A Novel Endodontic Approach in Removing Smear Layer Using Nano and Submicron Diamonds with Intracanal Oscillation Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation of Teeth Specimens
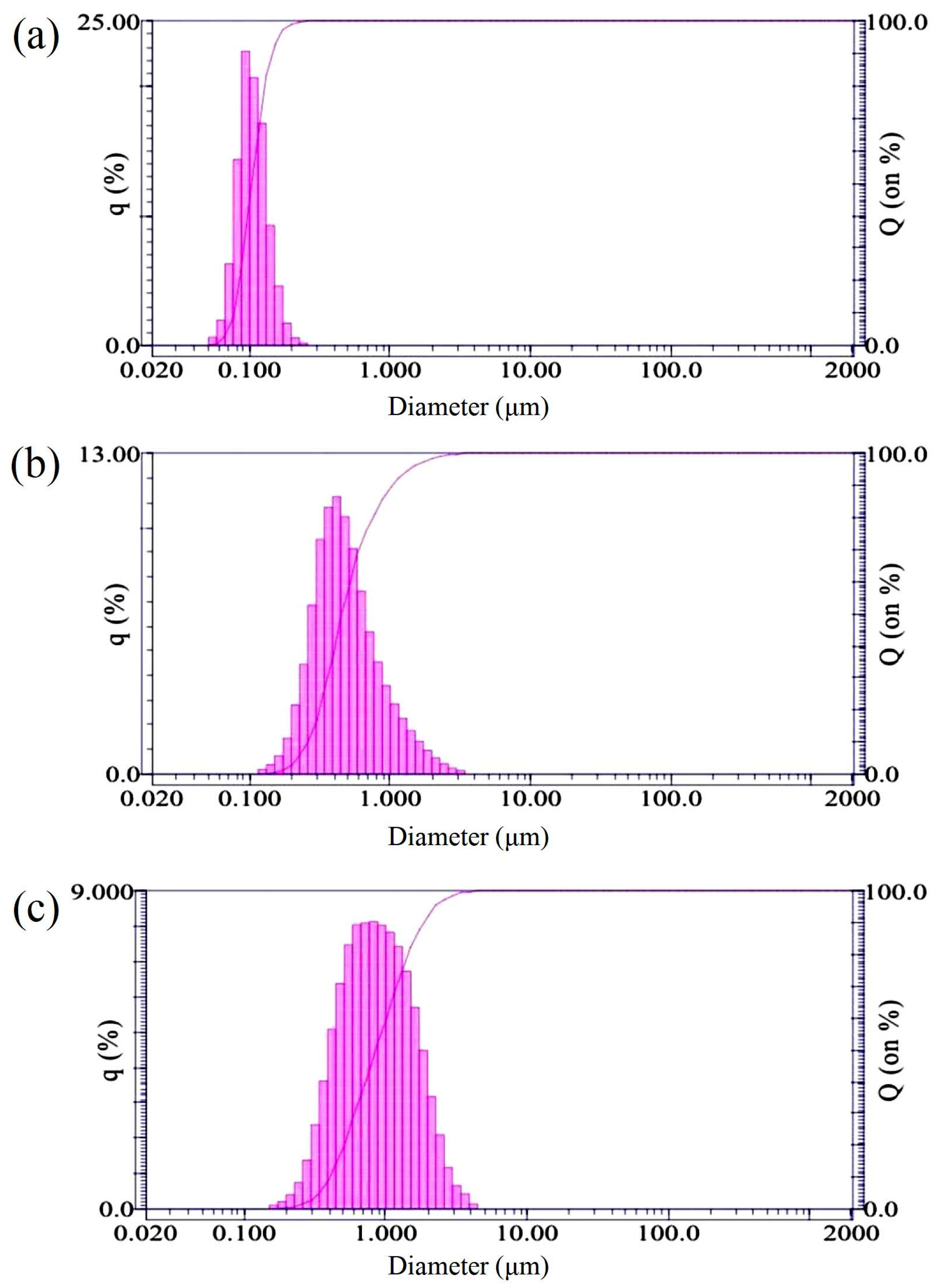
2.2. Preparation of Nano and Submicron Diamonds
2.3. Root Canal Irrigation Solutions
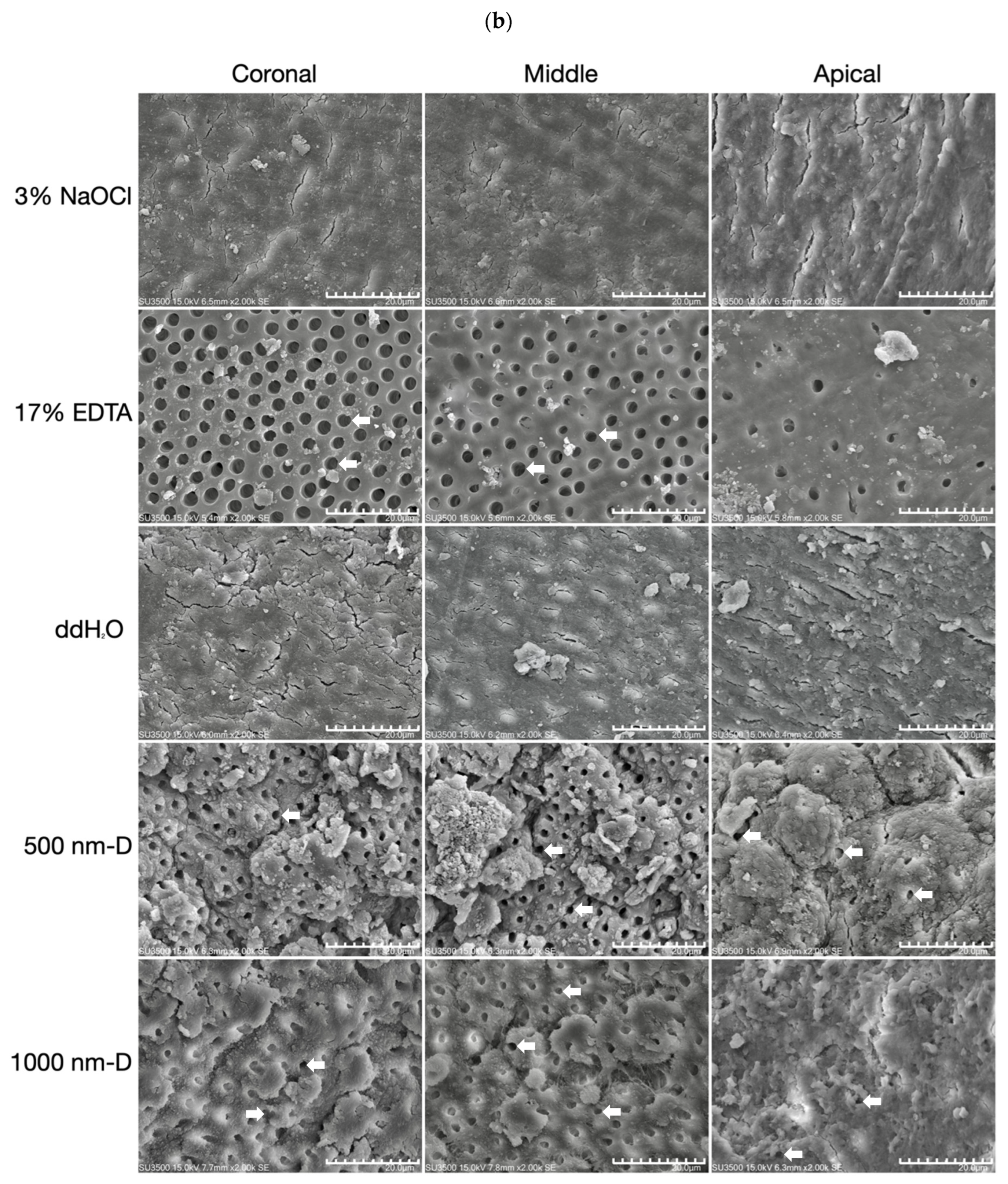
- Group 1 (Control): 3% NaOCl;
- Group 2 (Positive control): 17% EDTA;
- Group 3 (Negative control): distilled water;
- Group 4 (10 nm): 3% NaOCl and 10 nm nanodiamonds with a concentration of 10 mg/mL;
- Group 5 (50 nm): 3% NaOCl and 50 nm nanodiamonds with a concentration of 10 mg/mL;
- Group 6 (100 nm): 3% NaOCl and 100 nm nanodiamonds with a concentration of 10 mg/mL;
- Group 7 (500 nm): 3% NaOCl and 500 nm submicron diamonds with a concentration of 10 mg/mL;
- Group 8 (1000 nm): 3% NaOCl and 1000 nm submicron diamonds with a concentration of 10 mg/mL.
