The Role of Integrins for Mediating Nanodrugs to Improve Performance in Tumor Diagnosis and Treatment
Abstract
1. Introduction
2. The Structure and Function of Integrins
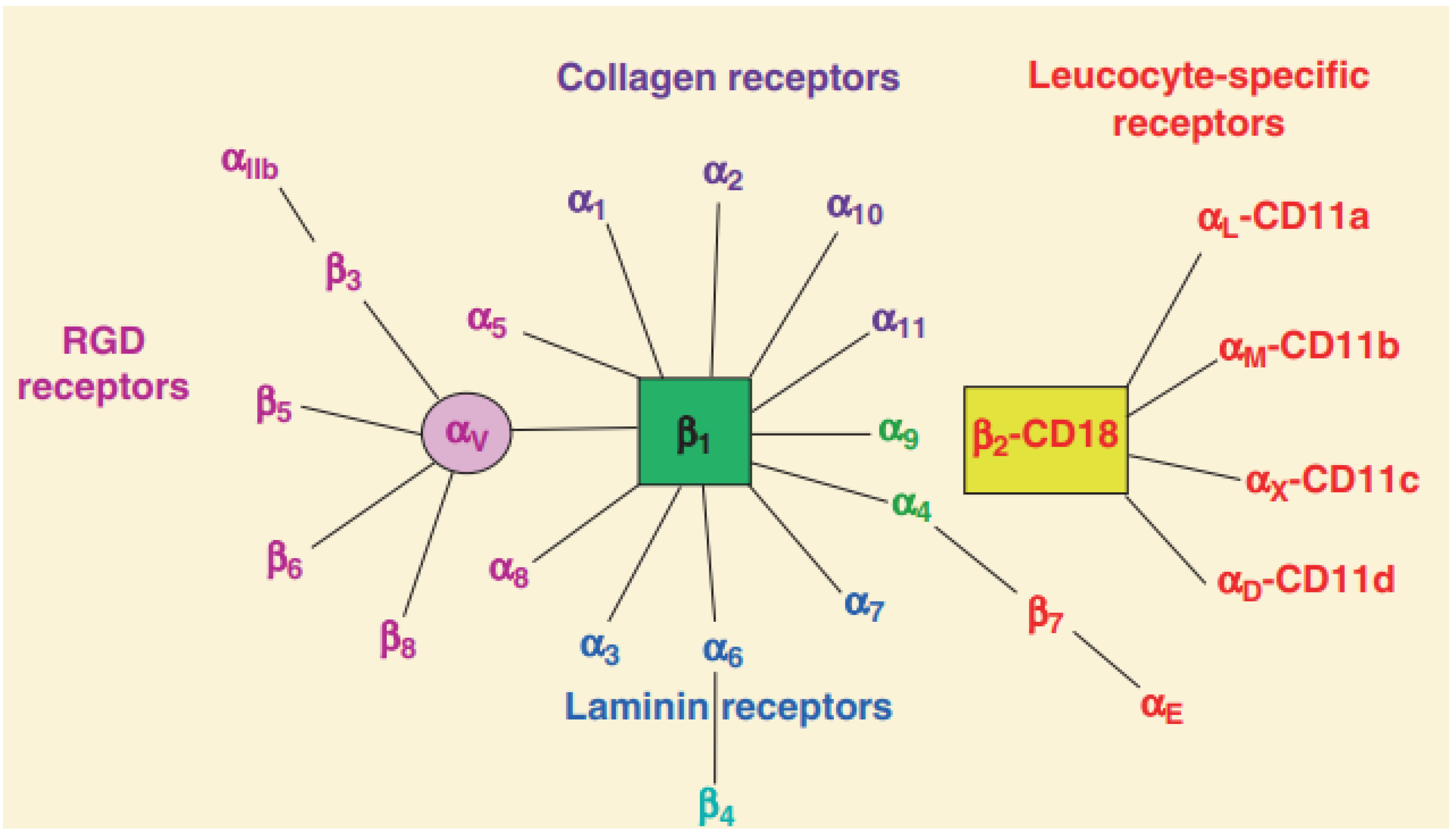
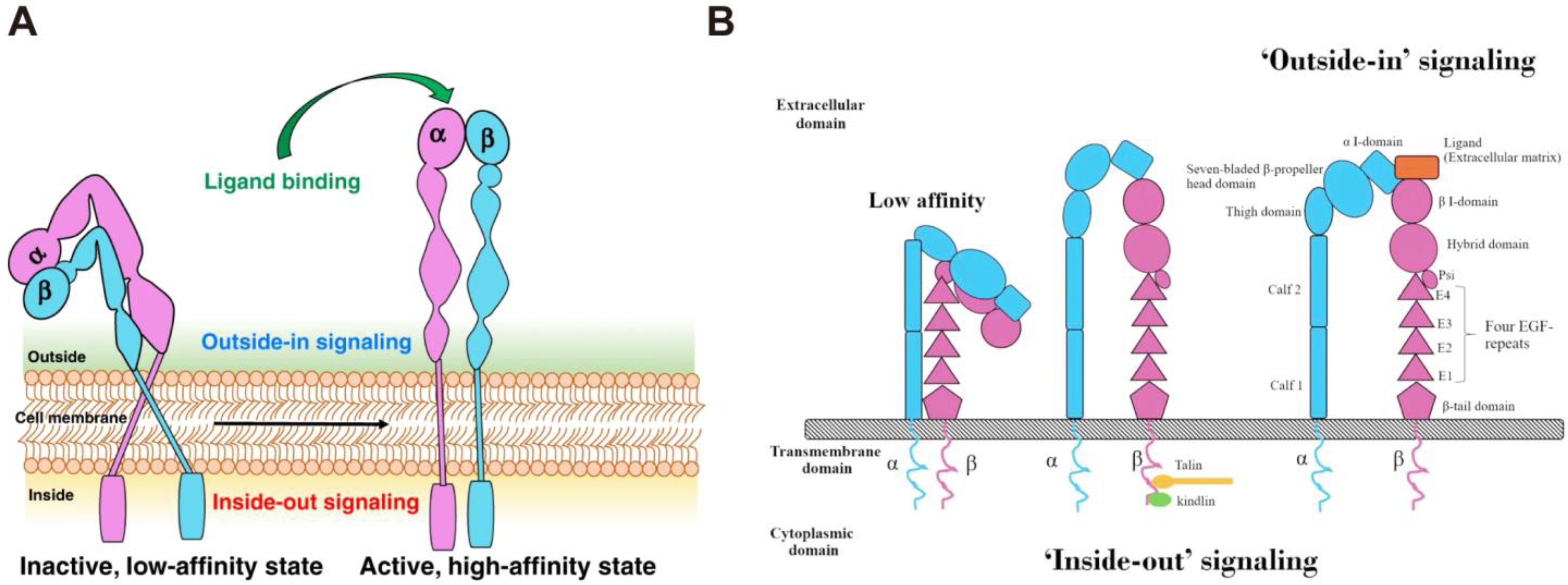
2.1. The Structure of Integrins
2.2. The Function of Integrins
2.3. The Effect of Integrins on Tumors
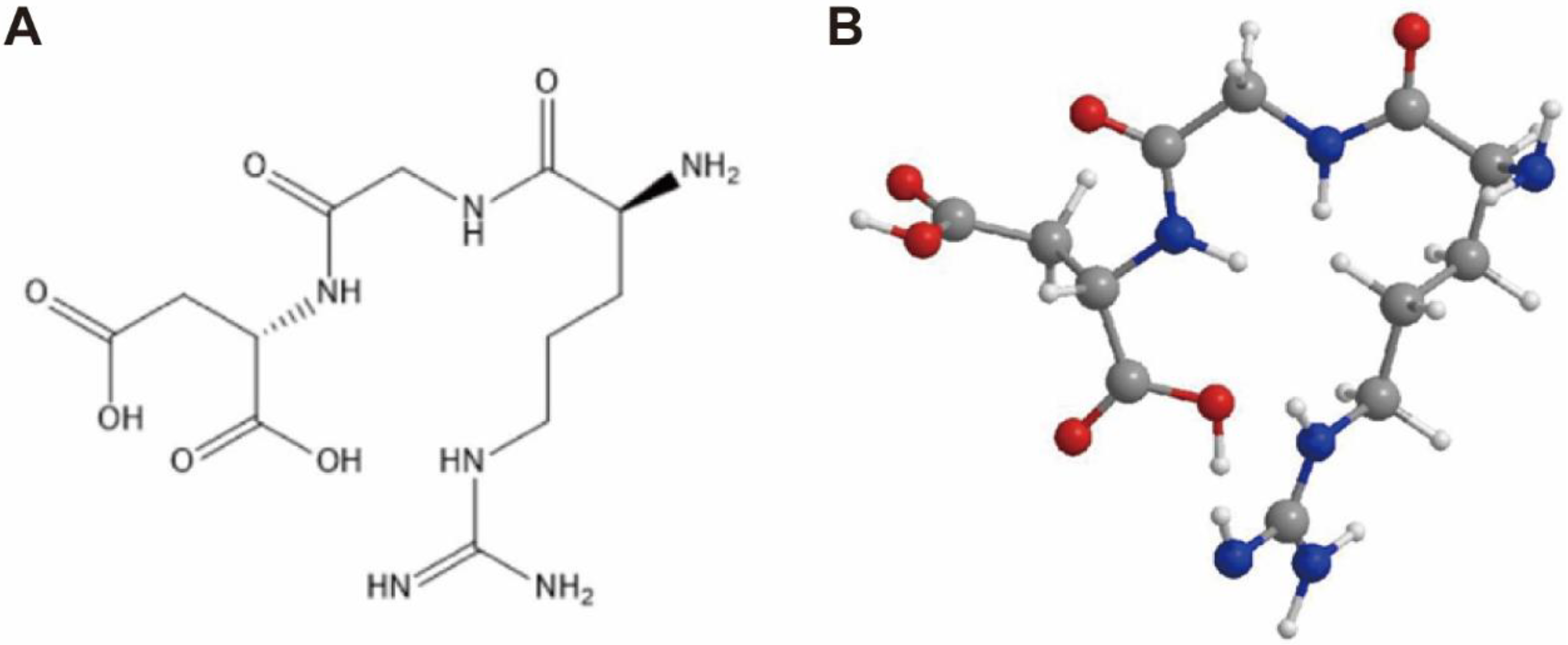
3. The Role of Integrin αvβ3 in Nanodrugs Antitumor Therapy
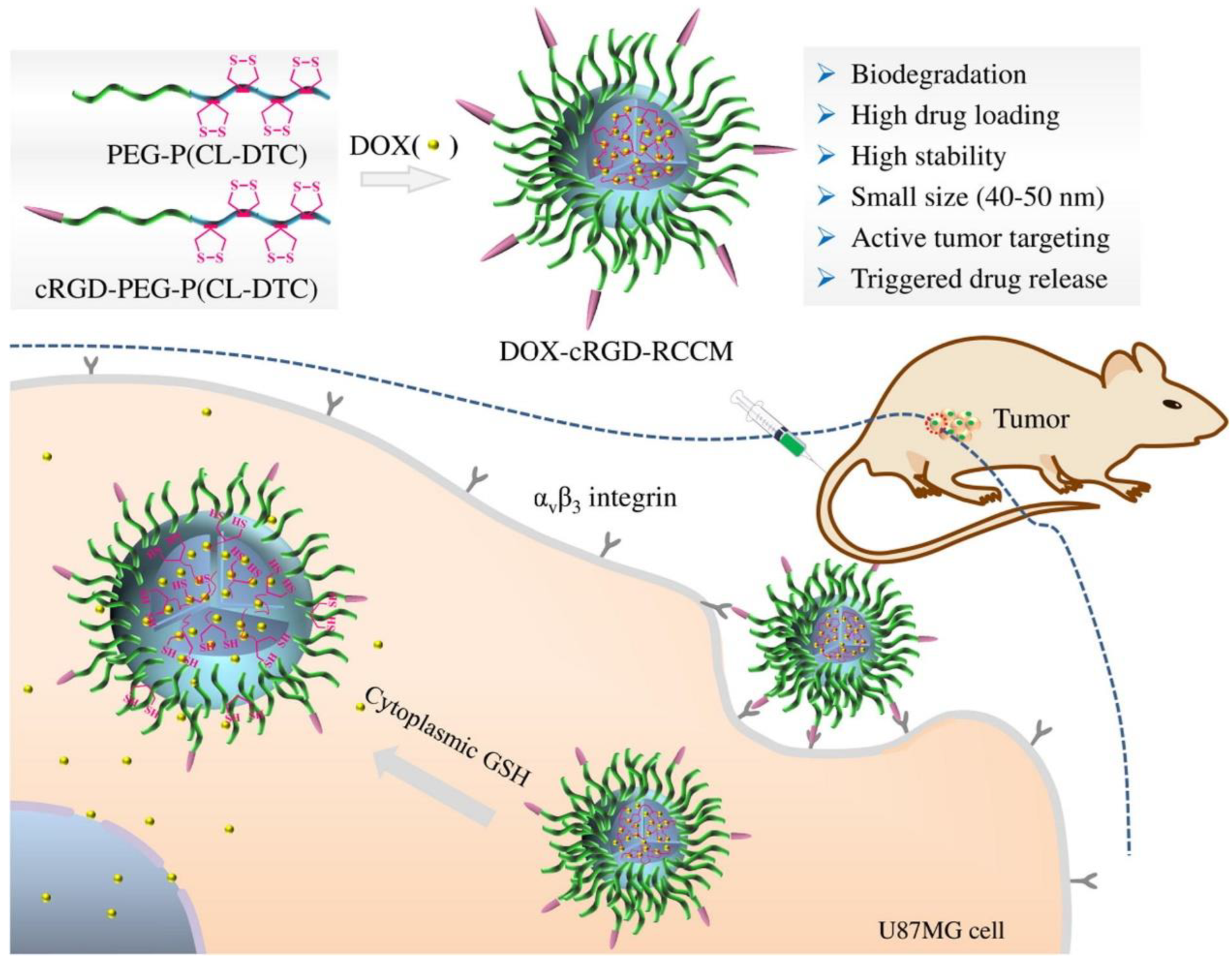
3.1. Chemotherapy
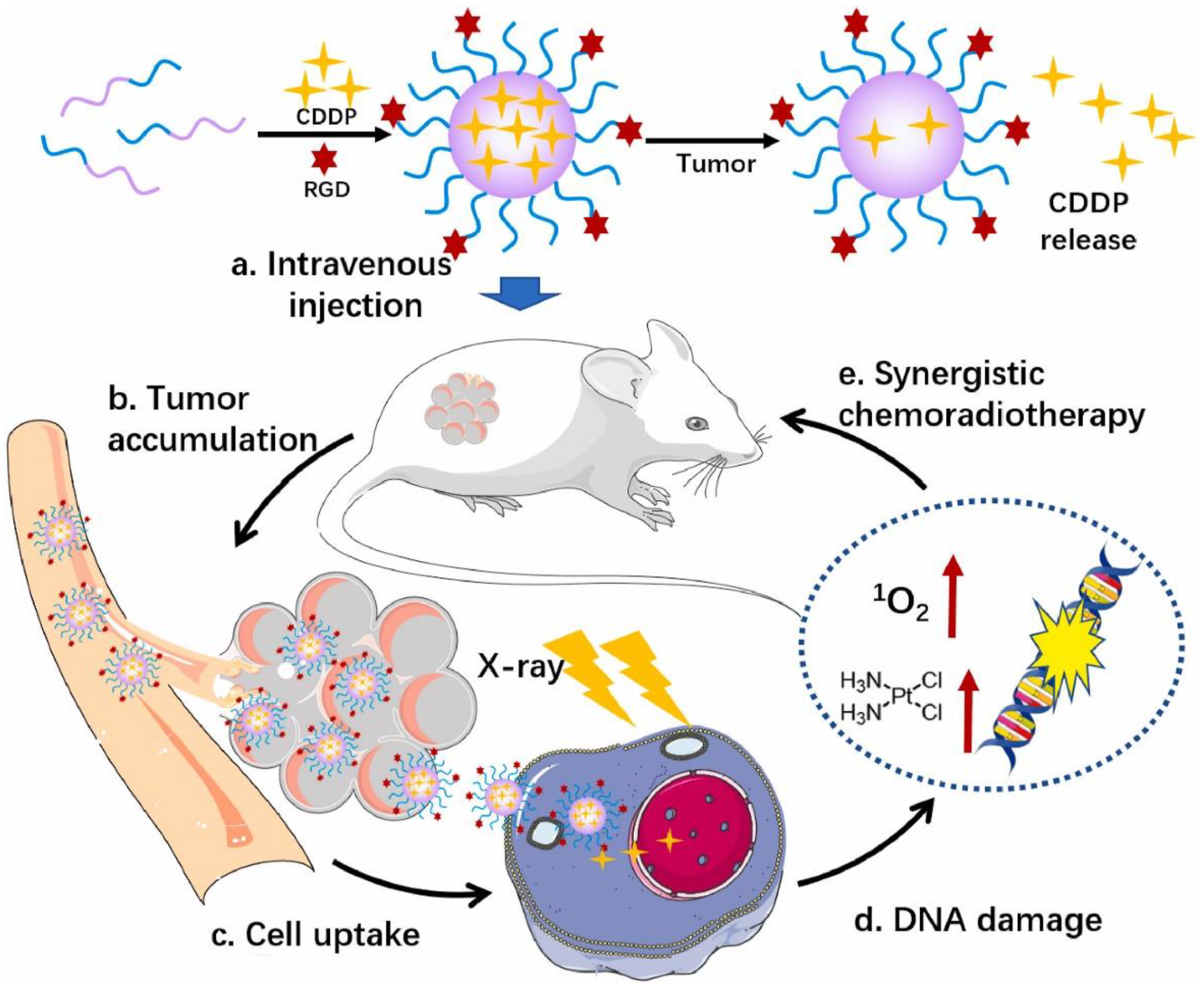
3.2. Radiotherapy
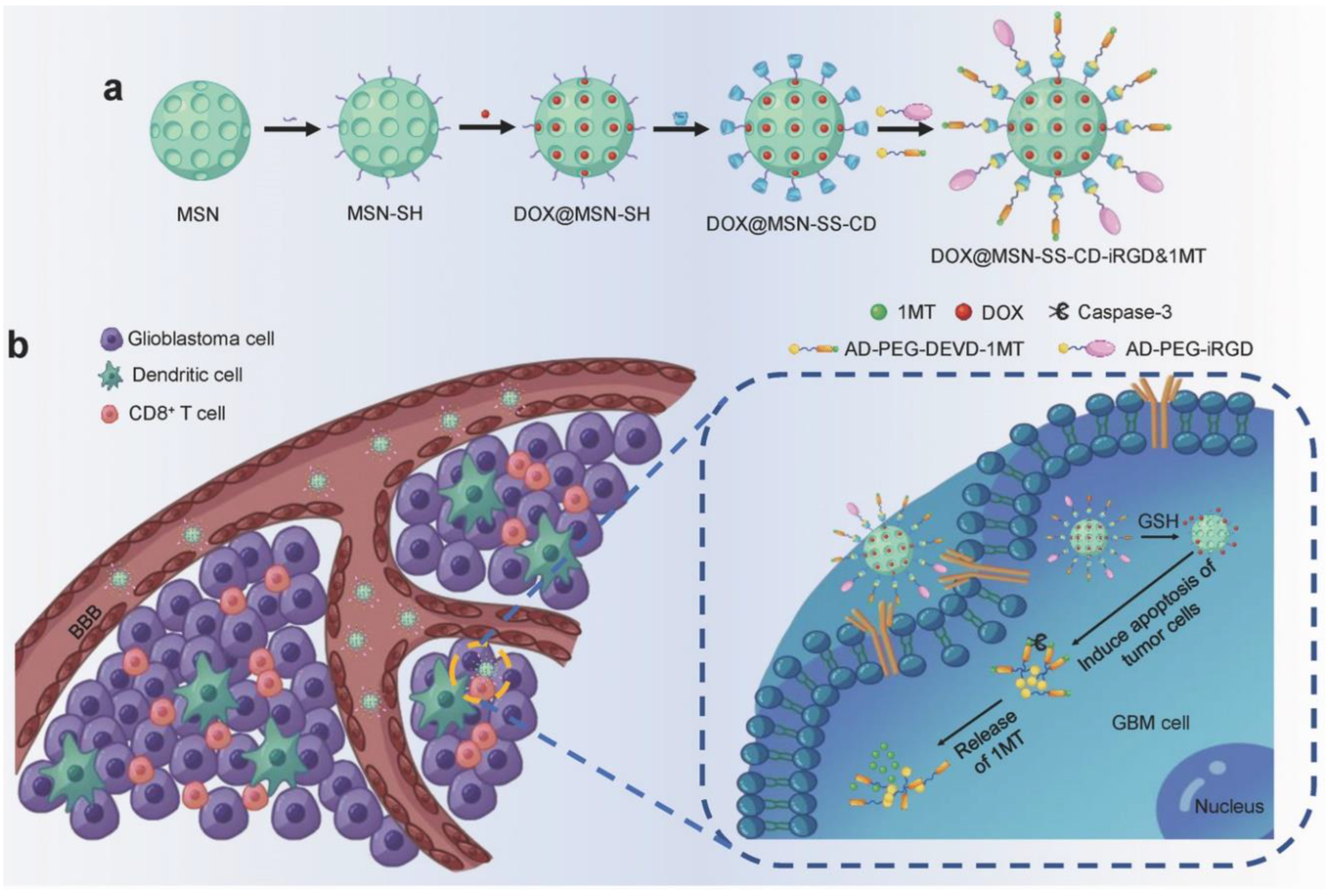
3.3. Immunotherapy
3.4. Other Treatment Strategies
4. The Nanoparticles Targeting Integrin αvβ3
4.1. Protein-Based Nano-Delivery System
4.2. Polymers
4.3. Inorganic Nanomaterials
4.4. Membrane
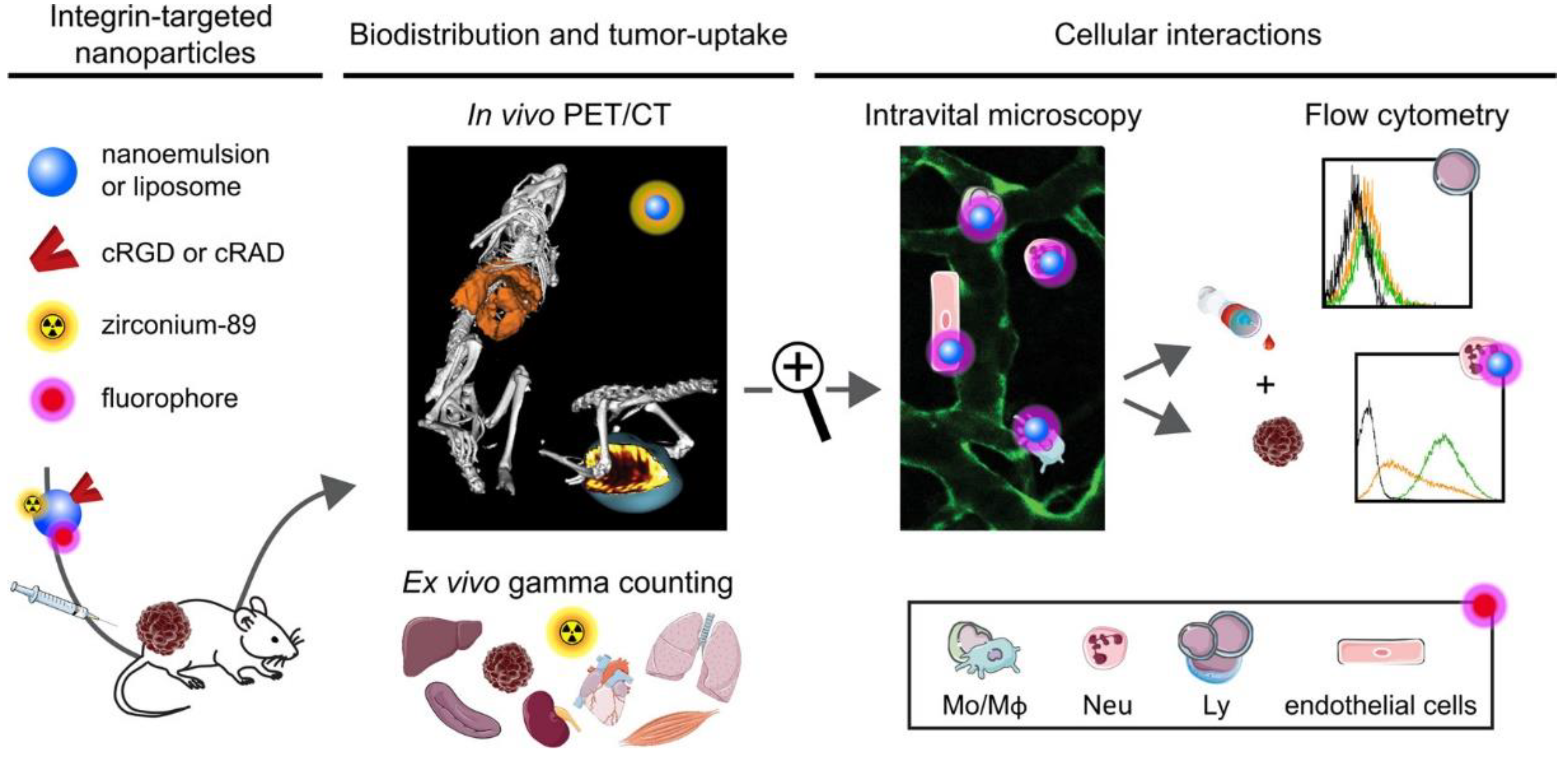
5. Physical Barriers to Integrin-Targeting Nanodrugs
5.1. Opsonization
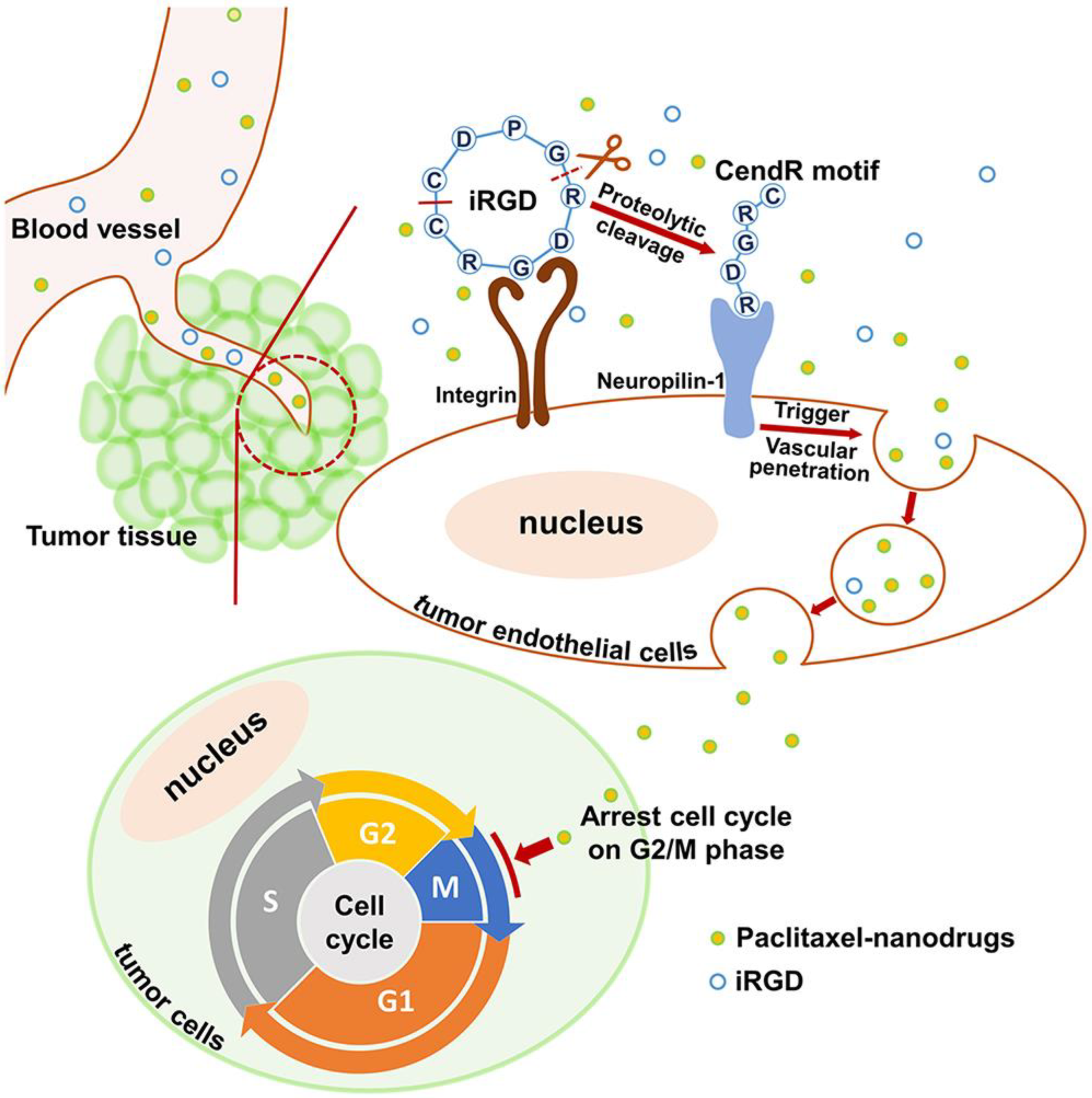
5.2. Penetration in Tumor
5.3. Endocytosis
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Xu, B.; Zhang, X. Tumor Metastasis: Mechanistic Insights and Therapeutic Interventions. MedComm 2021, 2, 587–617. [Google Scholar] [CrossRef] [PubMed]
- Meirson, T.; Gil-Henn, H.; Samson, A.O. Invasion and Metastasis: The Elusive Hallmark of Cancer. Oncogene 2020, 39, 2024–2026. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Sig. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-Cell Invasion and Migration: Diversity and Escape Mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Wittekind, C.; Neid, M. Cancer Invasion and Metastasis. Oncology 2005, 69 (Suppl. 1), 14–16. [Google Scholar] [CrossRef]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The Role of Integrins in Inflammation and Angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Danen, E.H.J. Integrins: An Overview of Structural and Functional Aspects; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Berman, A.E.; Kozlova, N.I. Integrins: Structure and Functions. Membr. Cell Biol. 2000, 13, 207–244. [Google Scholar]
- van der Flier, A.; Sonnenberg, A. Function and Interactions of Integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Why Integrin as a Primary Target for Imaging and Therapy. Theranostics 2011, 1, 30–47. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The Integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Shi, J.; Wang, F.; Liu, S. Radiolabeled Cyclic RGD Peptides as Radiotracers for Tumor Imaging. Biophys. Rep. 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Shimaoka, M.; Springer, T.A. Therapeutic Antagonists and Conformational Regulation of Integrin Function. Nat. Rev. Drug Discov. 2003, 2, 703–716. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Humphries, M.J.; Symonds, E.J.; Mould, A.P. Mapping Functional Residues onto Integrin Crystal Structures. Curr. Opin. Struct. Biol. 2003, 13, 236–243. [Google Scholar] [CrossRef]
- Arimori, T.; Miyazaki, N.; Mihara, E.; Takizawa, M.; Taniguchi, Y.; Cabañas, C.; Sekiguchi, K.; Takagi, J. Structural Mechanism of Laminin Recognition by Integrin. Nat. Commun. 2021, 12, 4012. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, Y.; Yuan, Z.; Qin, G. Research Advances on Structure and Biological Functions of Integrins. SpringerPlus 2016, 5, 1094. [Google Scholar] [CrossRef]
- Kolasangiani, R.; Bidone, T.C.; Schwartz, M.A. Integrin Conformational Dynamics and Mechanotransduction. Cells 2022, 11, 3584. [Google Scholar] [CrossRef]
