Size Effect of a Piezoelectric Material as a Separator Coating Layer for Suppressing Dendritic Li Growth in Li Metal Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of the Different Sized PM
2.3. Preparation of PM-Coated Separator
2.4. Characterization
2.5. Measurement of Electrochemcial Performance
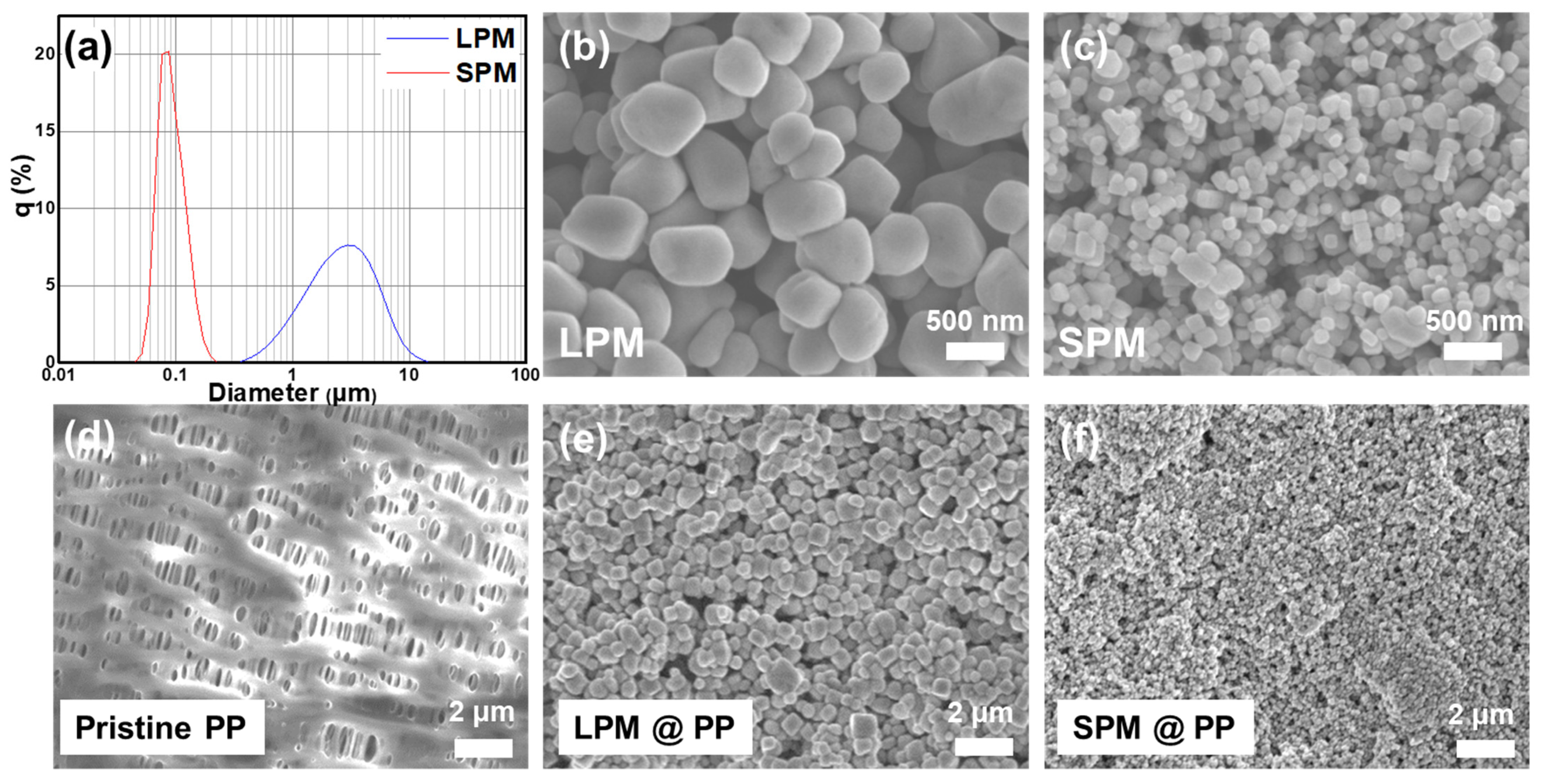
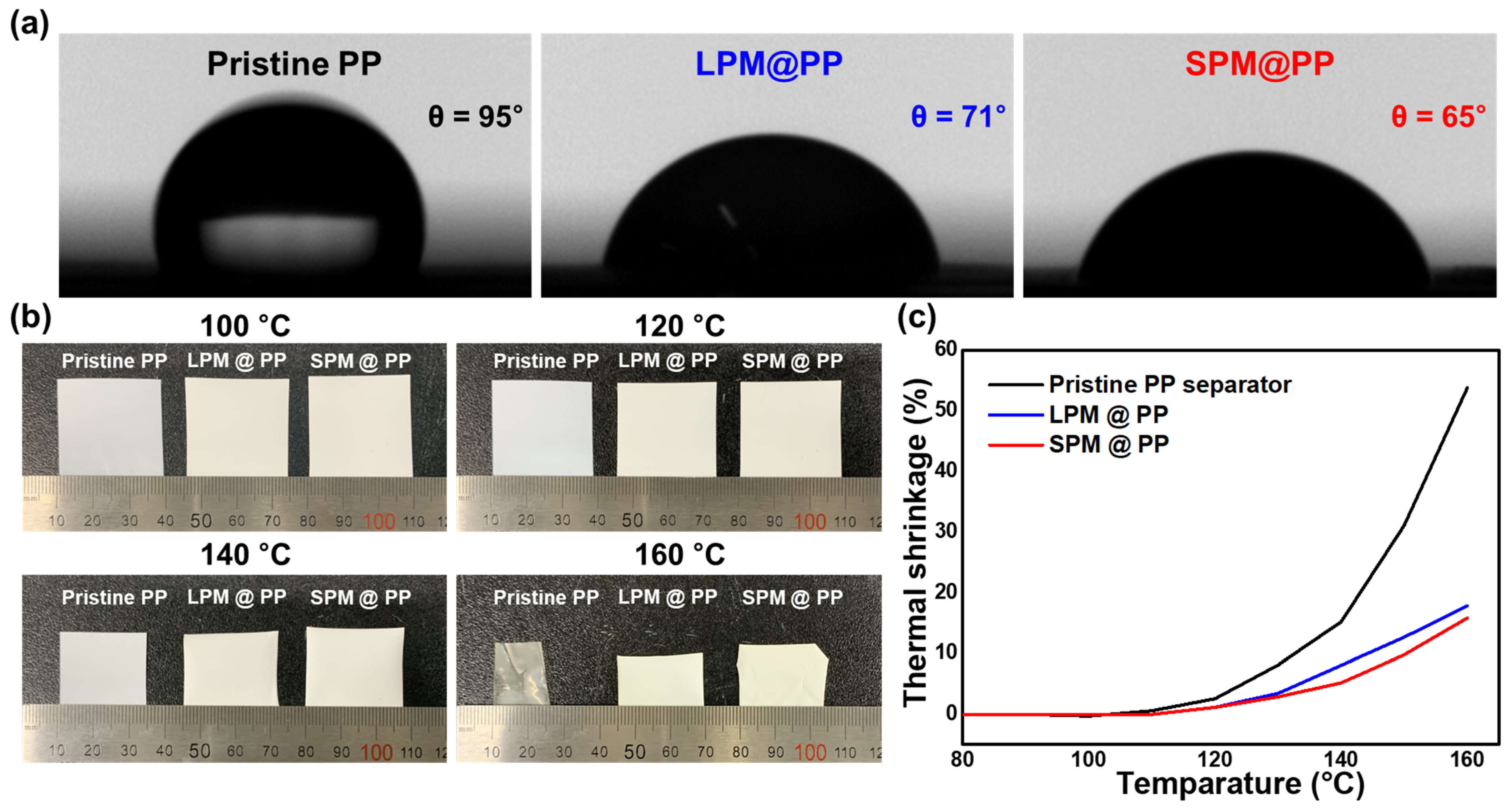
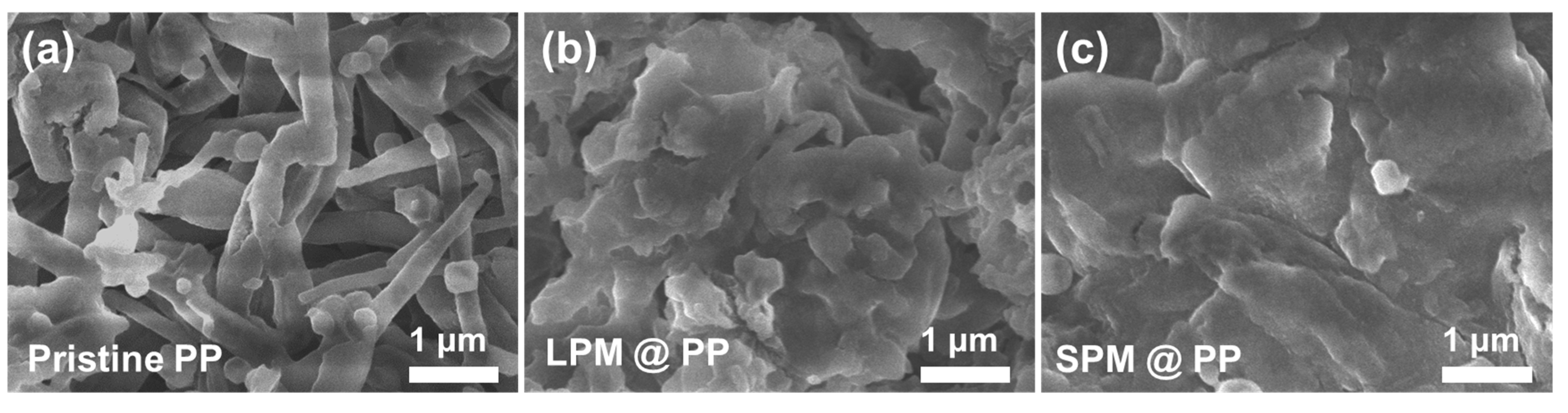
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J. Global warming. Rep. Prog. Phys. 2005, 68, 1343. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Guo, J. A review of the theoretical research and practical progress of carbon neutrality. Sustain. Oper. Comput. 2022, 3, 54–66. [Google Scholar] [CrossRef]
- Karjalainen, T.; Asikainen, A. Greenhouse gas emissions from the use of primary energy in forest operations and long-distance transportation of timber in Finland. For. Int. J. For. Res. 1996, 69, 215–228. [Google Scholar] [CrossRef]
- Aggarwal, P.; Jain, S. Energy demand and CO2 emissions from urban on-road transport in Delhi: Current and future projections under various policy measures. J. Clean. Prod. 2016, 128, 48–61. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building safe lithium-ion batteries for electric vehicles: A review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar]
- Chen, X.; Shen, W.; Vo, T.T.; Cao, Z.; Kapoor, A. An overview of lithium-ion batteries for electric vehicles. In Proceedings of the 2012 10th International Power & Energy Conference (IPEC), Ho Chi Minh City, Vietnam, 12–14 December 2012; pp. 230–235. [Google Scholar]
- Wang, C.-Y.; Liu, T.; Yang, X.-G.; Ge, S.; Stanley, N.V.; Rountree, E.S.; Leng, Y.; McCarthy, B.D. Fast charging of energy-dense lithium-ion batteries. Nature 2022, 611, 485–490. [Google Scholar] [CrossRef]
