Plasmon-Enhanced Photocatalytic CO2 Reduction for Higher-Order Hydrocarbon Generation Using Plasmonic Nano-Finger Arrays
Abstract
:1. Introduction
2. Materials and Methods
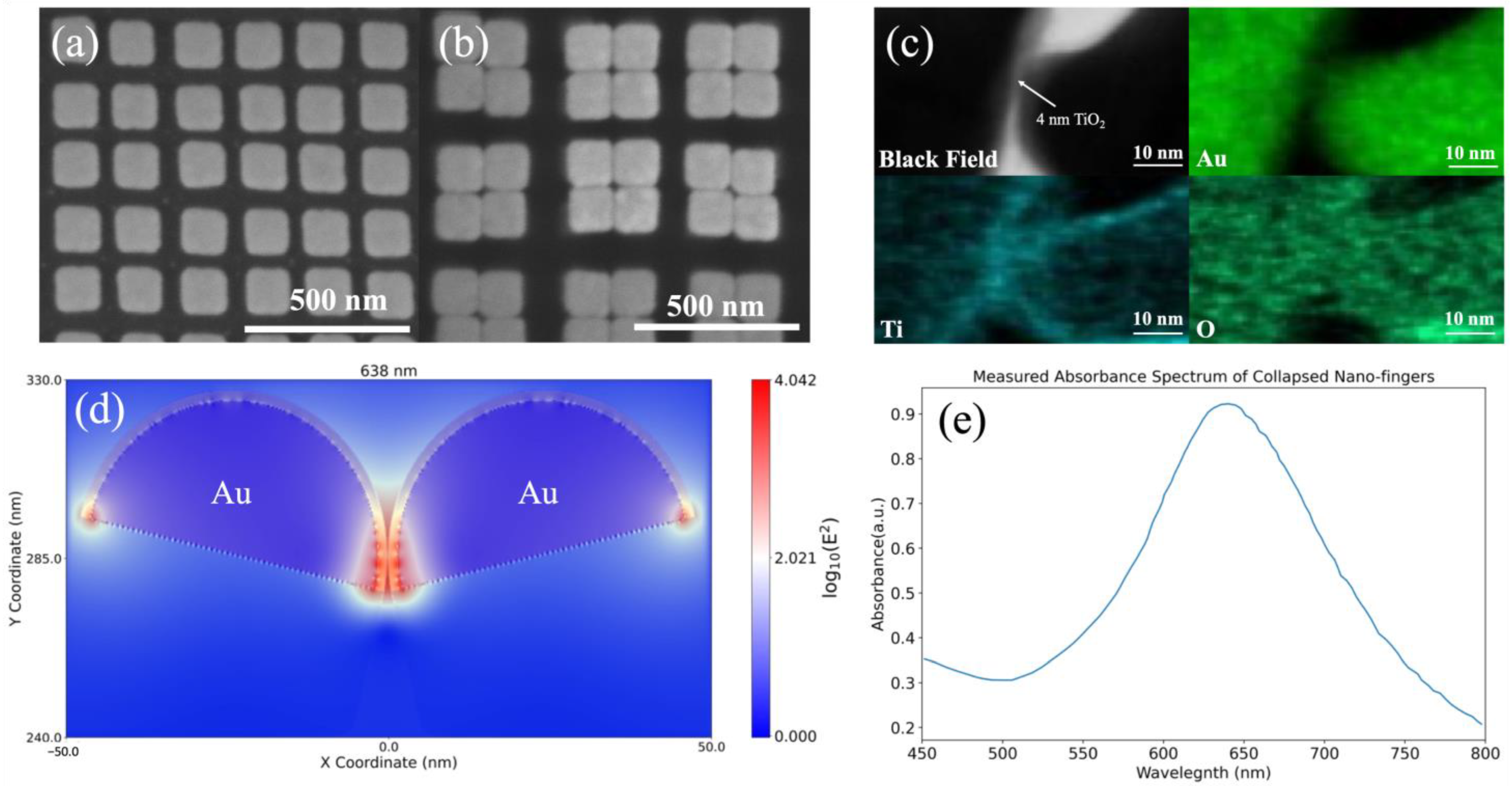
2.1. Fabrication of the Device
2.2. Characterization