- Tong, D.; Soley, N.; Kolasangiani, R.; Schwartz, M.A.; Bidone, T.C. Integrin AIIbβ3 Intermediates: From Molecular Dynamics to Adhesion Assembly. Biophys. J. 2023, 122, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Assa-Munt, N.; Jia, X.; Laakkonen, P.; Ruoslahti, E. Solution Structures and Integrin Binding Activities of an RGD Peptide with Two Isomers. Biochemistry 2001, 40, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of Integrins in Cell Invasion and Migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Estrada, J.C.; David, V.; Wermelinger, L.S.; Almeida, F.C.L.; Zingali, R.B. Structure-Function Relationship of the Disintegrin Family: Sequence Signature and Integrin Interaction. Front. Mol. Biosci. 2021, 8, 1178. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Iacob, R.E.; Li, J.; Engen, J.R.; Springer, T.A. Dynamics of Integrin α5β1, Fibronectin, and Their Complex Reveal Sites of Interaction and Conformational Change. J. Biol. Chem. 2022, 298, 102323. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting Integrin Pathways: Mechanisms and Advances in Therapy. Sig. Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Thomas Parsons, J.; Slack-Davis, J.K.; Tilghman, R.W.; Iwanicki, M.; Martin, K.H. Chapter 66—Integrin Signaling: Cell Migration, Proliferation, and Survival. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 491–499. ISBN 978-0-12-374145-5. [Google Scholar]
- Takagi, J.; Springer, T.A. Integrin Activation and Structural Rearrangement. Immunol. Rev. 2002, 186, 141–163. [Google Scholar] [CrossRef]
- Kim, M.; Carman, C.V.; Springer, T.A. Bidirectional Transmembrane Signaling by Cytoplasmic Domain Separation in Integrins. Science 2003, 301, 1720–1725. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Calderwood, D.A. Organization, Dynamics and Mechanoregulation of Integrin-Mediated Cell-ECM Adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef]
- Kulke, M.; Langel, W. Molecular Dynamics Simulations to the Bidirectional Adhesion Signaling Pathway of Integrin αVβ3. Proteins Struct. Funct. Bioinform. 2020, 88, 679–688. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and Kindlins: Partners in Integrin-Mediated Adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef]
- Bouti, P.; Webbers, S.D.S.; Fagerholm, S.C.; Alon, R.; Moser, M.; Matlung, H.L.; Kuijpers, T.W. β2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function. Front. Immunol. 2021, 11, 619925. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, J.; Sun, Y.; Li, D.; Meng, Z.; Wang, B.; Fan, P.; Liu, Z.; Jin, X.; Wu, H. Overexpressed ITGA2 Promotes Malignant Tumor Aggression by Up-Regulating PD-L1 Expression through the Activation of the STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 485. [Google Scholar] [CrossRef]
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a Potential Nanotherapeutic Target for Glioblastoma. Sci. Rep. 2019, 9, 6195. [Google Scholar] [CrossRef]
- Xiong, J.; Yan, L.; Zou, C.; Wang, K.; Chen, M.; Xu, B.; Zhou, Z.; Zhang, D. Integrins Regulate Stemness in Solid Tumor: An Emerging Therapeutic Target. J. Hematol. Oncol. 2021, 14, 177. [Google Scholar] [CrossRef]
- Rattanasinchai, C.; Navasumrit, P.; Ruchirawat, M. Elevated ITGA2 Expression Promotes Collagen Type I-Induced Clonogenic Growth of Intrahepatic Cholangiocarcinoma. Sci. Rep. 2022, 12, 22429. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging Therapeutic Opportunities for Integrin Inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z. The Roles of Integrin Avβ6 in Cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, Q.; Tian, H.; Su, Y.; Fu, G.-H.; Sun, T. Integrin β3 Promotes Cardiomyocyte Proliferation and Attenuates Hypoxia-Induced Apoptosis via Regulating the PTEN/Akt/MTOR and ERK1/2 Pathways. Int. J. Biol. Sci. 2020, 16, 644–654. [Google Scholar] [CrossRef]