2.4. Sonic and Ultrasonic Vibration Oscillation
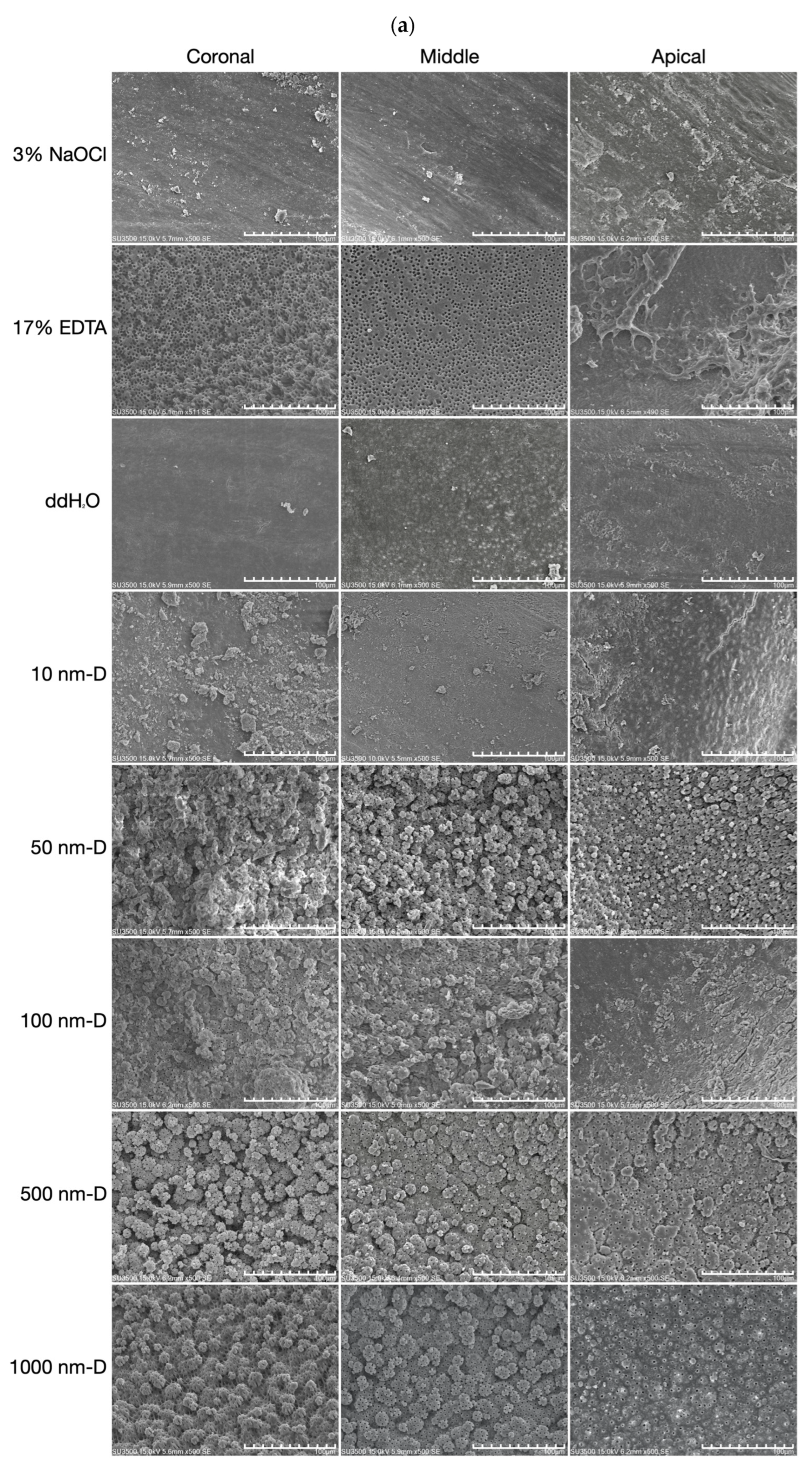
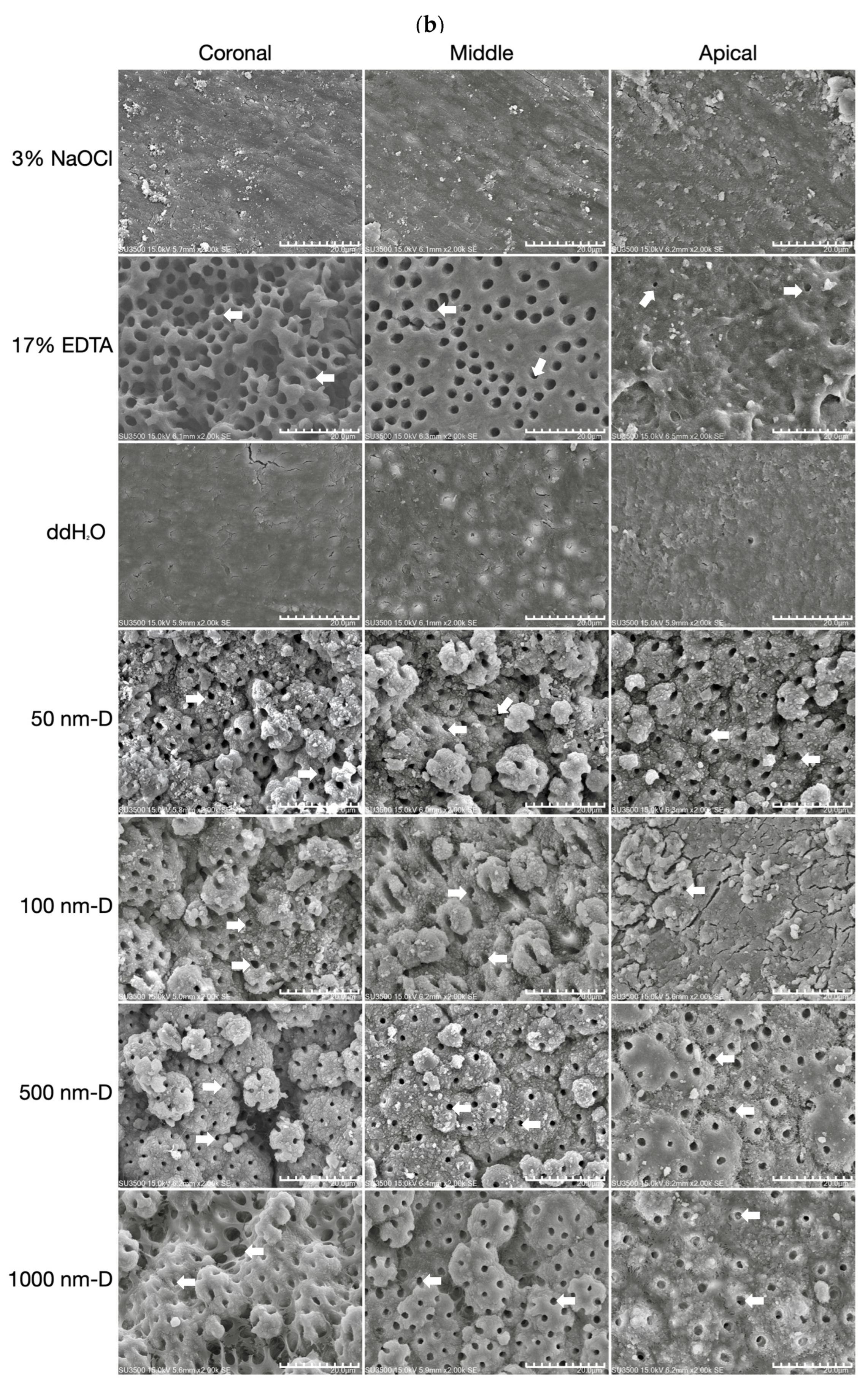
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Statistical Analysis
3. Results
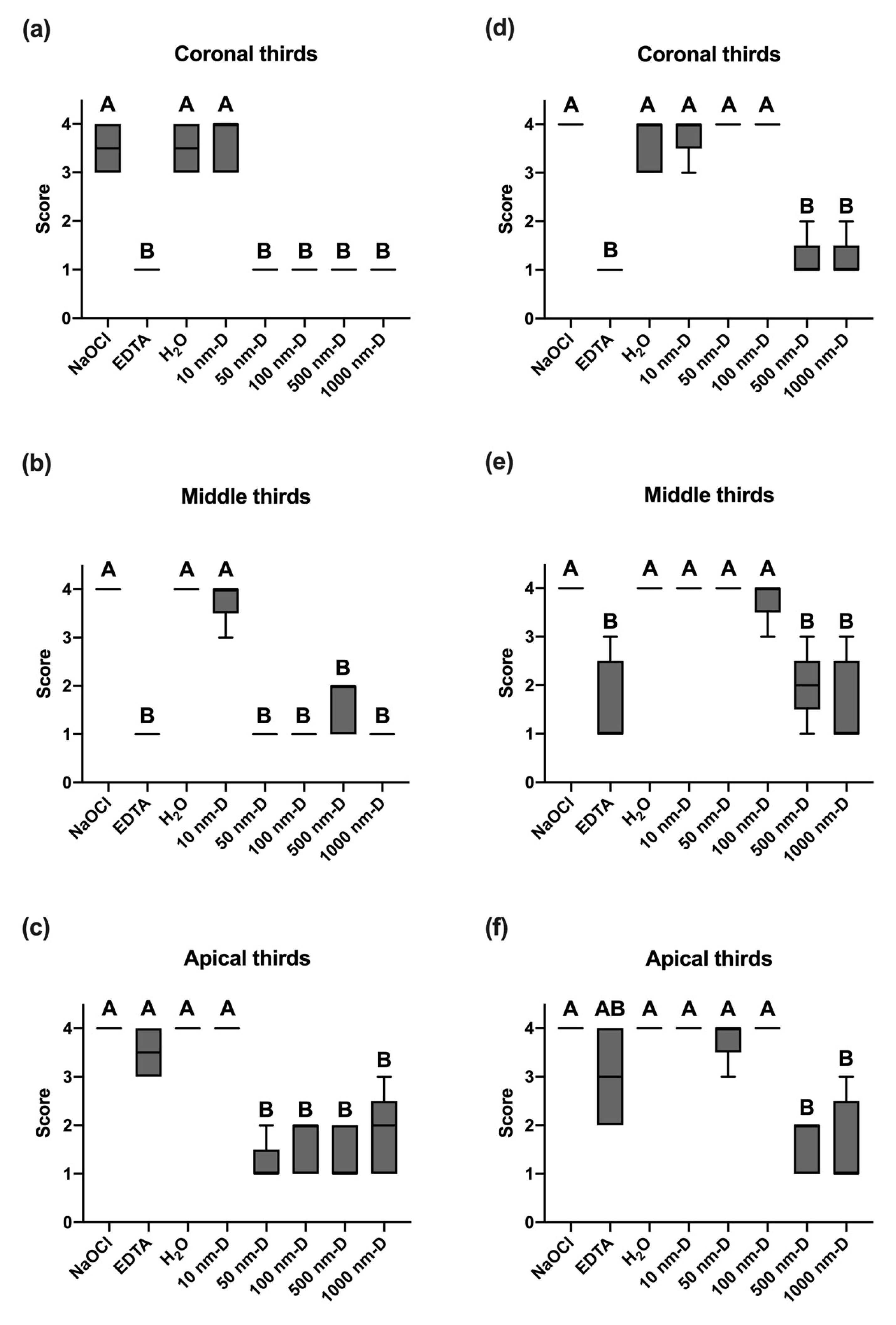
3.1. Examiner Calibration
3.2. Smear Layer Removal of Irrigants under Sonic Oscillation
3.3. Smear Layer Removal of Irrigants under Ultrasonic Oscillation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pashley, D.H.; Michelich, V.; Kehl, T. Dentin permeability: Effects of smear layer removal. J. Prosthet. Dent. 1981, 46, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Mader, C.L.; Baumgartner, J.C.; Peters, D.D. Scanning electron microscopic investigation of the smeared layer on root canal walls. J. Endod. 1984, 10, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.A. The synergistic relationship between ultrasound and sodium hypochlorite: A scanning electron microscope evaluation. J. Endod. 1987, 13, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Safavi, K.E.; Spångberg, L.S.; Costa, N.S., Jr.; Sapounas, G. An in vitro method for longitudinal evaluation of toxicity of endodontic sealers. J. Endod. 1989, 15, 484–486. [Google Scholar] [CrossRef]
- Meryon, S.D.; Brook, A.M. Penetration of dentine by three oral bacteria in vitro and their associated cytotoxicity. Int. Endod. J. 1990, 23, 196–202. [Google Scholar] [CrossRef]
- Michelich, V.J.; Schuster, G.S.; Pashley, D.H. Bacterial penetration of human dentin in vitro. J. Dent. Res. 1980, 59, 1398–1403. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Birang, R.; Feizianfard, M.; Nasouri, M. A comparative study of the removal of smear layer by two endodontic irrigants and Nd:YAG laser: A scanning electron microscopic study. ISRN Dent. 2012, 2012, 620951. [Google Scholar] [CrossRef]
- Olgart, L.; Brännström, M.; Johnson, G. Invasion of bacteria into dentinal tubules. Experiments in vivo and in vitro. Acta Odontol. Scand. 1974, 32, 61–70. [Google Scholar] [CrossRef]
- Akpata, E.S.; Blechman, H. Bacterial invasion of pulpal dentin wall in vitro. J. Dent. Res. 1982, 61, 435–438. [Google Scholar] [CrossRef]
- Williams, S.; Goldman, M. Penetrability of the smeared layer by a strain of Proteus vulgaris. J. Endod. 1985, 11, 385–388. [Google Scholar] [CrossRef]
- George, S.; Kishen, A.; Song, K.P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J. Endod. 2005, 31, 867–872. [Google Scholar] [CrossRef] [PubMed]
