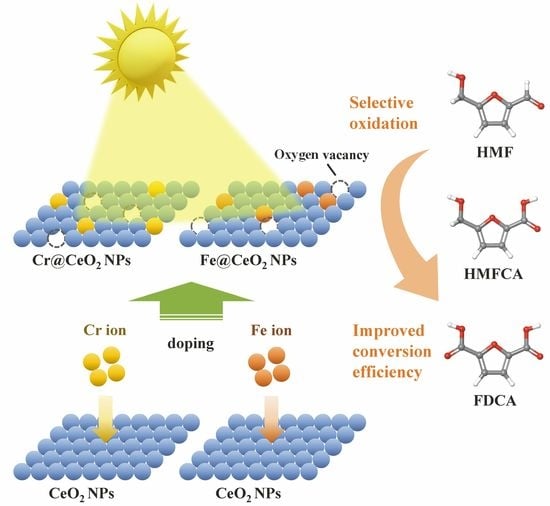
Photocatalysis of Cr- and Fe-Doped CeO2 Nanoparticles to Selective Oxidation of 5-Hydroxymethylfurfural
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and NP Preparation
2.2. Characterization
2.3. Photocatalytic Experiments
3. Results
3.1. Characterization of TM@CeO2 NPs
3.2. Electronic Properties of TM@CeO2 NPs
3.3. Selective Production of HMFCA and High Conversion Efficiency to FDCA by Photolysis of HMF in the Presence of TM@CeO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Li, Q.; Song, L.; Liang, Z.; Sun, M.; Wu, T.; Huang, B.; Luo, F.; Du, Y.; Yan, C.-H. A Review on CeO2-Based Electrocatalyst and Photocatalyst in Energy Conversion. Adv. Energy Sustain. Res. 2021, 2, 2000063. [Google Scholar] [CrossRef]
- Wang, F.; Wei, M.; Evans, D.G.; Duan, X. CeO2-based heterogeneous catalysts toward catalytic conversion of CO2. J. Mater. Chem. A 2016, 4, 5773–5783. [Google Scholar] [CrossRef]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron Localization Determines Defect Formation on Ceria Substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen Vacancies and Catalysis on Ceria Surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Bui, H.T.; Weon, S.; Bae, J.W.; Kim, E.-J.; Kim, B.; Ahn, Y.-Y.; Kim, K.; Lee, H.; Kim, W. Oxygen vacancy engineering of cerium oxide for the selective photocatalytic oxidation of aromatic pollutants. J. Hazard. Mater. 2021, 404, 123976. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.-S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Armandi, M.; Arletti, R.; Piumetti, M.; Bensaid, S.; Manzoli, M.; Panda, S.R.; Bonelli, B. Pure and Fe-doped CeO2 nanoparticles obtained by microwave assisted combustion synthesis: Physico-chemical properties ruling their catalytic activity towards CO oxidation and soot combustion. Appl. Catal. B 2017, 211, 31–45. [Google Scholar] [CrossRef]
- Lanzafame, P.; Centi, G.; Perathoner, S. Catalysis for biomass and CO2 use through solar energy: Opening new scenarios for a sustainable and low-carbon chemical production. Chem. Soc. Rev. 2014, 43, 7562–7580. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Uhrich, A.; Shen, Z.; Peng, B.; Ji, Z.; Muhler, M.; Zhao, G.; Wang, X.; Xu, X. Steering accessible oxygen vacancies for alcohol oxidation over defective Nb2O5 under visible light illumination. Appl. Catal. B 2021, 298, 120584. [Google Scholar] [CrossRef]
- Ventura, M.; Aresta , M.; Dibenedetto, A. Selective Aerobic Oxidation of 5-(Hydroxymethyl)furfural to 5-Formyl-2-furancarboxylic Acid in Water. ChemSusChem 2016, 9, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Nocito, F.; Ventura, M.; Aresta, M.; Dibenedetto, A. Selective Oxidation of 5-(Hydroxymethyl)furfural to DFF Using Water as Solvent and Oxygen as Oxidant with Earth-Crust-Abundant Mixed Oxides. ACS Omega 2018, 3, 18724–18729. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.P.H.; Pham, M.-T.; Bui, X.-T.; Wang, Y.-F.; You, S.-J. CeO2 as a photocatalytic material for CO2 conversion: A review. Sol. Energy 2022, 240, 443–466. [Google Scholar] [CrossRef]