2.3. Electromagnetics Simulation
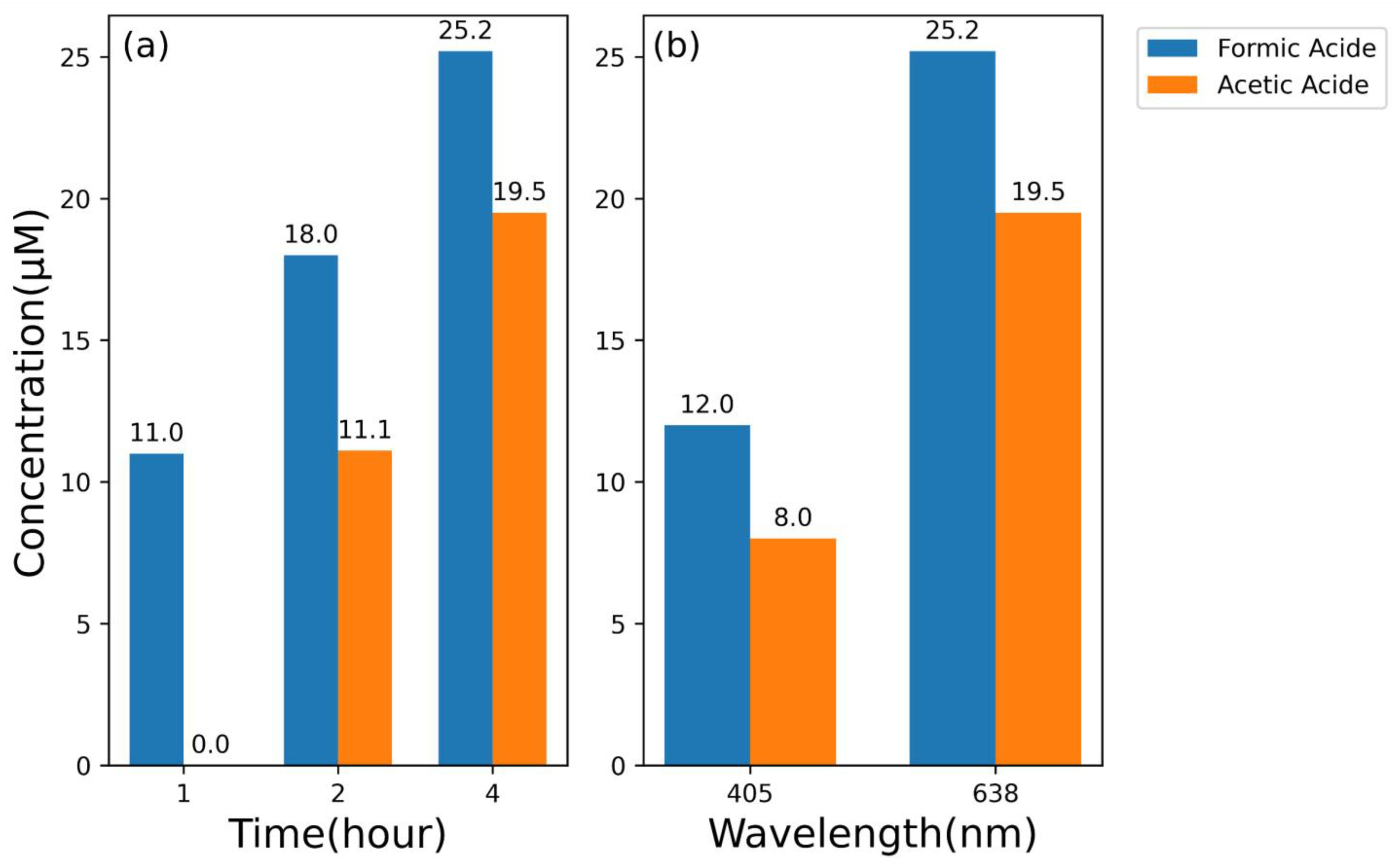
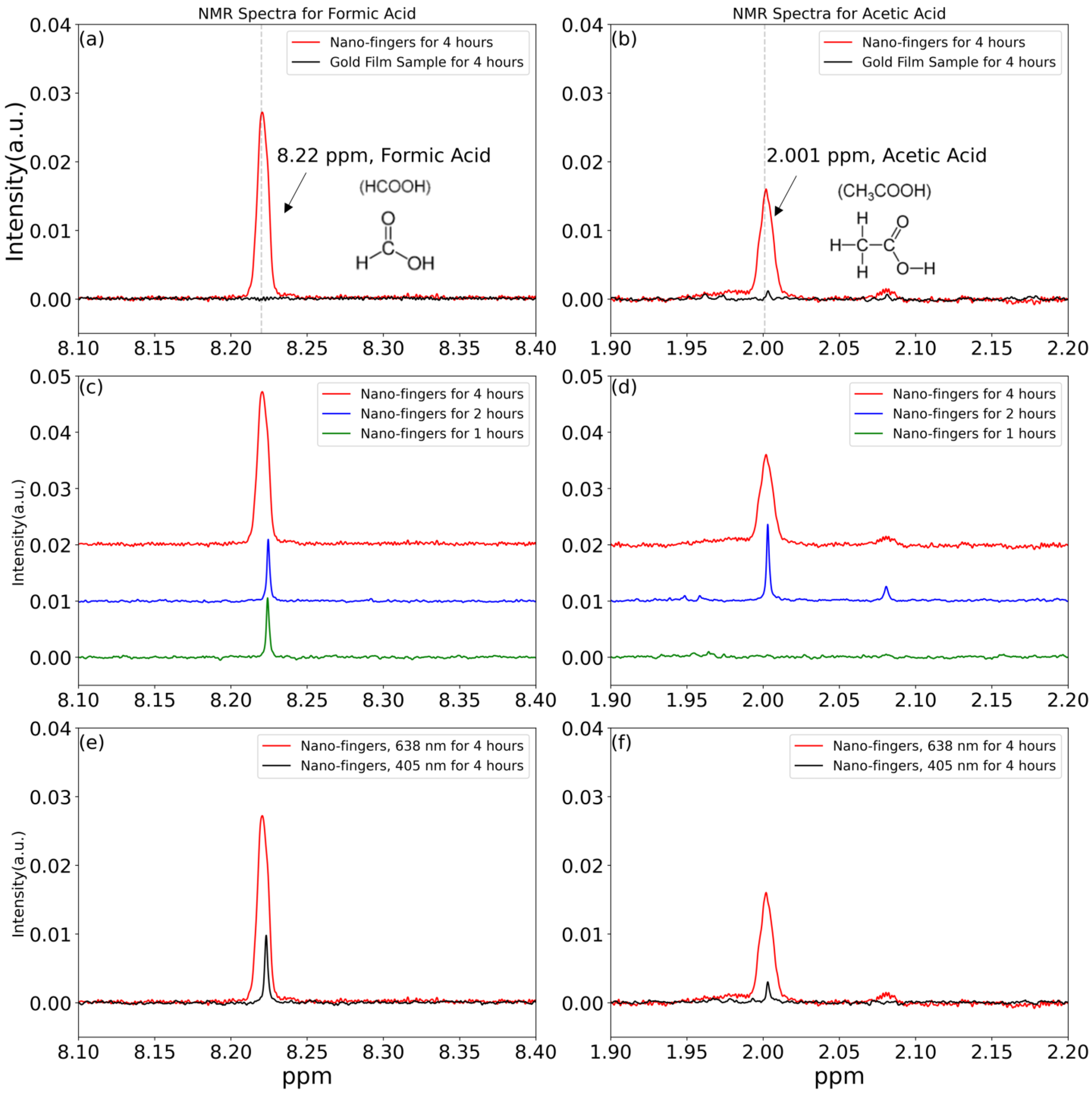
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Our World in Data: CO2 Emission. Available online: https://ourworldindata.org/co2-emissions (accessed on 17 May 2023).
- International Energy Agency Global CO2 Emissions Rebounded to Their Highest Level in History in 2021. Available online: https://www.iea.org/news/global-co2-emissions-rebounded-to-their-highest-level-in-history-in-2021 (accessed on 17 May 2023).