- McComb, D.; Smith, D.C. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J. Endod. 1975, 1, 238–242. [Google Scholar] [CrossRef]
- Outhwaite, W.C.; Livingston, M.J.; Pashley, D.H. Effects of changes in surface area, thickness, temperature and post-extraction time on human dentine permeability. Arch. Oral Biol. 1976, 21, 599–603. [Google Scholar] [CrossRef]
- Goldberg, F.; Abramovich, A. Analysis of the effect of EDTAC on the dentinal walls of the root canal. J. Endod. 1977, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wayman, B.E.; Kopp, W.M.; Pinero, G.J.; Lazzari, E.P. Citric and lactic acids as root canal irrigants in vitro. J. Endod. 1979, 5, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.S.; Armas, A.; Goldman, M.; Lin, P.S. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J. Endod. 1983, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Clark-Holke, D.; Drake, D.; Walton, R.; Rivera, E.; Guthmiller, J.M. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J. Dent. 2003, 31, 275–281. [Google Scholar] [CrossRef]
- White, R.R.; Goldman, M.; Lin, P.S. The influence of the smeared layer upon dentinal tubule penetration by plastic filling materials. J. Endod. 1984, 10, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.H.; Pişkin, B.; Baran, N. The effect of tubular penetration of root canal sealers on dye microleakage. Int. Endod. J. 1996, 29, 23–28. [Google Scholar] [CrossRef]
- Shahravan, A.; Haghdoost, A.-A.; Adl, A.; Rahimi, H.; Shadifar, F. Effect of smear layer on sealing ability of canal obturation: A systematic review and meta-analysis. J. Endod. 2007, 33, 96–105. [Google Scholar] [CrossRef]
- Calt, S.; Serper, A. Time-dependent effects of EDTA on dentin structures. J. Endod. 2002, 28, 17–19. [Google Scholar] [CrossRef]
- Torabinejad, M.; Khademi, A.A.; Babagoli, J.; Cho, Y.; Johnson, W.B.; Bozhilov, K.; Kim, J.; Shabahang, S. A new solution for the removal of the smear layer. J. Endod. 2003, 29, 170–175. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Erdemir, A.; Belli, S. Effect of EDTA and citric acid solutions on the microhardness and the roughness of human root canal dentin. J. Endod. 