Reagentless Electrochemical Detection of Tumor Biomarker Based on Stable Confinement of Electrochemical Probe in Bipolar Silica Nanochannel Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurements and Instrumentations
2.3. Preparation of n-SNF/ITO
2.4. Preparation of bp-SNF/ITO
2.5. Immobilization of MB and an Aptamer on bp-SNF/ITO Electrode
2.6. Electrochemical Detection of CEA
3. Results and Discussion
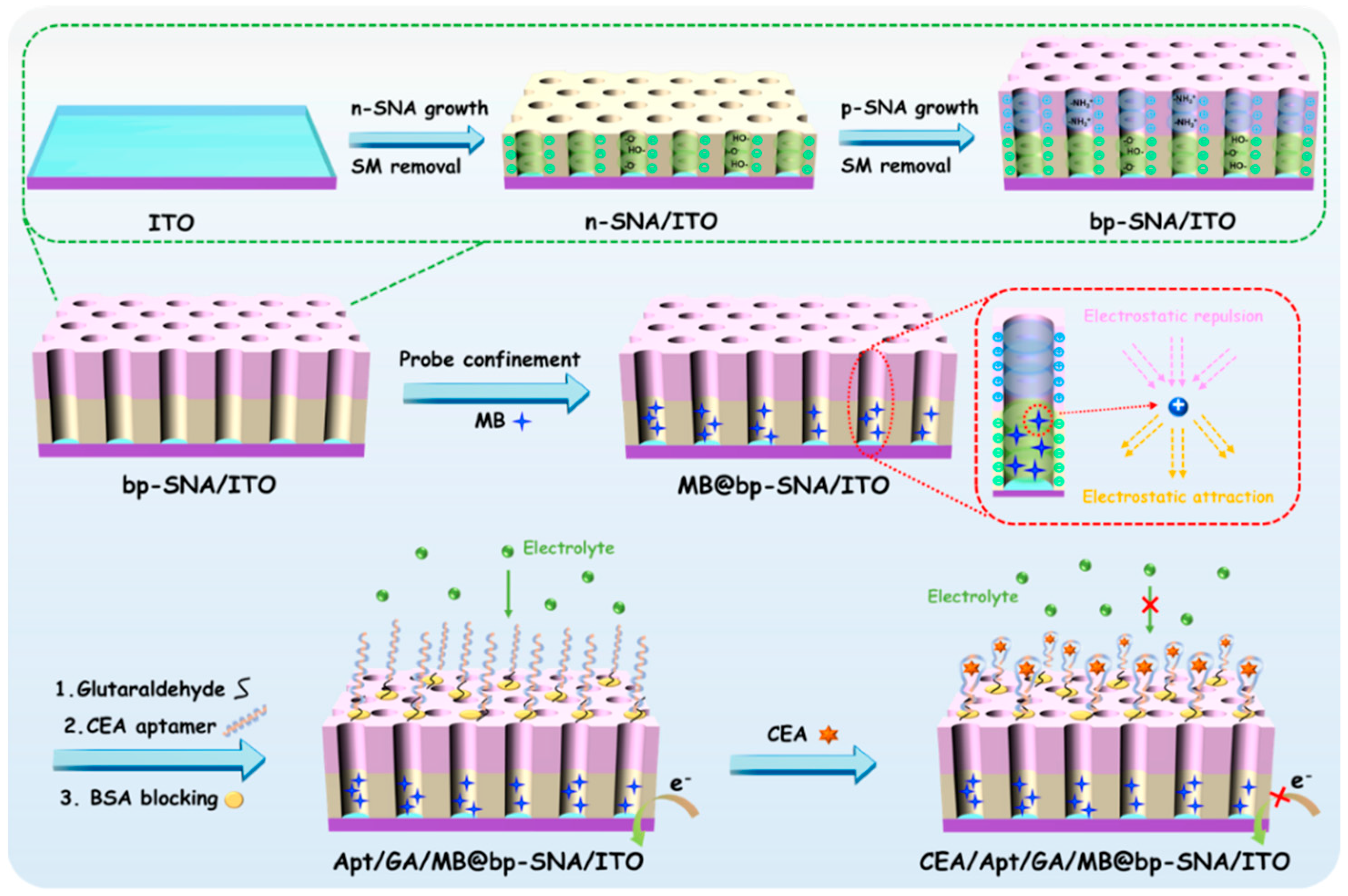
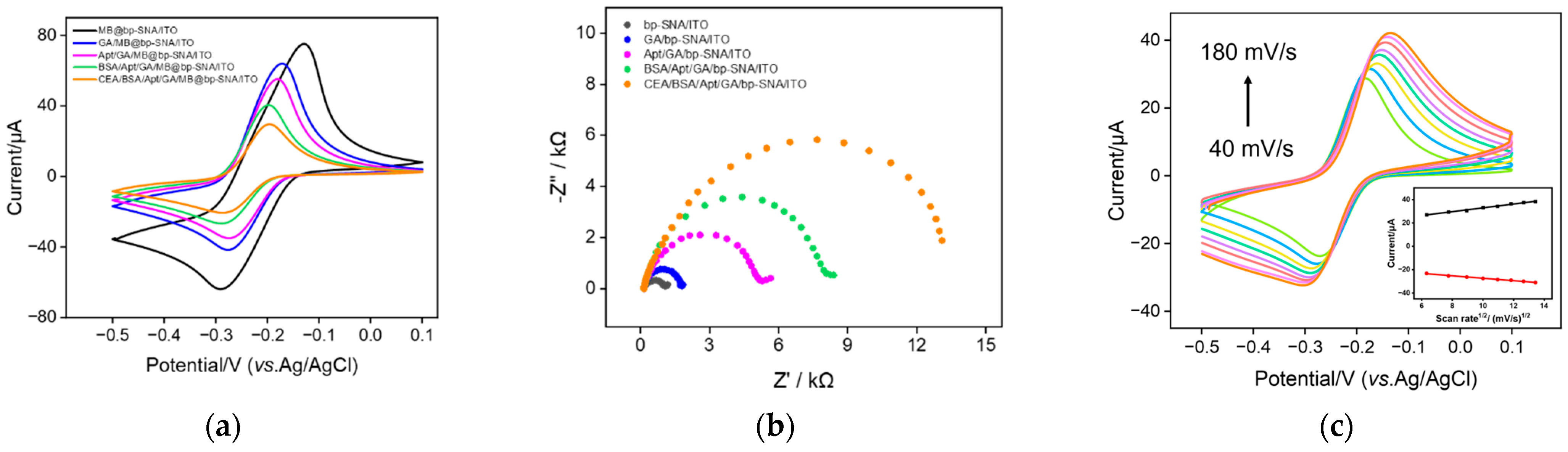
3.1. Construction of Electrochemical Aptamer Sensor with Integrated Electrochemical Probe
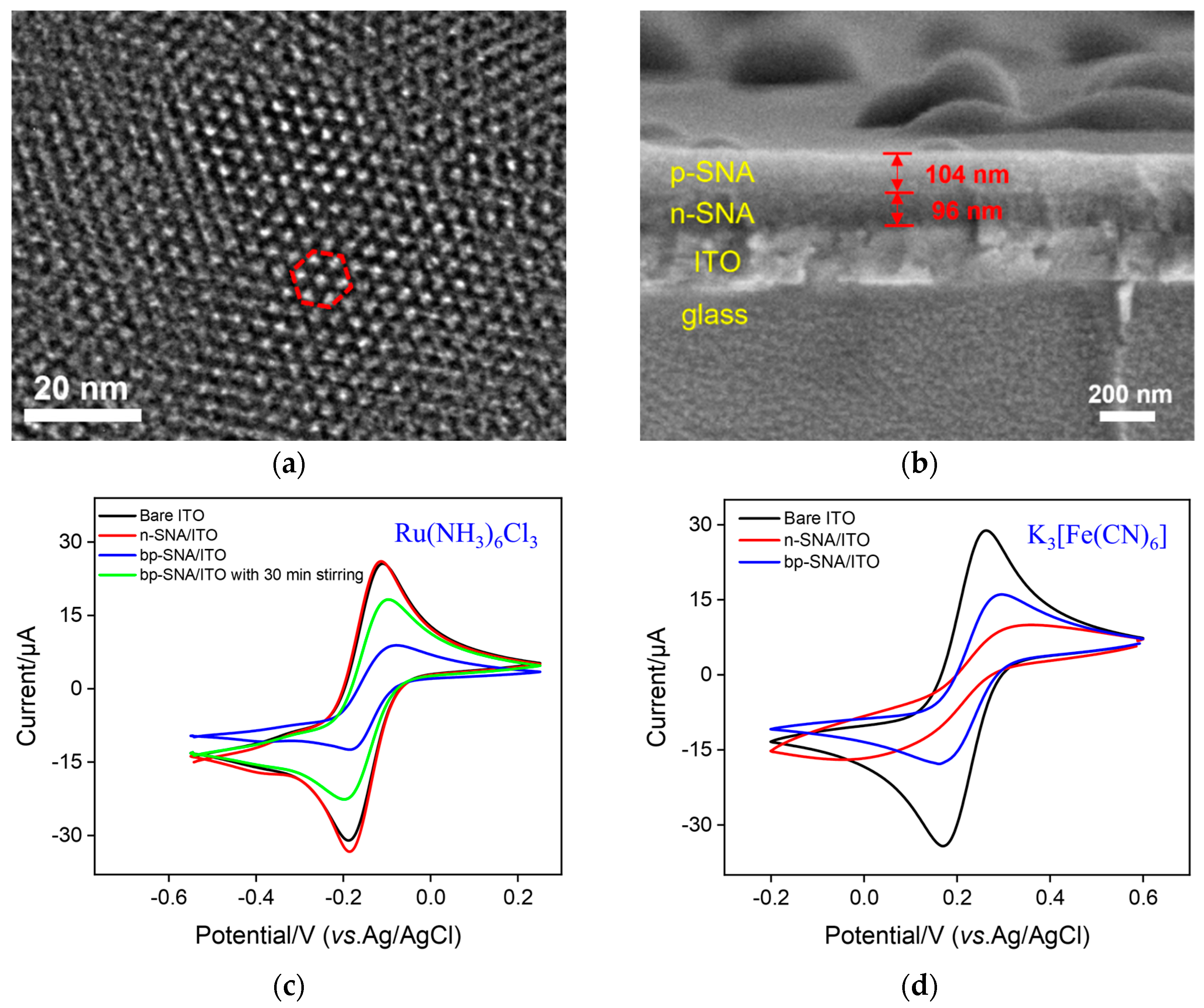
3.2. Characterization of bp-SNF Modified Electrode
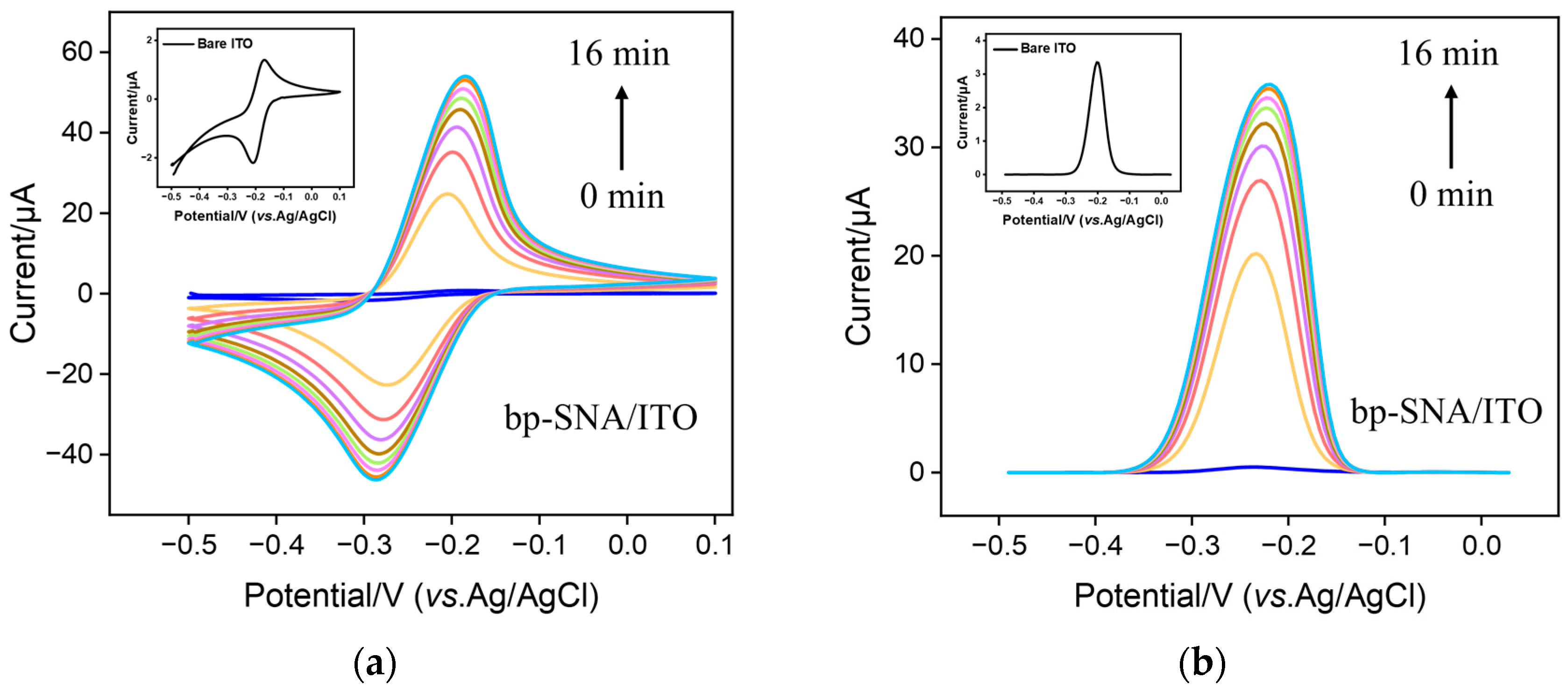
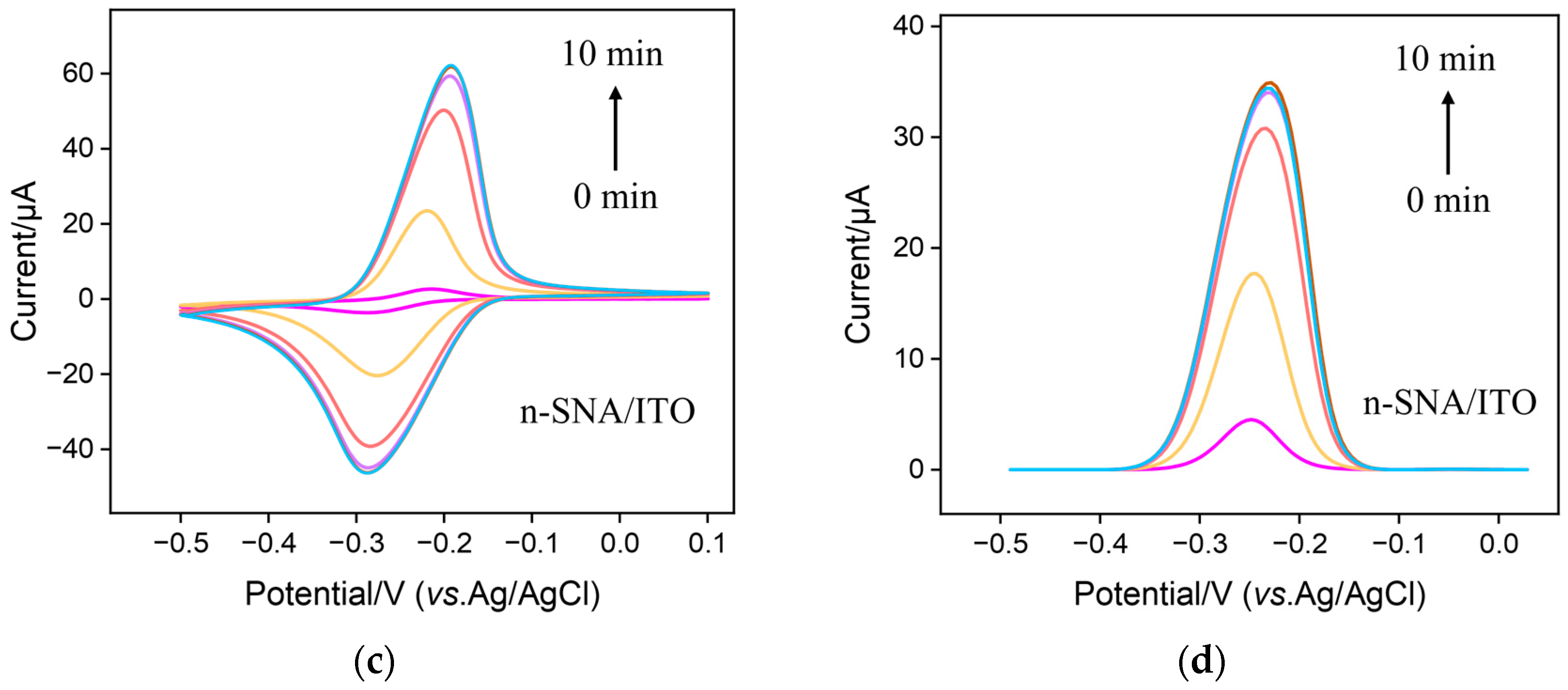
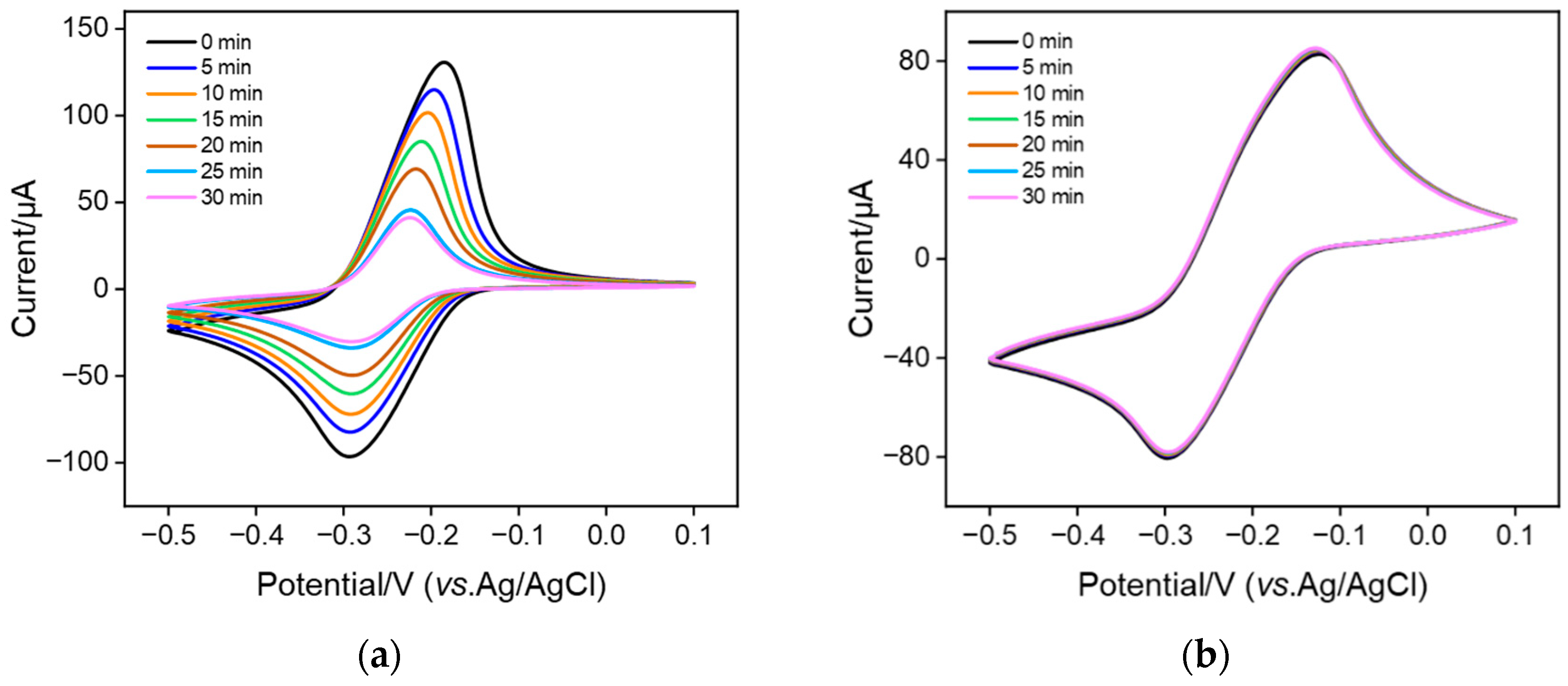
3.3. Stable Confinement of MB on bp-SNF/ITO
3.4. Fabrication of Aptamer Sensor for Electrochemical Detection of CEA
3.5. Electrochemical Detection of CEA Using the Fabricated Aptamer Sensor
3.6. Selectivity, Reproductivity, and Stability of the Fabricated Aptamer Sensor
3.7. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dalton, W.S.; Friend, S.H. Cancer biomarkers—An invitation to the table. Science 2006, 312, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Uludag, Y.; Gurbuz, Y.; Tothill, I.E. Surface plasmon resonance based immunosensor for the detection of the cancer biomarker