchkov, D.V.; Martelet, C.; Jaffrezic-Renault, N. New enzyme potentiometric sensor for hypochlorite species detection. Sens. Actuators B Chem. 1997, 43, 99–104. [Google Scholar] [CrossRef]
- Wakigawa, K.; Gohda, A.; Fukushima, S.; Mori, T.; Niidome, T.; Katayama, Y. Rapid and selective determination of free chlorine in aqueous solution using electrophilic addition to styrene by gas chromatography/mass spectrometry. Talanta 2013, 103, 81–85. [Google Scholar] [CrossRef]
- Leggett, D.J.; Chen, N.H.; Mahadevappa, D.S. Rapid determination of residual chlorine by flow injection analysis. Analyst 1982, 107, 433–441. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Szili, M.; Kasik, I.; Matejec, V.; Nagy, G.; Kovacs, B. Poly(luminol) based sensor array for determination of dissolved chlorine in water. Sens. Actuators B Chem. 2014, 192, 92–98. [Google Scholar] [CrossRef]
- Murata, M.; Ivandini, T.A.; Shibata, M.; Nomura, S.; Fujishima, A.; Einaga, Y. Electrochemical detection of free chlorine at highly boron-doped diamond electrodes. J. Electroanal. Chem. 2008, 612, 29–36. [Google Scholar] [CrossRef]
- Kolnel, A.S.; Pathiratne, S.S.S.; Jayasena, E.M.C.M. Linear sweep voltammetric determination of free chlorine in waters using graphite working electrodes. J. Natl. Sci. Found. 2008, 36, 7. [Google Scholar] [CrossRef]
- Olivé-Monllau, R.; Orozco, J.; Fernández-Sánchez, C.; Baeza, M.; Bartrolí, J.; Jimenez-Jorquera, C.; Céspedes, F. Flow injection analysis system based on amperometric thin-film transducers for free chlorine detection in swimming pool waters. Talanta 2009, 77, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Zen, J.-M. Free chlorine detection based on EC’ mechanism at an electroactive polymelamine-modified electrode. Electrochem. Commun. 2014, 46, 87–90. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kesavan, S.; Nguyen, T.T.; Hwang, J.; Lamiel, C.; Shim, J.-J. Polydopamine@electrochemically reduced graphene oxide-modified electrode for electrochemical detection of free-chlorine. Sens. Actuators B Chem. 2017, 240, 818–828. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Wu, Z.-Y.; Chen, S.-M. Amperometric determination of sodium hypochlorite at poly MnTAPP-nano Au film modified electrode. J. Electroanal. Chem. 2011, 661, 322–328. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; García-García, F.J.; González-Mora, J.L.; González-Elipe, A.R. A novel and improved surfactant-modified Prussian Blue electrode for amperometric detection of free chlorine in water. Sens. Actuators B Chem. 2015, 213, 116–123. [Google Scholar] [CrossRef]
- Campo, F.J.D.; Ordeig, O.; Muñoz, F.J. Improved free chlorine amperometric sensor chip for drinking water applications. Anal. Chim. Acta 2005, 554, 98–104. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Zare, H.R.; Golabi, S.M. Caffeic acid modified glassy carbon electrode for electrocatalytic oxidation of reduced nicotinamide adenine dinucleotide (NADH). J. Solid State Electrochem. 2000, 4, 87–94. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Zhao, L.; Shen, S.; Yuan, M.; Liu, W.; Tu, Q.; Yu, R.; Wang, J. Au nanoparticles/poly(caffeic acid) composite modified glassy carbon electrode for voltammetric determination of acetaminophen. Talanta 2016, 159, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Li, N.B.; Ren, W.; Luo, H.Q. Simultaneous voltammetric measurement of ascorbic acid and dopamine on poly(caffeic acid)-modified glassy carbon electrode. J. Solid State Electrochem. 2008, 12, 693–699. [Google Scholar] [CrossRef]