2005, 31, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Heredia, M.; Ferrer-Luque, C.M.; González-Rodríguez, M.P.; Martín-Peinado, F.J.; González-López, S. Decalcifying effect of 15% EDTA, 15% citric acid, 5% phosphoric acid and 2.5% sodium hypochlorite on root canal dentine. Int. Endod. J. 2008, 41, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.C.; Cuenin, P.R. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J. Endod. 1992, 18, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maezono, H.; Shen, Y.; Haapasalo, M. Evaluation of root canal dentin erosion after different irrigation methods using energy-dispersive x-ray spectroscopy. J. Endod. 2016, 42, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Baldasso, F.E.R.; Roleto, L.; Silva, V.D.D.; Morgental, R.D.; Kopper, P.M.P. Effect of final irrigation protocols on microhardness reduction and erosion of root canal dentin. Braz. Oral Res. 2017, 31, e40. [Google Scholar] [CrossRef]
- Retana-Lobo, C.; Ramírez-Mora, T.; Murillo-Gómez, F.; Maria Guerreiro-Tanomaru, J.; Tanomaru-Filho, M.; Reyes-Carmona, J. Final irrigation protocols affect radicular dentin DMP1-CT expression, microhardness, and biochemical composition. Clin. Oral Investig. 2022, 26, 5491–5501. [Google Scholar] [CrossRef]
- Grawehr, M.; Sener, B.; Waltimo, T.; Zehnder, M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int. Endod. J. 2003, 36, 411–417. [Google Scholar] [CrossRef]
- Girard, S.; Paqué, F.; Badertscher, M.; Sener, B.; Zehnder, M. Assessment of a gel-type chelating preparation containing 1-hydroxyethylidene-1, 1-bisphosphonate. Int. Endod. J. 2005, 38, 810–816. [Google Scholar] [CrossRef]
- Zehnder, M.; Schmidlin, P.; Sener, B.; Waltimo, T. Chelation in root canal therapy reconsidered. J. Endod. 2005, 31, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.P.; Yiu, C.K.Y.; Matinlinna, J.P.; Kishen, A.; Neelakantan, P. The effects of sequential and continuous chelation on dentin. Dent. Mater. 2020, 36, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Varughese, A.A.; Sharma, S.; Subbarao, C.V.; Zehnder, M.; De-Deus, G. Continuous chelation irrigation improves the adhesion of epoxy resin-based root canal sealer to root dentine. Int. Endod. J. 2012, 45, 1097–1102. [Google Scholar] [CrossRef]
- Gu, L.-S.; Kim, J.R.; Ling, J.; Choi, K.K.; Pashley, D.H.; Tay, F.R. Review of contemporary irrigant agitation techniques and devices. J. Endod. 2009, 35, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, M.H.; Bernardineli, N.; Cavenago, B.C.; Marciano, M.; del Carpio-Perochena, A.; de Moraes, I.G.; Duarte, M.H.; Bramante, C.M.; Ordinola-Zapata, R. Micro–computed tomography study of the internal anatomy of mesial root canals of mandibular molars. J. Endod. 2011, 37, 1682–1686. [Google Scholar] [CrossRef]