carcinoembryonic antigen. Talanta 2011, 86, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Aatif, M. The footprints of cancer development: Cancer biomarkers. Cancer Treat. Rev. 2009, 35, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer biomarkers and their biosensors: A comprehensive review. TrAC Trends Anal. Chem. 2023, 158, 116813. [Google Scholar] [CrossRef]
- Gulhati, P.; Yin, J.; Pederson, L.; Schmoll, H.J.; Hoff, P.; Douillard, J.Y.; Hecht, J.R.; Tournigand, C.; Tebbut, N.; Chibaudel, B.; et al. Threshold change in CEA as a predictor of non-progression to first-line systemic therapy in metastatic colorectal cancer patients with elevated CEA. J. Natl. Cancer Inst. 2020, 112, 1127–1136. [Google Scholar] [CrossRef]
- Kabel, A.M. Tumor markers of breast cancer: New prospectives. J. Oncol. Sci. 2017, 3, 5–11. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Su, B.B.; Shi, H.; Wan, J. Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World J. Gastroenterol. 2012, 18, 2121–2126. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Feng, J.; Wang, P.; Dong, Y. The label-free immunosensor based on rhodium@palladium nanodendrites/sulfo group functionalized multi-walled carbon nanotubes for the sensitive analysis of carcino embryonic antigen. Anal. Chim. Acta 2018, 1007, 61–70. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, B.; Tang, J.; Liu, B.; Lai, W.; Tang, D. Sandwich-type immunosensors and immunoassays exploiting nanostructure labels: A review. Anal. Chim. Acta 2013, 758, 1–18. [Google Scholar] [CrossRef]