- Ilanidis, D.; Wu, G.; Stagge, S.; Martín, C.; Jönsson, L.J. Effects of redox environment on hydrothermal pretreatment of lignocellulosic biomass under acidic conditions. Bioresour. Technol. 2021, 319, 124211. [Google Scholar] [CrossRef]
- Su, T.; Liu, Q.; Lü, H.; Ali Alasmary, F.; Zhao, D.; Len, C. Selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid using silver oxide supported on calcium carbonate. Mol. Catal. 2021, 502, 111374. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Z.; Xin, X.; Zhang, J.; Feng, Y.; Lv, H. Selective Valorization of 5-Hydroxymethylfurfural to 2,5-Diformylfuran Using Atmospheric O2 and MAPbBr3 Perovskite under Visible Light. ACS Catal. 2020, 10, 14793–14800. [Google Scholar] [CrossRef]
- Zhao, D.; Su, T.; Wang, Y.; Varma, R.S.; Len, C. Recent advances in catalytic oxidation of 5-hydroxymethylfurfural. Molecular Catalysis 2020, 495, 111133. [Google Scholar] [CrossRef]
- de Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. In Biobased Monomers, Polymers, and Materials; American Chemical Society: Washington, DC, USA, 2012; Volume 1105, pp. 1–13. [Google Scholar]
- Zhao, D.; Rodriguez-Padron, D.; Luque, R.; Len, C. Insights into the Selective Oxidation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-furancarboxylic Acid Using Silver Oxide. ACS Sustain. Chem. Eng. 2020, 8, 8486–8495. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Tian, Z.; Weng, Y.; Wang, C.; Ma, J.; Zhu, C.; Li, W.; Liu, Q.; Ma, L. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au/CeO2 catalysts: The morphology effect of CeO2. Catal. Sci. Technol. 2019, 9, 1570–1580. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Yan, C.; Song, H.; Zhang, Y.; Wei, Y.; Wang, K.; Li, B.; Yuan, S.; Yan, Y. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over MnOx-CeO2 Supported Palladium Nanocatalyst under Aqueous Conditions. ChemistrySelect 2020, 5, 10156–10162. [Google Scholar] [CrossRef]
- Yan, Y.; Li, K.; Zhao, J.; Cai, W.; Yang, Y.; Lee, J.-M. Nanobelt-arrayed vanadium oxide hierarchical microspheres as catalysts for selective oxidation of 5-hydroxymethylfurfural toward 2,5-diformylfuran. Appl. Catal. B 2017, 207, 358–365. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Damma, D.; Jampaiah, D.; Mukherjee, D.; Vithal, M.; Reddy, B.M. Cr-Doped CeO2 Nanorods for CO Oxidation: Insights into Promotional Effect of Cr on Structure and Catalytic Performance. Catal. Lett. 2020, 150, 948–962. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Samiee, S.; Nancarrow, P. Fabrication of cerium oxide nanoparticles: Characterization and optical properties. J. Colloid Interface Sci. 2011, 356, 473–480. [Google Scholar] [CrossRef]
- Sreeremya, T.S.; Krishnan, A.; Peer Mohamed, A.; Hareesh, U.S.; Ghosh, S. Synthesis and characterization of cerium oxide based nanofluids: An efficient coolant in heat transport applications. Chem. Eng. J. 2014, 255, 282–289. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Qin, F.; Dai, Q.; Wang, X. Fe doped CeO2 nanosheets as Fenton-like heterogeneous catalysts for degradation of salicylic acid. Chem. Eng. J. 2018, 333, 226–239. [Google Scholar] [CrossRef]