- Lee, P.T.; Ward, K.R.; Tschulik, K.; Chapman, G.; Compton, R.G. Electrochemical Detection of Glutathione Using a Poly(caffeic acid) Nanocarbon Composite Modified Electrode. Electroanalysis 2014, 26, 366–373. [Google Scholar] [CrossRef]
- Ren, W.; Luo, H.Q.; Li, N.B. Simultaneous voltammetric measurement of ascorbic acid, epinephrine and uric acid at a glassy carbon electrode modified with caffeic acid. Biosens. Bioelectron. 2006, 21, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, N.B.; Ren, W.; Luo, H.Q. Caffeic Acid-Modified Glassy Carbon Electrode for the Simultaneous Determination of Epinephrine and Dopamine. Electroanalysis 2007, 19, 1496–1502. [Google Scholar] [CrossRef]
- Ren, W.; Luo, H.Q.; Li, N.B. Electrochemical Behavior of Epinephrine at a Glassy Carbon Electrode Modified by Electrodeposited Films of Caffeic Acid. Sensors 2006, 6, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.-Q.; Zhang, Q.; Zhu, L.; Wang, H.-F.; Shi, J.-L.; Wei, F. CaO-Templated Growth of Hierarchical Porous Graphene for High-Power Lithium–Sulfur Battery Applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Kesavan, S.; Abraham John, S. Stable determination of paracetamol in the presence of uric acid in human urine sample using melamine grafted graphene modified electrode. J. Electroanal. Chem. 2016, 760, 6–14. [Google Scholar] [CrossRef]
- Kesavan, S.; Raj, M.A.; John, S.A. Formation of electrochemically reduced graphene oxide on melamine electrografted layers and its application toward the determination of methylxanthines. Anal. Biochem. 2016, 496, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Rafighi, P.; Tavahodi, M.; Haghighi, B. Fabrication of a third-generation glucose biosensor using graphene-polyethyleneimine-gold nanoparticles hybrid. Sens. Actuators B Chem. 2016, 232, 454–461. [Google Scholar] [CrossRef]
- Kesavan, S.; John, S.A. A novel approach to fabricate stable graphene layers on electrode surfaces using simultaneous electroreduction of diazonium cations and graphene oxide. RSC Adv. 2016, 6, 62876–62883. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef]
- Marchezi, P.E.; Sonai, G.G.; Hirata, M.K.; Schiavon, M.A.; Nogueira, A.F. Understanding the Role of Reduced Graphene Oxide in the Electrolyte of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 23368–23376. [Google Scholar] [CrossRef]
- Kumar, D.R.; Nguyen, T.T.; Lamiel, C.; Shim, J.-J. Layered 2-D Bi2Se3 nanosheets intercalated by Ni(OH)2 and their supercapacitor performance. Mater. Lett. 2016, 165, 257–262. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile Synthesis of Three-Dimensional Ternary ZnCo2O4/Reduced Graphene Oxide/NiO Composite Film on Nickel Foam for Next Generation Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kannan, R.; Kim, A.R.; Kumar, G.G.; Nahm, K.S.; Yoo, D.J. Facile enhancement in proton conductivity of sulfonated poly (ether ether ketone) using functionalized graphene oxide—Synthesis, characterization, and application towards proton exchange membrane fuel cells. Colloid Polym. Sci. 2016, 294, 1197–1207. [Google Scholar] [CrossRef]