- Yokoyama, S.; Takeuchi, A.; Yamaguchi, S.; Mitani, Y.; Watanabe, T.; Matsuda, K.; Hotta, T.; Shively, J.E.; Yamaue, H. Clinical implications of carcinoembryonic antigen distribution in serum exosomal fraction-Measurement by ELISA. PLoS ONE 2017, 12, e0183337–e0183346. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Hara, H.; Araya, J.; Ichikawa, A.; Fujita, Y.; Utsumi, H.; Hashimoto, M.; Wakui, H.; Minagawa, S.; Numata, T.; et al. Prostaglandin e-major urinary metabolite (PGE-MUM) as a tumor marker for lung adenocarcinoma. Cancers 2019, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C. Methods developed for SELEX. Anal. Bioanal. Chem. 2007, 387, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef] [PubMed]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef]
- Wu, K.; Chu, C.; Ma, C.; Yang, H.; Yan, M.; Ge, S.; Yu, J.; Song, X. Immunoassay for carcinoembryonic antigen based on the Zn2+-enhanced fluorescence of magnetic-fluorescent nanocomposites. Sens. Actuators B Chem. 2015, 206, 43–49. [Google Scholar] [CrossRef]
- Su, Y.; Lai, W.; Liang, Y.; Zhang, C. Novel cloth-based closed bipolar solid-state electrochemiluminescence (CBP-SS-ECL) aptasensor for detecting carcinoembryonic antigen. Anal. Chim. Acta 2022, 1206, 339789–339798. [Google Scholar] [CrossRef]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450–1121457. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, S.; Xi, F. Disposal immunosensor for sensitive electrochemical detection of prostate-specific antigen based on amino-rich nanochannels array-modified patterned indium tin oxide electrode. Nanomaterials 2022, 12, 3810. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Yu, G.; Liu, L.; Yan, F. Highly sensitive electrochemical detection of paraquat in environmental water samples using a vertically ordered mesoporous silica film and a nanocarbon composite. Nanomaterials 2022, 12, 3632. [Google Scholar] [CrossRef]
- Chang, Q.; Huang, J.; He, L.; Xi, F. Simple immunosensor for ultrasensitive electrochemical determination of biomarker of the bone metabolism in human serum. Front. Chem. 2022, 10, 940795. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Han, Y.; Fan, L.; Guo, Y. Sandwich electrochemical carcinoembryonic antigen aptasensor based on signal amplification of polydopamine functionalized graphene conjugate Pd-Pt nanodendrites. Bioelectrochemistry 2021, 142, 107947. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, G.; Yu, S.; Huang, R.; Fan, C. A potentiometric aptasensor for carcinoembryonic antigen (CEA) on graphene oxide nanosheets using catalytic recycling of DNase I with signal amplification. Anal. Methods. 2018, 10, 5364–5371. [Google Scholar] [CrossRef]