- Kim, P.J. Surface-functionalized separator for stable and reliable lithium metal batteries: A review. Nanomaterials 2021, 11, 2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.-T.; Popovic, J.; Lim, K.; Yin, Y.-X.; Maier, J.; Guo, Y.-G. Towards better Li metal anodes: Challenges and strategies. Mater. Today 2020, 33, 56–74. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Hagos, T.T.; Huang, C.-J.; Teshager, M.A.; Cheng, J.-H.; Su, W.-N.; Hwang, B.-J. Nucleation and Growth Mechanism of Lithium Metal Electroplating. J. Am. Chem. Soc. 2019, 141, 18612–18623. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, X.B.; Xu, R.; Zhang, X.Q.; Yan, C.; Huang, J.Q.; Zhang, Q. Plating/stripping behavior of actual lithium metal anode. Adv. Energy Mater. 2019, 9, 1902254. [Google Scholar] [CrossRef]
- Oyakhire, S.T.; Huang, W.; Wang, H.; Boyle, D.T.; Schneider, J.R.; de Paula, C.; Wu, Y.; Cui, Y.; Bent, S.F. Revealing and Elucidating ALD-Derived Control of Lithium Plating Microstructure. Adv. Energy Mater. 2020, 10, 2002736. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Pol, V.G. Surface Functionalization of a Conventional Polypropylene Separator with an Aluminum Nitride Layer toward Ultrastable and High-Rate Lithium Metal Anodes. ACS Appl. Mater. Interfaces 2019, 11, 3917–3924. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Y.; Cui, Z.; Tao, Y.; Li, B.; Wang, W. Recent progress of functional separators in dendrite inhibition for lithium metal batteries. Energy Storage Mater. 2021, 35, 157–168. [Google Scholar] [CrossRef]
- Varzi, A.; Raccichini, R.; Passerini, S.; Scrosati, B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J. Mater. Chem. A 2016, 4, 17251–17259. [Google Scholar] [CrossRef]
- Fan, M.; Chen, B.; Wang, K.; Yu, Q.; Ding, Y.; Lei, Z.; Liu, F.; Shen, Y.; He, G. Robust silver nanowire membrane with high porosity to construct stable Li metal anodes. Mater. Today Energy 2021, 21, 100751. [Google Scholar] [CrossRef]
- Zhang, S.S. Improved cyclability of liquid electrolyte lithium/sulfur batteries by optimizing electrolyte/sulfur ratio. Energies 2012, 5, 5190–5197. [Google Scholar] [CrossRef]