- Aksorn, N.; Chanvorachote, P. Integrin as a Molecular Target for Anti-Cancer Approaches in Lung Cancer. Anticancer Res. 2019, 39, 541–548. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Li, M.; Wu, X.; Setrerrahmane, S.; Xu, H. Integrins as Attractive Targets for Cancer Therapeutics. Acta. Pharm. Sin. B 2021, 11, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, X.; Kan, C.; Chen, B.; Qu, N.; Hou, N.; Liu, Y.; Han, F. COL1A1: A Novel Oncogenic Gene and Therapeutic Target in Malignancies. Pathol. Res. Pract. 2022, 236, 154013. [Google Scholar] [CrossRef] [PubMed]
- Baltes, F.; Pfeifer, V.; Silbermann, K.; Caspers, J.; Wantoch von Rekowski, K.; Schlesinger, M.; Bendas, G. β1-Integrin Binding to Collagen Type 1 Transmits Breast Cancer Cells into Chemoresistance by Activating ABC Efflux Transporters. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118663. [Google Scholar] [CrossRef]
- Reis, J.S.D.; Santos, M.A.R.d.C.; da Costa, K.M.; Freire-de-Lima, C.G.; Morrot, A.; Previato, J.O.; Previato, L.M.; da Fonseca, L.M.; Freire-de-Lima, L. Increased Expression of the Pathological O-Glycosylated Form of Oncofetal Fibronectin in the Multidrug Resistance Phenotype of Cancer Cells. Matrix Biol. 2023, 118, 47–68. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in Metastasis: An Updated Review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative Splicing and Cancer: A Systematic Review. Signal. Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G. The Roles of Integrins in Cancer. Fac. Rev. 2021, 10, 45. [Google Scholar] [CrossRef]
- Moschos, S.J.; Drogowski, L.M.; Reppert, S.L.; Kirkwood, J.M. Integrins and Cancer. Oncology 2007, 21, 13–20. [Google Scholar]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Cordes, N.; Park, C.C. Beta1 Integrin as a Molecular Therapeutic Target. Int. J. Radiat. Biol. 2007, 83, 753–760. [Google Scholar] [CrossRef]
- Blandin, A.-F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 Integrins as Therapeutic Targets to Disrupt Hallmarks of Cancer. Front. Pharmacol. 2015, 6, 279. [Google Scholar] [CrossRef]
- Hou, J.; Yan, D.; Liu, Y.; Huang, P.; Cui, H. The Roles of Integrin α5β1 in Human Cancer. Onco Targets Ther. 2020, 13, 13329–13344. [Google Scholar] [CrossRef]
- Fang, Z.; Yao, W.; Fu, Y.; Wang, L.-Y.; Li, Z.; Yang, Y.; Shi, Y.; Qiu, S.; Fan, J.; Zha, X. Increased Integrin A5β1 Heterodimer Formation and Reduced C-Jun Expression Are Involved in Integrin β1 Overexpression-Mediated Cell Growth Arrest. J. Cell. Biochem. 2010, 109, 383–395. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-Formulations of Drugs: Recent Developments, Impact and Challenges. Biochimie 2016, 128–129, 99–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced Materials and Processing for Drug Delivery: The Past and the Future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Preat, V. RGD-Based Strategies To Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Al Mazeedi, M.A.M.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Chen, Y. Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Peng, J.Q.; Fumoto, S.; Suga, T.; Miyamoto, H.; Kuroda, N.; Kawakami, S.; Nishida, K. Targeted Co-Delivery of Protein and Drug to a Tumor in Vivo by Sophisticated RGD-Modified Lipid-Calcium Carbonate Nanoparticles. J. Control. Release 2019, 302, 42–53. [Google Scholar] [CrossRef]
- Filipczak, N.; Joshi, U.; Attia, S.A.; Berger Fridman, I.; Cohen, S.; Konry, T.; Torchilin, V. Hypoxia-Sensitive Drug Delivery to Tumors. J. Control. Release 2022, 341, 431–442. [Google Scholar] [CrossRef]
- Li, Y.; Hong, W.; Zhang, H.; Zhang, T.T.; Chen, Z.; Yuan, S.; Peng, P.; Xiao, M.; Xu, L. Photothermally Triggered Cytosolic Drug Delivery of Glucose Functionalized Polydopamine Nanoparticles in Response to Tumor Microenvironment for the GLUT1-Targeting Chemo-Phototherapy. J. Control. Release 2020, 317, 232–245. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Carvalho, S.M.; Lobato, Z.I.P.; Leite, M.D.F.; Cunha, A.d.S.; Mansur, H.S. Design and Development of Polysaccharide-Doxorubicin-Peptide Bioconjugates for Dual Synergistic Effects of Integrin-Targeted and Cell-Penetrating Peptides for Cancer Chemotherapy. Bioconjugate Chem. 2018, 29, 1973–2000. [Google Scholar] [CrossRef]