- Kesavan, S.; Kumar, D.R.; Lee, Y.R.; Shim, J.-J. Determination of tetracycline in the presence of major interference in human urine samples using polymelamine/electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2017, 241, 455–465. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Sun, J.; Wang, Y.; Zhang, J. Stable Nafion-functionalized graphene dispersions for transparent conducting films. Nanotechnology 2009, 20, 465605. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.A.; John, S.A. Fabrication of Electrochemically Reduced Graphene Oxide Films on Glassy Carbon Electrode by Self-Assembly Method and Their Electrocatalytic Application. J. Phys. Chem. C 2013, 117, 4326–4335. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Han, H.S.; Jeon, S. One-step chemical reduction of graphene oxide with oligothiophene for improved electrocatalytic oxygen reduction reactions. Carbon 2013, 61, 164–172. [Google Scholar] [CrossRef]
- Fletcher, S.; Black, V.J.; Kirkpatrick, I. A universal equivalent circuit for carbon-based supercapacitors. J. Solid State Electrochem. 2014, 18, 1377–1387. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, Y. A water-soluble sulfonate-BODIPY based fluorescent probe for selective detection of HOCl/OCl− in aqueous media. Analyst 2014, 139, 2986–2989. [Google Scholar] [CrossRef]







| Electrode | pH | Applied Potential (V) | Type of Reaction | Linear Range (μM) | Sensitivity (μA μM−1) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| Graphite electrode | 10 | +1.03 | Oxidation | 19.4–5830 | − | 19.4 | [16] |
| BDD electrode a | 8 | +1.40 | Oxidation | 0.39–1940 | 0.038 | 0.16 | [15] |
| Polymelamine/SPCE b | 7 | +0.125 | EC | 10–7000 | 0.058 | 5.5 | [18] |
| Au microelectrode | 5.5 | +0.35 | Reduction | 3.89–97.17 | 0.012 | 0.39 | [17] |
| Polydopamine/ERGO/GC | 7.2 | +0.15 | EC | 9.9–215.2 | 0.0071 | 0.044 | [19] |
| polyMnTAPP-Au/GC c | 10.4 | −0.15 | Reduction | 24–10,700 | − | 24.0 | [20] |
| p-CFA@ERGO/GC | 7.2 | +0.17 | EC’ | 20–20,000 | 0.0361 | 0.030 | This work |
| Samples | Free Chlorine Concentration before Addition (µmol L−1) | Electrochemical Method | DPD Method | ||||
|---|---|---|---|---|---|---|---|
| Added (µmol L−1) | Found (µmol L−1) | Recovery (RSD) (%) | Added (µmol L−1) | Found (µmol L−1) | Recovery (RSD) (%) | ||
| Drinking water-1 | 0 | 10 | 9.9 | 99.0 (1.428) | 10 | 10.1 | 101 (2.562) |
| Drinking water-2 | 0 | 20 | 19.9 | 99.5 (0.683) | 20 | 19.8 | 99.0 (1.241) |
| Tap water-1 | 0.50 | 10 | 9.85 | 98.5 (0.117) | 10 | 9.9 | 99.0 (0.354) |
| Tap water-2 | 0.56 | 20 | 19.95 | 99.9 (2.177) | 20 | 20.40 | 102 (1.935) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesavan, S.; Kumar, D.R.; Dhakal, G.; Kim, W.K.; Lee, Y.R.; Shim, J.-J. Poly(caffeic acid) Redox Couple Decorated on Electrochemically Reduced Graphene Oxide for Electrocatalytic Sensing Free Chlorine in Drinking Water. Nanomaterials 2023, 13, 151. https://doi.org/10.3390/nano13010151
Kesavan S, Kumar DR, Dhakal G, Kim WK, Lee YR, Shim J-J. Poly(caffeic acid) Redox Couple Decorated on Electrochemically Reduced Graphene Oxide for Electrocatalytic Sensing Free Chlorine in Drinking Water. Nanomaterials. 2023; 13(1):151. https://doi.org/10.3390/nano13010151
Chicago/Turabian StyleKesavan, Srinivasan, Deivasigamani Ranjith Kumar, Ganesh Dhakal, Woo Kyoung Kim, Yong Rok Lee, and Jae-Jin Shim. 2023. "Poly(caffeic acid) Redox Couple Decorated on Electrochemically Reduced Graphene Oxide for Electrocatalytic Sensing Free Chlorine in Drinking Water" Nanomaterials 13, no. 1: 151. https://doi.org/10.3390/nano13010151
APA StyleKesavan, S., Kumar, D. R., Dhakal, G., Kim, W. K., Lee, Y. R., & Shim, J.-J. (2023). Poly(caffeic acid) Redox Couple Decorated on Electrochemically Reduced Graphene Oxide for Electrocatalytic Sensing Free Chlorine in Drinking Water. Nanomaterials, 13(1), 151. https://doi.org/10.3390/nano13010151