- De Gregorio, C.; Estevez, R.; Cisneros, R.; Heilborn, C.; Cohenca, N. Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: An in vitro study. J. Endod. 2009, 35, 891–895. [Google Scholar] [CrossRef]
- Haupt, F.; Meinel, M.; Gunawardana, A.; Hülsmann, M. Effectiveness of different activated irrigation techniques on debris and smear layer removal from curved root canals: A SEM evaluation. Aust. Endod. J. 2020, 46, 40–46. [Google Scholar] [CrossRef]
- Ahmad, M.; Pitt Ford, T.R.; Crum, L.A. Ultrasonic debridement of root canals: Acoustic streaming and its possible role. J. Endod. 1987, 13, 490–499. [Google Scholar] [CrossRef]
- Mancini, M.; Cerroni, L.; Iorio, L.; Armellin, E.; Conte, G.; Cianconi, L. Smear layer removal and canal cleanliness using different irrigation systems (EndoActivator, EndoVac, and passive ultrasonic irrigation): Field emission scanning electron microscopic evaluation in an in vitro study. J. Endod. 2013, 39, 1456–1460. [Google Scholar] [CrossRef]
- Jiang, L.M.; Lak, B.; Eijsvogels, L.M.; Wesselink, P.; van der Sluis, L.W.M. Comparison of the cleaning efficacy of different final irrigation techniques. J. Endod. 2012, 38, 838–841. [Google Scholar] [CrossRef]
- Urban, K.; Donnermeyer, D.; Schäfer, E.; Bürklein, S. Canal cleanliness using different irrigation activation systems: A SEM evaluation. Clin. Oral Investig. 2017, 21, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Kishen, A.; Felitti, R.; Bhagirath, A.Y.; Medapati, M.R.; Lai, C.; Cunha, R.S. Antibacterial properties of chitosan nanoparticles and propolis associated with calcium hydroxide against single- and multispecies biofilms: An in vitro and in situ study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Kim, D.; Liu, Y.; Karabucak, B.; Koo, H. Novel endodontic disinfection approach using catalytic nanoparticles. J. Endod. 2018, 44, 806–812. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.Y.; Shi, W.; Hong, C.; Tetradis, S.; Kim, Y.; et al. Nanodiamond-gutta percha composite biomaterials for root canal therapy. ACS Nano 2015, 9, 11490–11501. [Google Scholar] [CrossRef]