- Ahmad, K.; Lee, E.J.; Shaikh, S.; Kumar, A.; Rao, K.M.; Park, S.-Y.; Jin, J.O.; Han, S.S.; Choi, I. Targeting Integrins for Cancer Management Using Nanotherapeutic Approaches: Recent Advances and Challenges. Semin. Cancer Biol. 2021, 69, 325–336. [Google Scholar] [CrossRef]
- Saifi, M.A.; Sathish, G.; Bazaz, M.R.; Godugu, C. Exploration of Tumor Penetrating Peptide IRGD as a Potential Strategy to Enhance Tumor Penetration of Cancer Nanotherapeutics. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188895. [Google Scholar] [CrossRef]
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q.; Li, L.; Hu, L.; Li, S.; Tan, H.; et al. Co-Administration of IRGD Enhances Tumor-Targeted Delivery and Anti-Tumor Effects of Paclitaxel-Loaded PLGA Nanoparticles for Colorectal Cancer Treatment. Int. J. Nanomed. 2019, 14, 8543–8560. [Google Scholar] [CrossRef]
- Liang, G.; Jin, X.; Zhang, S.; Xing, D. RGD Peptide-Modified Fluorescent Gold Nanoclusters as Highly Efficient Tumor-Targeted Radiotherapy Sensitizers. Biomaterials 2017, 144, 95–104. [Google Scholar] [CrossRef]
- Ding, Y.; Xiao, X.; Zeng, L.; Shang, Q.; Jiang, W.; Xiong, S.; Duan, X.; Shen, J.; Wang, R.; Guo, J.; et al. Platinum-Crosslinking Polymeric Nanoparticle for Synergetic Chemoradiotherapy of Nasopharyngeal Carcinoma. Bioact. Mater. 2021, 6, 4707–4716. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, J.; Yang, M.; Wang, H.; Chu, X.; Zhou, J.; Huo, M.; Yin, T. Biomineralization-Inspired Dasatinib Nanodrug with Sequential Infiltration for Effective Solid Tumor Treatment. Biomaterials 2021, 267, 120481. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy Toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Hallahan, D.E.; Qu, S.; Geng, L.; Cmelak, A.; Chakravarthy, A.; Martin, W.; Scarfone, C.; Giorgio, T. Radiation-Mediated Control of Drug Delivery. Am. J. Clin. Oncol. 2001, 24, 473–480. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Cui, L.; Her, S.; Borst, G.R.; Bristow, R.G.; Jaffray, D.A.; Allen, C. Radiosensitization by Gold Nanoparticles: Will They Ever Make It to the Clinic? Radiother. Oncol. 2017, 124, 344–356. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Cai, J.; Li, X.; Cheng, D.; Su, H.; Zhang, J.; Liu, S.; Shi, H.; Zhang, Y.; et al. Tumor Angiogenesis Targeted Radiosensitization Therapy Using Gold Nanoprobes Guided by MRI/SPECT Imaging. ACS Appl. Mater. Interfaces 2016, 8, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, K.; Gao, H.; Luo, Q.; Wang, X.; Li, X.; Tong, W.; Zhang, X.; Luo, C.; Yang, G.; et al. A 64 Cu-Porphyrin-Based Dual-Modal Molecular Probe with Integrin Av Β3 Targeting Function for Tumour Imaging. J. Labelled. Comp. Radiopharm. 2020, 63, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xin, J. Advances in Clinical Oncology Research on 99mTc-3PRGD2 SPECT Imaging. Front. Oncol. 2022, 12, 898764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Targeted Radionuclide Therapy: A Historical and Personal Review. Semin. Nucl. Med. 2020, 50, 87–97. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. When Radionuclides Meet Nanoparticles. Nat. Nanotech 2018, 13, 359–360. [Google Scholar] [CrossRef]