- Niu, C.; Lin, X.; Jiang, X.; Guo, F.; Liu, J.; Liu, X.; Huang, H.; Huang, Y. An electrochemical aptasensor for highly sensitive detection of CEA based on exonuclease III and hybrid chain reaction dual signal amplification. Bioelectrochemistry 2022, 143, 107986. [Google Scholar] [CrossRef]
- Si, Z.; Xie, B.; Chen, Z.; Tang, C.; Li, T.; Yang, M. Electrochemical aptasensor for the cancer biomarker CEA based on aptamer induced current due to formation of molybdophosphate. Microchim. Acta 2017, 184, 3215–3221. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC. Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Tang, H.; Xi, F. Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film. Front. Chem. 2022, 10, 954748. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef]
- Wei, X.; Luo, X.; Xu, S.; Xi, F.; Zhao, T. A flexible electrochemiluminescence sensor equipped with vertically ordered mesoporous silica nanochannel film for sensitive detection of clindamycin. Front. Chem. 2022, 10, 872582. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, G.; Dou, Y.; Li, W.; Mou, C.-Y.; Zhang, X.; Asiri, A.M.; Zhao, D. Highly ordered mesoporous silica films with perpendicular mesochannels by a simple stöber-solution growth approach. Angew. Chem. Int. Edit. 2012, 51, 2173–2177. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101–131109. [Google Scholar] [CrossRef]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica nanochannel membranes for electrochemical analysis and molecular sieving: A comprehensive review. Crit. Rev. Anal. Chem. 2020, 50, 424–444. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563–114570. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, L.; Su, B. Vertically ordered silica mesochannels as preconcentration materials for the electrochemical detection of methylene blue. Sci. China Chem. 2015, 58, 1593–1599. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-ordered mesoporous silica films for electrochemical detection of Hg(II) ion in pharmaceuticals and soil samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhou, L.; Chen, J.; Yan, F.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Nanochannel-confined graphene quantum dots for ultrasensitive electrochemical analysis of complex samples. ACS Nano 2018, 12, 12673–12681. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, W.; Lu, S.; Guo, C.; Wang, P.; Zhang, D.; Ma, W. A label-free electrochemical immunosensor for CEA detection on a novel signal amplification platform of Cu2S/Pd/CuO nanocomposites. Front. Bioeng. Biotech. 2021, 9, 767717–767725. [Google Scholar] [CrossRef]
- Fan, X.; Deng, D.; Chen, Z.; Qi, J.; Li, Y.; Han, B.; Huan, K.; Luo, L. A sensitive amperometric immunosensor for the detection of carcinoembryonic antigen using ZnMn2O4@reduced graphene oxide composites as signal amplifier. Sens. Actuators B Chem. 2021, 339, 129852–129860. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Song, D.; Xu, J.; Zhang, M. Electrochemical aptasensor of carcinoembryonic antigen based on concanavalin a-functionalized magnetic copper silicate carbon microtubes and gold-nanocluster-assisted signal amplification. ACS. Appl. Nano. Mater. 2020, 3, 3449–3458. [Google Scholar] [CrossRef]
- Song, J.; Teng, H.; Xu, Z.; Liu, N.; Xu, L.; Liu, L.; Gao, F.; Luo, X. Free-standing electrochemical biosensor for carcinoembryonic antigen detection based on highly stable and flexible conducting polypyrrole nanocomposite. Mikrochim. Acta 2021, 188, 217–225. [Google Scholar] [CrossRef] [PubMed]

| Electrode | Method | Linear Range (ng/mL) | LOD (pg/mL) | Sample | Preparation Time of Sensor * (h) | Ref. |
|---|---|---|---|---|---|---|
| CEA/BSA/Ab/Cu2S/Pd/CuO/GCE | I-t | 10−4–100 | 0.03311 | Human serum | 13.3 | [50] |
| PDA@Gr/Pd-PtNDs/ hemin/G4/CEA/Apt1/PDA@Gr/GCE | DPV | 0.05–103 | 6.3 | Human serum | 15.5 | [27] |
| CEA/BSA/Ab/Au/ZnMn2O4@rGO/GCE | DPV | 0.01–50 | 1.93 | Human serum | >27 | [51] |
| hybrid DNA/CEA-H1/BSA/MCH/H2/Au | DPV | 0.01–100 | 0.84 | Human serum | 15 | [29] |
| NCMTs@Fe3O4@Cusilicate/ConA/CEA/AuNCs-aptamer | DPV | 0.03–6 | 5.38 | Human serum | >16.5 | [52] |
| CEA/MCH/aptamer/AuNPs/PPy | EIS | 0.1–103 | 33 | Fetal bovine serum | >14 | [53] |