- Ares, J.R.; Pascual, A.; Ferrer, I.J.; Sánchez, C. Grain and crystallite size in polycrystalline pyrite thin films. Thin Solid Films 2005, 480–481, 477–481. [Google Scholar] [CrossRef]
- Phokha, S.; Prabhakaran, D.; Boothroyd, A.; Pinitsoontorn, S.; Maensiri, S. Ferromagnetic induced in Cr-doped CeO2 particles. Microelectron. Eng. 2014, 126, 93–98. [Google Scholar] [CrossRef]
- Schmitt, R.; Nenning, A.; Kraynis, O.; Korobko, R.; Frenkel, A.I.; Lubomirsky, I.; Haile, S.M.; Rupp, J.L.M. A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 2020, 49, 554–592. [Google Scholar] [CrossRef]
- Laguna, O.H.; Centeno, M.A.; Boutonnet, M.; Odriozola, J.A. Fe-doped ceria solids synthesized by the microemulsion method for CO oxidation reactions. Appl. Catal. B 2011, 106, 621–629. [Google Scholar] [CrossRef]
- Verma, R.; Samdarshi, S.K.; Bojja, S.; Paul, S.; Choudhury, B. A novel thermophotocatalyst of mixed-phase cerium oxide (CeO2/Ce2O3) homocomposite nanostructure: Role of interface and oxygen vacancies. Sol. Energy Mater. Sol. Cells 2015, 141, 414–422. [Google Scholar] [CrossRef]
- Agarwal, S.; Lefferts, L.; Mojet, B.L. Ceria Nanocatalysts: Shape Dependent Reactivity and Formation of OH. ChemCatChem 2013, 5, 479–489. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Mojet, B.L.; Lefferts, L. Surface-Dependence of Defect Chemistry of Nanostructured Ceria. J. Phys. Chem. C 2015, 119, 12423–12433. [Google Scholar] [CrossRef]
- Paidi, V.K.; Brewe, D.L.; Freeland, J.W.; Roberts, C.A.; van Lierop, J. Role of Ce 4f hybridization in the origin of magnetism in nanoceria. Phys. Rev. B 2019, 99, 180403. [Google Scholar] [CrossRef]
- Wu, L.; Wiesmann, H.J.; Moodenbaugh, A.R.; Klie, R.F.; Zhu, Y.; Welch, D.O.; Suenaga, M. Oxidation state and lattice expansion of CeO2-x nanoparticles as a function of particle size. Phys. Rev. B 2004, 69, 125415. [Google Scholar] [CrossRef]
- Song, K.; Schmid, H.; Srot, V.; Gilardi, E.; Gregori, G.; Du, K.; Maier, J.; van Aken, P.A. Cerium reduction at the interface between ceria and yttria-stabilised zirconia and implications for interfacial oxygen non-stoichiometry. APL Mater. 2014, 2, 032104. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, R.-J.; Lee, W.; Dong, C.-L.; Gloter, A. Spectromicroscopic evidence of interstitial and substitutional dopants in association with oxygen vacancies in Sm-doped ceria nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 3274–3281. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Kim, J.-Y.; Liu, G.; Iglesias-Juez, A.; Fernández-García, M. Properties of CeO2 and Ce1-xZrxO2 Nanoparticles: X-ray Absorption Near-Edge Spectroscopy, Density Functional, and Time-Resolved X-ray Diffraction Studies. J. Phys. Chem. B 2003, 107, 3535–3543. [Google Scholar] [CrossRef]