- Dash, A.; Russ Knapp, F.F.; Pillai, M.R.A. Targeted Radionuclide Therapy—An Overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Fu, K.; Lin, Q.; Wen, X.; Jacobson, O.; Sun, L.; Wu, H.; Zhang, X.; Guo, Z.; et al. Integrin αvβ3-Targeted Radionuclide Therapy Combined with Immune Checkpoint Blockade Immunotherapy Synergistically Enhances Anti-Tumor Efficacy. Theranostics 2019, 9, 7948–7960. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Huang, W.; He, L.; Ouyang, J.; Chen, Q.; Liu, C.; Tao, W.; Chen, T. Triangle-Shaped Tellurium Nanostars Potentiate Radiotherapy by Boosting Checkpoint Blockade Immunotherapy. Matter 2020, 3, 1725–1753. [Google Scholar] [CrossRef]
- Yuasa, H.J.; Ball, H.J.; Austin, C.J.D.; Hunt, N.H. 1-L-Methyltryptophan Is a More Effective Inhibitor of Vertebrate IDO2 Enzymes than 1-D-Methyltryptophan. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 10–15. [Google Scholar] [CrossRef]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.-P.; Tao, J.; Liu, C.-J.; He, X.-H.; Zhang, X.-Z. iRGD Modified Chemo-Immunotherapeutic Nanoparticles for Enhanced Immunotherapy against Glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Zheng, J.; He, W.; Li, J.; Feng, X.; Li, Y.; Cheng, B.; Zhou, Y.; Li, M.; Liu, K.; Shao, X.; et al. Bifunctional Compounds as Molecular Degraders for Integrin-Facilitated Targeted Protein Degradation. J. Am. Chem. Soc. 2022, 144, 21831–21836. [Google Scholar] [CrossRef]
- Busenhart, P.; Montalban-Arques, A.; Katkeviciute, E.; Morsy, Y.; Van Passen, C.; Hering, L.; Atrott, K.; Lang, S.; Garzon, J.F.G.; Naschberger, E.; et al. Inhibition of Integrin αvβ6 Sparks T-Cell Antitumor Response and Enhances Immune Checkpoint Blockade Therapy in Colorectal Cancer. J. Immunother. Cancer 2022, 10, e003465. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, S.; Yuan, B.; Wu, Y.; Gao, Y.; Wan, G.; Li, G. A Novel CTLA-4 Affinity Peptide for Cancer Immunotherapy by Increasing the Integrin Avβ3 Targeting. Discov. Oncol. 2022, 13, 99. [Google Scholar] [CrossRef]
- Pan, X.; Yi, M.; Liu, C.; Jin, Y.; Liu, B.; Hu, G.; Yuan, X. Cilengitide, an αvβ3-Integrin Inhibitor, Enhances the Efficacy of Anti-Programmed Cell Death-1 Therapy in a Murine Melanoma Model. Bioengineered 2022, 13, 4557–4572. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Yu, M.; Cao, R.; Ma, Z.; Zhu, M. Development of “Smart” Drug Delivery Systems for Chemo/PDT Synergistic Treatment. J. Mater. Chem. B 2023, 11, 1416–1433. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Peng, J.; Wang, R.; Sun, W.; Huang, M.; Wang, R.; Li, Y.; Wang, P.; Sun, G.; Xie, S. Delivery of MiR-320a-3p by Gold Nanoparticles Combined with Photothermal Therapy for Directly Targeting Sp1 in Lung Cancer. Biomater. Sci. 2021, 9, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhu, J.; Yang, Z.; Li, L.; Fan, W.; He, L.; Tang, W.; Deng, L.; Mu, J.; Ma, Y.; et al. A Phototheranostic Strategy to Continuously Deliver Singlet Oxygen in the Dark and Hypoxic Tumor Microenvironment. Angew. Chem. Int. Ed. Engl. 2020, 59, 8833–8838. [Google Scholar] [CrossRef] [PubMed]
- Dayan, A.; Fleminger, G.; Ashur-Fabian, O. RGD-Modified Dihydrolipoamide Dehydrogenase Conjugated to Titanium Dioxide Nanoparticles—Switchable Integrin-Targeted Photodynamic Treatment of Melanoma Cells. RSC Adv. 2018, 8, 9112–9119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, P.; Wang, X.; Hou, X.; Liu, F.; Jiang, X. Integrin αvβ3-Targeted Polydopamine-Coated Gold Nanostars for Photothermal Ablation Therapy of Hepatocellular Carcinoma. Regen. Biomater. 2021, 8, rbab046. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Targeted Drug Delivery in Cancer Therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef]
- Dai, L.; Liu, J.; Luo, Z.; Li, M.; Cai, K. Tumor Therapy: Targeted Drug Delivery Systems. J. Mater. Chem. B 2016, 4, 6758–6772. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-Based Nanoparticles as Potential Controlled Release Drug Delivery Systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Ming, H.; Fang, L.; Gao, J.; Li, C.; Ji, Y.; Shen, Y.; Hu, Y.; Li, N.; Chang, J.; Li, W.; et al. Antitumor Effect of Nanoparticle 131I-Labeled Arginine-Glycine-Aspartate-Bovine Serum Albumin-Polycaprolactone in Lung Cancer. AJR Am. J. Roentgenol. 2017, 208, 1116–1126. [Google Scholar] [CrossRef]