| CEA/BSA/Apt/GA/MB@bp-SNA/ITO | DPV | 10−3–103 | 0.22 | Fetal bovine serum | 5.2 | This work |
| Sample | Added a (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| serum | 0.0100 | 0.00989 | 98.9 | 3.5 |
| 1.00 | 1.03 | 103 | 2.5 | |
| 100 | 98.9 | 98.9 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless Electrochemical Detection of Tumor Biomarker Based on Stable Confinement of Electrochemical Probe in Bipolar Silica Nanochannel Film. Nanomaterials 2023, 13, 1645. https://doi.org/10.3390/nano13101645
Zhou X, Han Q, Zhou J, Liu C, Liu J. Reagentless Electrochemical Detection of Tumor Biomarker Based on Stable Confinement of Electrochemical Probe in Bipolar Silica Nanochannel Film. Nanomaterials. 2023; 13(10):1645. https://doi.org/10.3390/nano13101645
Chicago/Turabian StyleZhou, Xile, Qianqian Han, Jinming Zhou, Chaoxu Liu, and Jiyang Liu. 2023. "Reagentless Electrochemical Detection of Tumor Biomarker Based on Stable Confinement of Electrochemical Probe in Bipolar Silica Nanochannel Film" Nanomaterials 13, no. 10: 1645. https://doi.org/10.3390/nano13101645
APA StyleZhou, X., Han, Q., Zhou, J., Liu, C., & Liu, J. (2023). Reagentless Electrochemical Detection of Tumor Biomarker Based on Stable Confinement of Electrochemical Probe in Bipolar Silica Nanochannel Film. Nanomaterials, 13(10), 1645. https://doi.org/10.3390/nano13101645