- Lee, W.; Chen, S.-Y.; Chen, Y.-S.; Dong, C.-L.; Lin, H.-J.; Chen, C.-T.; Gloter, A. Defect Structure Guided Room Temperature Ferromagnetism of Y-Doped CeO2 Nanoparticles. J. Phys. Chem. C 2014, 118, 26359–26367. [Google Scholar] [CrossRef]
- D’Angelo, A.M.; Chaffee, A.L. Correlations between Oxygen Uptake and Vacancy Concentration in Pr-Doped CeO2. ACS Omega 2017, 2, 2544–2551. [Google Scholar] [CrossRef]
- Choi, J.H.; Hong, J.-A.; Son, Y.R.; Wang, J.; Kim, H.S.; Lee, H.; Lee, H. Comparison of Enhanced Photocatalytic Degradation Efficiency and Toxicity Evaluations of CeO2 Nanoparticles Synthesized through Double-Modulation. Nanomaterials 2020, 10, 1543. [Google Scholar] [CrossRef]
- Drozd, V.; Liu, G.Q.; Liu, R.S.; Kuo, H.T.; Shen, C.H.; Shy, D.S.; Xing, X.K. Synthesis, electrochemical properties, and characterization of LiFePO4/C composite by a two-source method. J. Alloys Compd. 2009, 487, 58–63. [Google Scholar] [CrossRef]
- Yitamben, E.N.; Lovejoy, T.C.; Pakhomov, A.B.; Heald, S.M.; Negusse, E.; Arena, D.; Ohuchi, F.S.; Olmstead, M.A. Correlation between morphology, chemical environment, and ferromagnetism in the intrinsic-vacancy dilute magnetic semiconductor Cr-doped Ga2Se3/Si(001). Phys. Rev. B 2011, 83, 045203. [Google Scholar] [CrossRef]
- Katayama, T.; Yasui, S.; Osakabe, T.; Hamasaki, Y.; Itoh, M. Ferrimagnetism and Ferroelectricity in Cr-Substituted GaFeO3 Epitaxial Films. Chem. Mater. 2018, 30, 1436–1441. [Google Scholar] [CrossRef]
- Mohamed, A.Y.; Park, W.G.; Cho, D.-Y. Chemical Structure and Magnetism of FeOx/Fe2O3 Interface Studied by X-ray Absorption Spectroscopy. Magnetochemistry 2020, 6, 33. [Google Scholar] [CrossRef]




| Type of NPs | Crystallite Size (nm) | a-Axis Lattice Parameter (Å) |
|---|---|---|
| CeO2 | 19.1 ± 1.0 | 5.4150 ± 0.0008 |
| Cr@CeO2 | 18.3 ± 1.1 | 5.4123 ± 0.0004 |
| Fe@CeO2 | 19.2 ± 0.5 | 5.4136 ± 0.0008 |
| Type of NPs | PCD Efficiency of HMF (C/C0) | Conversion Efficiency from HMF to FDCA (%) |
|---|---|---|
| CeO2 | 0.41 ± 0.05 | 10.8 ± 0.51 |
| Cr@CeO2 | 0.08 ± 0.01 | 39.2 ± 0.78 |
| Fe@CeO2 | 0.03 ± 0.01 | 40.4 ± 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.-W.; Pham, V.N.; Ha, J.M.; Shin, M.; Lee, H.; Youn, Y.-S. Photocatalysis of Cr- and Fe-Doped CeO2 Nanoparticles to Selective Oxidation of 5-Hydroxymethylfurfural. Nanomaterials 2023, 13, 44. https://doi.org/10.3390/nano13010044
Nam J-W, Pham VN, Ha JM, Shin M, Lee H, Youn Y-S. Photocatalysis of Cr- and Fe-Doped CeO2 Nanoparticles to Selective Oxidation of 5-Hydroxymethylfurfural. Nanomaterials. 2023; 13(1):44. https://doi.org/10.3390/nano13010044
Chicago/Turabian StyleNam, Jeong-Woo, Vy Ngoc Pham, Jeong Min Ha, Minjeong Shin, Hangil Lee, and Young-Sang Youn. 2023. "Photocatalysis of Cr- and Fe-Doped CeO2 Nanoparticles to Selective Oxidation of 5-Hydroxymethylfurfural" Nanomaterials 13, no. 1: 44. https://doi.org/10.3390/nano13010044
APA StyleNam, J.-W., Pham, V. N., Ha, J. M., Shin, M., Lee, H., & Youn, Y.-S. (2023). Photocatalysis of Cr- and Fe-Doped CeO2 Nanoparticles to Selective Oxidation of 5-Hydroxymethylfurfural. Nanomaterials, 13(1), 44. https://doi.org/10.3390/nano13010044