- Hülsmann, M.; Heckendorff, M.; Schäfers, F. Comparative in-vitro evaluation of three chelator pastes. Int. Endod. J. 2002, 35, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Vishwanath, V.; Taschieri, S.; Corbella, S. Present status and future directions: Minimally invasive root canal preparation and periradicular surgery. Int. Endod. J. 2022, 55, 845–871. [Google Scholar] [CrossRef]
- Pintor, A.V.B.; Dos Santos, M.R.M.; Ferreira, D.M.; Barcelos, R.; Primo, L.G.; Maia, L.C. Does smear layer removal influence root canal therapy outcome? A systematic review. J. Clin. Pediatr. Dent. 2016, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Fedele, G.; Doğramaci, E.J.; Guastalli, A.R.; Steier, L.; de Figueiredo, J.A. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J. Endod. 2012, 38, 426–431. [Google Scholar] [CrossRef]
- Von der Fehr, F.R.; Östby, B.N. Effect of edtac and sulfuric acid on root canal dentine. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 199–205. [Google Scholar] [CrossRef]
- Teixeira, C.S.; Felippe, M.C.S.; Felippe, W.T. The effect of application time of EDTA and NaOCl on intracanal smear layer removal: An SEM analysis. Int. Endod. J. 2005, 38, 285–290. [Google Scholar] [CrossRef]
- Lumley, P.J.; Walmsley, A.D.; Laird, W.R. Streaming patterns produced around endosonic files. Int. Endod. J. 1991, 24, 290–297. [Google Scholar] [CrossRef]
- Lussi, A.; Nussbächer, U.; Grosrey, J. A novel noninstrumented technique for cleansing the root canal system. J. Endod. 1993, 19, 549–553. [Google Scholar] [CrossRef]
- Roy, R.A.; Ahmad, M.; Crum, L.A. Physical mechanisms governing the hydrodynamic response of an oscillating ultrasonic file. Int. Endod. J. 1994, 27, 197–207. [Google Scholar] [CrossRef]
- Rödig, T.; Döllmann, S.; Konietschke, F.; Drebenstedt, S.; Hülsmann, M. Effectiveness of Different Irrigant Agitation Techniques on Debris and Smear Layer Removal in Curved Root Canals: A Scanning Electron Microscopy Study. J. Endod. 2010, 36, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Stamos, D.E.; Sadeghi, E.M.; Haasch, G.C.; Gerstein, H. An in vitro comparison study to quantitate the debridement ability of hand, sonic, and ultrasonic instrumentation. J. Endod. 1987, 13, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Sabins, R.A.; Johnson, J.D.; Hellstein, J.W. A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J. Endod. 2003, 29, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ruddle, C.J. Endodontic disinfection: The sonic advantage. Dent. Today 2017, 36, 84–87. [Google Scholar]