- Yang, G.; Frisco, S.; Tao, R.; Philip, N.; Bennett, T.H.; Stetson, C.; Zhang, J.-G.; Han, S.-D.; Teeter, G.; Harvey, S.P.; et al. Robust Solid/Electrolyte Interphase (SEI) Formation on Si Anodes Using Glyme-Based Electrolytes. ACS Energy Lett. 2021, 6, 1684–1693. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.-R.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. 2017, 56, 7764–7768. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, J.; Xiao, P.; Agboola, P.O.; Shakir, I.; Xu, Y. Graphene anchored on Cu foam as a lithiophilic 3D current collector for a stable and dendrite-free lithium metal anode. J. Mater. Chem. A 2018, 6, 9899–9905. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Menkin, S.; Golodnitsky, D.; Peled, E. Artificial solid-electrolyte interphase (SEI) for improved cycleability and safety of lithium–ion cells for EV applications. Electrochem. Commun. 2009, 11, 1789–1791. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.; Chen, X.; Legut, D.; Fan, Y.; Zhang, R.; Lu, Y.; Cheng, X.; Zhang, Q. Rational design of graphitic-inorganic Bi-layer artificial SEI for stable lithium metal anode. Energy Storage Mater. 2019, 16, 426–433. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Kim, P.J. A new approach to stabilize the electrochemical performance of Li metal batteries through the structure alteration of CNT scaffolds. Carbon 2023, 203, 426–435. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, D.O.; Kim, K.M.; Oh, J.; Kim, J.; Kang, S.H.; Lee, M.J.; Lee, Y.-G. Graphene Oxide Induced Surface Modification for Functional Separators in Lithium Secondary Batteries. Sci. Rep. 2019, 9, 2464. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ren, Y.; Sokolowski, J.; Zhu, X.; Wu, G. Mechanistic understanding of the role separators playing in advanced lithium-sulfur batteries. InfoMat 2020, 2, 483–508. [Google Scholar] [CrossRef]
- Orendorff, C.J. The role of separators in lithium-ion cell safety. Electrochem. Soc. Interface 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Choi, J.; Kim, P.J. A roadmap of battery separator development: Past and future. Curr. Opin. Electrochem. 2022, 31, 100858. [Google Scholar] [CrossRef]