- Mu, J.; Zhong, H.; Zou, H.; Liu, T.; Yu, N.; Zhang, X.; Xu, Z.; Chen, Z.; Guo, S. Acid-Sensitive PEGylated Paclitaxel Prodrug Nanoparticles for Cancer Therapy: Effect of PEG Length on Antitumor Efficacy. J. Control. Release 2020, 326, 265–275. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Huo, M.; Wang, H.; Zhang, Y.; Cai, H.; Zhang, P.; Li, L.; Zhou, J.; Yin, T. Co-Delivery of Silybin and Paclitaxel by Dextran-Based Nanoparticles for Effective Anti-Tumor Treatment through Chemotherapy Sensitization and Microenvironment Modulation. J. Control. Release 2020, 321, 198–210. [Google Scholar] [CrossRef]
- Shi, Q.; Tong, Y.; Zheng, Y.; Liu, Y.; Yin, T. PDT-Sensitized ROS-Responsive Dextran Nanosystem for Maximizing Antitumor Potency of Multi-Target Drugs. Int. J. Pharm. 2023, 633, 122567. [Google Scholar] [CrossRef]
- Du, Q.; Lv, F.; Huang, J.; Tang, X.; Zhao, Z.; Chen, J. A Multiple Environment-Sensitive Prodrug Nanomicelle Strategy Based on Chitosan Graftomer for Enhanced Tumor Therapy of Gambogic Acid. Carbohydr. Polym. 2021, 267, 118229. [Google Scholar] [CrossRef]
- Raj, A.; Saraf, P.; Javali, N.M.; Li, X.; Jasti, B. Binding and Uptake of Novel RGD Micelles to the αvβ3 Integrin Receptor for Targeted Drug Delivery. J. Drug Target. 2014, 22, 518–527. [Google Scholar] [CrossRef]
- Xu, W.; Yan, X.; Liu, N.; Wu, G. P1c Peptide Decorated Liposome Targeting αvβ3-Expressing Tumor Cells In Vitro and In Vivo. RSC Adv. 2018, 8, 25575–25583. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. 8—Inorganic Nanoparticles for Targeted Drug Delivery. In Biointegration of Medical Implant Materials; Sharma, C.P., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2010; pp. 204–235. ISBN 978-1-84569-509-5. [Google Scholar]
- Liu, Q.; Kim, Y.-J.; Im, G.-B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic Nanoparticles Applied as Functional Therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Jeon, J.; Hong, S.H.; Rhim, W.-K.; Lee, Y.-S.; Youn, H.; Chung, J.-K.; Lee, M.C.; Lee, D.S.; Kang, K.W.; et al. Tumor Targeting and Imaging Using Cyclic RGD-PEGylated Gold Nanoparticle Probes with Directly Conjugated Iodine-125. Small 2011, 7, 2052–2060. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. RGD-Modified Polymer and Liposome Nanovehicles: Recent Research Progress for Drug Delivery in Cancer Therapeutics. Eur. J. Pharm. Sci. 2019, 128, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Dang, Y.; Liang, G.; Liu, G. Iodine-125-Labeled CRGD-Gold Nanoparticles as Tumor-Targeted Radiosensitizer and Imaging Agent. Nanoscale Res. Lett. 2015, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Chen, M.; Liu, L.; Li, Y. Applications of Quantum Dots in Cancer Detection and Diagnosis: A Review. J. Biomed. Nanotechnol. 2017, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Kurian, P. Quantum Probes in Cancer Research. Nat. Rev. Cancer 2022, 22, 378–379. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Lei, X.; Zhang, J.; Yang, H.; Li, K.; Xu, C. Peptide-Conjugated Quantum Dots Act as the Target Marker for Human Pancreatic Carcinoma Cells. Cell. Physiol. Biochem. 2016, 38, 1121–1128. [Google Scholar] [CrossRef]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y. Cell Membrane Coated Nanoparticles: Next-Generation Therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, X.; Zhang, L.; Liu, C.; Liu, G. Cell Membrane-Encapsulated Nanoparticles for Vaccines and Immunotherapy. Particuology 2022, 64, 35–42. [Google Scholar] [CrossRef]