- Jiang, L.M.; Verhaagen, B.; Versluis, M.; van der Sluis, L.W. Evaluation of a sonic device designed to activate irrigant in the root canal. J. Endod. 2010, 36, 143–146. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Neuhaus, K.W.; Liebi, M.; Stauffacher, S.; Eick, S.; Lussi, A. Antibacterial efficacy of a new sonic irrigation device for root canal disinfection. J. Endod. 2016, 42, 1799–1803. [Google Scholar] [CrossRef]
- Caron, G.; Nham, K.; Bronnec, F.; Machtou, P. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J. Endod. 2010, 36, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Reis, C.; Paciornik, S. Critical appraisal of published smear layer-removal studies: Methodological issues. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Muwaquet-Rodríguez, S.; Albero-Monteagudo, A. Update of the therapeutic planning of irrigation and intracanal medication in root canal treatment. A literature review. J. Clin. Exp. Dent. 2019, 11, e185–e193. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-S.; Hsiao, C.-H.; Chang, Y.-C.; Chang, C.-H.; Yang, J.-C.; Gutmann, J.L.; Chang, H.-C.; Huang, H.-M.; Hsieh, S.-C. A Novel Endodontic Approach in Removing Smear Layer Using Nano and Submicron Diamonds with Intracanal Oscillation Irrigation. Nanomaterials 2023, 13, 1646. https://doi.org/10.3390/nano13101646
Huang C-S, Hsiao C-H, Chang Y-C, Chang C-H, Yang J-C, Gutmann JL, Chang H-C, Huang H-M, Hsieh S-C. A Novel Endodontic Approach in Removing Smear Layer Using Nano and Submicron Diamonds with Intracanal Oscillation Irrigation. Nanomaterials. 2023; 13(10):1646. https://doi.org/10.3390/nano13101646
Chicago/Turabian StyleHuang, Ching-Shuan, Chih-Hsun Hsiao, Yu-Chia Chang, Chih-Hsiang Chang, Jen-Chang Yang, James L. Gutmann, Huan-Cheng Chang, Haw-Ming Huang, and Sung-Chih Hsieh. 2023. "A Novel Endodontic Approach in Removing Smear Layer Using Nano and Submicron Diamonds with Intracanal Oscillation Irrigation" Nanomaterials 13, no. 10: 1646. https://doi.org/10.3390/nano13101646
APA StyleHuang, C.-S., Hsiao, C.-H., Chang, Y.-C., Chang, C.-H., Yang, J.-C., Gutmann, J. L., Chang, H.-C., Huang, H.-M., & Hsieh, S.-C. (2023). A Novel Endodontic Approach in Removing Smear Layer Using Nano and Submicron Diamonds with Intracanal Oscillation Irrigation. Nanomaterials, 13(10), 1646. https://doi.org/10.3390/nano13101646