- Vijatović, M.; Bobić, J.; Stojanović, B.D. History and challenges of barium titanate: Part I. Sci. Sinter. 2008, 40, 155–165. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Thijs, M.; Ooms, F.G.B.; Ganapathy, S.; Wagemaker, M. High dielectric barium titanate porous scaffold for efficient Li metal cycling in anode-free cells. Nat. Commun. 2021, 12, 6536. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, M.; Ma, R.; Yuan, Q.; Yang, D.; Cui, B.; Ma, C.; Liu, M.; Hu, D. Design strategy of barium titanate/polyvinylidene fluoride-based nanocomposite films for high energy storage. J. Mater. Chem. A 2020, 8, 884–917. [Google Scholar] [CrossRef]
- Liu, G.; Wang, D.; Zhang, J.; Kim, A.; Lu, W. Preventing Dendrite Growth by a Soft Piezoelectric Material. ACS Mater. Lett. 2019, 1, 498–505. [Google Scholar] [CrossRef]
- Du, P.; Li, B.; Mao, Z.; Nan, Y.; Guo, D.; Wu, S. Regulating lithium-ion flow by piezoelectric effect of the poled-BaTiO3 film for dendrite-free lithium metal anode. J. Electroanal. Chem. 2022, 919, 116538. [Google Scholar] [CrossRef]
- Xia, S.; Zhao, Y.; Yan, J.; Yu, J.; Ding, B. Dynamic Regulation of Lithium Dendrite Growth with Electromechanical Coupling Effect of Soft BaTiO3 Ceramic Nanofiber Films. ACS Nano 2021, 15, 3161–3170. [Google Scholar] [CrossRef]
- Cui, B.; Yu, P.; Wang, X. Preparation and Characterization of BaTiO3 Powders and Ceramics by Sol–Gel Process Using Decanedioic Acid. J. Alloys Compd. 2008, 459, 589–593. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Bae, S.Y.; Jeon, S.P.; Song, J.H.; Wang, S.E.; Jung, D.S.; Jang, J.; Park, H.; Kim, P.J.; et al. Polyanion-assisted ionic-electronic conductive agents designed for high density Si-based anodes. J. Power Sources 2022, 541, 231728. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Z.; Kong, Q.; Yao, J.; Zhang, C.; Han, P.; Cui, G. A high temperature operating nanofibrous polyimide separator in Li-ion battery. Solid State Ion. 2013, 232, 44–48. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. TiO2 ceramic-grafted polyethylene separators for enhanced thermostability and electrochemical performance of lithium-ion batteries. J. Membr. Sci. 2016, 504, 97–103. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. A Highly Thermostable Ceramic-Grafted Microporous Polyethylene Separator for Safer Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef] [PubMed]
- Yim, T.; Han, S.H.; Park, N.H.; Park, M.-S.; Lee, J.H.; Shin, J.; Choi, J.W.; Jung, Y.; Jo, Y.N.; Yu, J.-S.; et al. Effective Polysulfide Rejection by Dipole-Aligned BaTiO3 Coated Separator in Lithium–Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 7817–7823. [Google Scholar] [CrossRef]
- Su, L.; Manthiram, A. Lithium-Metal Batteries via Suppressing Li Dendrite Growth and Improving Coulombic Efficiency. Small Struct. 2022, 3, 2200114. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kwon, K.; Kim, K.; Han, S.; Kim, P.J.; Choi, J. Size Effect of a Piezoelectric Material as a Separator Coating Layer for Suppressing Dendritic Li Growth in Li Metal Batteries. Nanomaterials 2023, 13, 90. https://doi.org/10.3390/nano13010090
Kim J, Kwon K, Kim K, Han S, Kim PJ, Choi J. Size Effect of a Piezoelectric Material as a Separator Coating Layer for Suppressing Dendritic Li Growth in Li Metal Batteries. Nanomaterials. 2023; 13(1):90. https://doi.org/10.3390/nano13010090
Chicago/Turabian StyleKim, Junghwan, Kihwan Kwon, Kwanghyun Kim, Seungmin Han, Patrick Joohyun Kim, and Junghyun Choi. 2023. "Size Effect of a Piezoelectric Material as a Separator Coating Layer for Suppressing Dendritic Li Growth in Li Metal Batteries" Nanomaterials 13, no. 1: 90. https://doi.org/10.3390/nano13010090
APA StyleKim, J., Kwon, K., Kim, K., Han, S., Kim, P. J., & Choi, J. (2023). Size Effect of a Piezoelectric Material as a Separator Coating Layer for Suppressing Dendritic Li Growth in Li Metal Batteries. Nanomaterials, 13(1), 90. https://doi.org/10.3390/nano13010090