- Zou, S.; Wang, B.; Wang, C.; Wang, Q.; Zhang, L. Cell Membrane-Coated Nanoparticles: Research Advances. Nanomedicine 2020, 15, 625–641. [Google Scholar] [CrossRef]
- Han, X.; Shen, S.; Fan, Q.; Chen, G.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red Blood Cell–Derived Nanoerythrosome for Antigen Delivery with Enhanced Cancer Immunotherapy. Sci. Adv. 2019, 5, eaaw6870. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Li, Z.; Ren, L.; Ma, X.; Zhu, H.; Gong, Q.; Zhang, H.; Gu, Z.; Luo, K. A Tumor Cell Membrane-Coated Self-Amplified Nanosystem as a Nanovaccine to Boost the Therapeutic Effect of Anti-PD-L1 Antibody. Bioact. Mater. 2023, 21, 299–312. [Google Scholar] [CrossRef]
- Ji, B.; Cai, H.; Yang, Y.; Peng, F.; Song, M.; Sun, K.; Yan, F.; Liu, Y. Hybrid Membrane Camouflaged Copper Sulfide Nanoparticles for Photothermal-Chemotherapy of Hepatocellular Carcinoma. Acta Biomater. 2020, 111, 363–372. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Neutrophils as an Emerging Therapeutic Target and Tool for Cancer Therapy. Life Sci. 2021, 285, 119952. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, L.; Wang, S.; Yang, J.; Lu, G.; Luo, N.; Gao, X.; Ma, G.; Xie, H.-Y.; Wei, W. Construction of a Biomimetic Magnetosome and Its Application as a SiRNA Carrier for High-Performance Anticancer Therapy. Adv. Funct. Mater. 2018, 28, 1703326. [Google Scholar] [CrossRef]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-Camouflaged Nanococktail: Simultaneous Inhibition of Drug-Resistant Tumor Growth and Metastasis via a Cancer Cells and Tumor Vasculature Dual-Targeting Strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Hodivala-Dilke, K. Avβ3 Integrin and Angiogenesis: A Moody Integrin in a Changing Environment. Curr. Opin. Cell Biol. 2008, 20, 514–519. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- LaFoya, B.; Munroe, J.A.; Miyamoto, A.; Detweiler, M.A.; Crow, J.J.; Gazdik, T.; Albig, A.R. Beyond the Matrix: The Many Non-ECM Ligands for Integrins. Int. J. Mol. Sci. 2018, 19, 449. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Calderwood, D.A. Chapter 22: Structural and Signaling Functions of Integrins. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183206. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in Wound Healing, Fibrosis and Tumor Stroma: High Potential Targets for Therapeutics and Drug Delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53. [Google Scholar] [CrossRef]
- Arosio, D.; Casagrande, C. Advancement in Integrin Facilitated Drug Delivery. Adv. Drug Deliv. Rev. 2016, 97, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Szebeni, J. Stealth Liposomes and Long Circulating Nanoparticles: Critical Issues in Pharmacokinetics, Opsonization and Protein-Binding Properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Griffin, L.; Banda, N.K.; Saba, L.M.; Groman, E.V.; Scheinman, R.; Moghimi, S.M.; Simberg, D. Complement Opsonization of Nanoparticles: Differences between Humans and Preclinical Species. J. Control. Release 2021, 338, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sosale, N.G.; Spinler, K.R.; Alvey, C.; Discher, D.E. Macrophage Engulfment of a Cell or Nanoparticle Is Regulated by Unavoidable Opsonization, a Species-Specific ‘Marker of Self’ CD47, and Target Physical Properties. Curr. Opin. Immunol. 2015, 35, 107–112. [Google Scholar] [CrossRef]
- Cox, N.; Kintzing, J.R.; Smith, M.; Grant, G.A.; Cochran, J.R. Integrin-Targeting Knottin Peptide-Drug Conjugates Are Potent Inhibitors of Tumor Cell Proliferation. Angew. Chem.-Int. Edit. 2016, 55, 9894–9897. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Guimaraes, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S. Liposome Protein Corona Characterization as a New Approach in Nanomedicine. Anal. Bioanal. Chem. 2019, 411, 4313–4326. [Google Scholar] [CrossRef]
- Onishchenko, N.; Tretiakova, D.; Vodovozova, E. Spotlight on the Protein Corona of Liposomes. Acta Biomater. 2021, 134, 57–78. [Google Scholar] [CrossRef]
- Foteini, P.; Pippa, N.; Naziris, N.; Demetzos, C. Physicochemical Study of the Protein-Liposome Interactions: Influence of Liposome Composition and Concentration on Protein Binding. J. Liposome Res. 2019, 29, 313–321. [Google Scholar] [CrossRef]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi, R.Z.; Laganà, A.; Amenitsch, H.; Masuelli, L.; et al. Interplay of Protein Corona and Immune Cells Controls Blood Residency of Liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Y.; Zou, Y.; Meng, F.; Zhang, J.; Deng, C.; Sun, H.; Zhong, Z. Targeted Glioma Chemotherapy by Cyclic RGD Peptide-Functionalized Reversibly Core-Crosslinked Multifunctional Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone) Micelles. Acta Biomater. 2017, 50, 396–406. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Li, Q.; Li, L.; Jia, Y.; Geng, F.; Zhou, J.; Yin, T. Tumor Microenvironment Remodeling-Based Penetration Strategies to Amplify Nanodrug Accessibility to Tumor Parenchyma. Adv. Drug. Deliv. Rev. 2021, 172, 80–103. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.; Park, S. IRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 1906. [Google Scholar] [CrossRef]
- Rios De La Rosa, J.M.; Spadea, A.; Donno, R.; Lallana, E.; Lu, Y.; Puri, S.; Caswell, P.; Lawrence, M.J.; Ashford, M.; Tirelli, N. Microfluidic-Assisted Preparation of RGD-Decorated Nanoparticles: Exploring Integrin-Facilitated Uptake in Cancer Cell Lines. Sci. Rep. 2020, 10, 14505. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Q.; Duan, T.; Hu, R.; Tang, J. The Fate of Nanoparticles In Vivo and the Strategy of Designing Stealth Nanoparticle for Drug Delivery. Curr. Drug. Targets 2021, 22, 922–946. [Google Scholar] [CrossRef]
- Glassman, P.M.; Hood, E.D.; Ferguson, L.T.; Zhao, Z.; Siegel, D.L.; Mitragotri, S.; Brenner, J.S.; Muzykantov, V.R. Red Blood Cells: The Metamorphosis of a Neglected Carrier into the Natural Mothership for Artificial Nanocarriers. Adv. Drug Deliv. Rev. 2021, 178, 113992. [Google Scholar] [CrossRef]
- Sofias, A.M.; Toner, Y.C.; Meerwaldt, A.E.; van Leent, M.M.T.; Soultanidis, G.; Elschot, M.; Gonai, H.; Grendstad, K.; Flobak, Å.; Neckmann, U.; et al. Tumor Targeting by Avβ3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano 2020, 14, 7832–7846. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, B.; Hou, S.; Sui, Y.; Yang, T.; Lv, M.; Zhou, Y.; Yu, H.; Li, S.; Peng, H.; et al. Delivery of RGD-Modified Liposome as a Targeted Colorectal Carcinoma Therapy and Its Autophagy Mechanism. J. Drug Target. 2021, 29, 863–874. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Jiang, W.; Li, B.; Hu, Y.; Liu, D. The Role of Integrins for Mediating Nanodrugs to Improve Performance in Tumor Diagnosis and Treatment. Nanomaterials 2023, 13, 1721. https://doi.org/10.3390/nano13111721
Yu C, Jiang W, Li B, Hu Y, Liu D. The Role of Integrins for Mediating Nanodrugs to Improve Performance in Tumor Diagnosis and Treatment. Nanomaterials. 2023; 13(11):1721. https://doi.org/10.3390/nano13111721
Chicago/Turabian StyleYu, Chi, Wei Jiang, Bin Li, Yong Hu, and Dan Liu. 2023. "The Role of Integrins for Mediating Nanodrugs to Improve Performance in Tumor Diagnosis and Treatment" Nanomaterials 13, no. 11: 1721. https://doi.org/10.3390/nano13111721
APA StyleYu, C., Jiang, W., Li, B., Hu, Y., & Liu, D. (2023). The Role of Integrins for Mediating Nanodrugs to Improve Performance in Tumor Diagnosis and Treatment. Nanomaterials, 13(11), 1721. https://doi.org/10.3390/nano13111721