- Zeng, G.; Qiu, J.; Hou, B.; Shi, H.; Lin, Y.; Hettick, M.; Javey, A.; Cronin, S.B. Enhanced Photocatalytic Reduction of CO2 to CO through TiO2 Passivation of InP in Ionic Liquids. Chem. A Eur. J. 2015, 21, 13502–13507. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Qiu, J.; Li, Z.; Pavaskar, P.; Cronin, S.B. CO2 Reduction to Methanol on TiO2-Passivated GaP Photocatalysts. ACS Catal 2014, 4, 3512–3516. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in Electrochemical Reduction of CO2 At Metal Electrodes in Aqueous Hydrogencarbonate Solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Creel, E.B.; Corson, E.R.; Eichhorn, J.; Kostecki, R.; Urban, J.J.; McCloskey, B.D. Directing Selectivity of Electrochemical Carbon Dioxide Reduction Using Plasmonics. ACS Energy Lett. 2019, 4, 1098–1105. [Google Scholar] [CrossRef]
- Chen, C.S.; Wan, J.H.; Yeo, B.S. Electrochemical Reduction of Carbon Dioxide to Ethane Using Nanostructured Cu2O-Derived Copper Catalyst and Palladium(II) Chloride. J. Phys. Chem. C 2015, 119, 26875–26882. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Meryem, S.S.; Nasreen, S.; Siddique, M.; Khan, R. An Overview of the Reaction Conditions for an Efficient Photoconversion of CO2. Rev. Chem. Eng. 2018, 34, 409–425. [Google Scholar] [CrossRef]
- Lu, S.; Lou, F.; Yu, Z. Recent Progress in Two-Dimensional Materials for Electrocatalytic CO2 Reduction. Catalysts 2022, 12, 288. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef]
- Mittal, D.; Ahlawat, M.; Govind Rao, V. Recent Progress and Challenges in Plasmon-Mediated Reduction of CO2 to Chemicals and Fuels. Adv. Mater. Interfaces 2022, 9, 1–24. [Google Scholar] [CrossRef]
- Ciocarlan, R.G.; Blommaerts, N.; Lenaerts, S.; Cool, P.; Verbruggen, S.W. Recent Trends in Plasmon-Assisted Photocatalytic CO2 Reduction. ChemSusChem 2023, 16, e202201647. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A Critical Review on TiO2 Based Photocatalytic CO2 Reduction System: Strategies to Improve Efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Heijkers, S.; Snoeckx, R.; Kozák, T.; Silva, T.; Godfroid, T.; Britun, N.; Snyders, R.; Bogaerts, A. CO2 Conversion in a Microwave Plasma Reactor in the Presence of N2: Elucidating the Role of Vibrational Levels. J. Phys. Chem. C 2015, 119, 12815–12828. [Google Scholar] [CrossRef]
- Mitsingas, C.M.; Rajasegar, R.; Hammack, S.; Do, H.; Lee, T. High Energy Efficiency Plasma Conversion of CO2 at Atmospheric Pressure Using a Direct-Coupled Microwave Plasma System. IEEE Trans. Plasma Sci. 2016, 44, 651–656. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, B.; Aravind, I.A.; Wang, Y.; Zhang, B.; Weng, S.; Cai, Z.; Li, R.; Baygi, A.Z.; Smith, A.; et al. CO2 Reduction to Higher Hydrocarbons by Plasma Discharge in Carbonated Water. ACS Energy Lett. 2021, 6, 3924–3930. [Google Scholar] [CrossRef]
- Yu, S.; Wilson, A.J.; Heo, J.; Jain, P.K. Plasmonic Control of Multi-Electron Transfer and C-C Coupling in Visible-Light-Driven CO2 Reduction on Au Nanoparticles. Nano Lett. 2018, 18, 2189–2194. [Google Scholar] [CrossRef]
- Corson, E.R.; Kas, R.; Kostecki, R.; Urban, J.J.; Smith, W.A.; McCloskey, B.D.; Kortlever, R. In Situ ATR-SEIRAS of Carbon Dioxide Reduction at a Plasmonic Silver Cathode. J. Am. Chem. Soc. 2020, 142, 11750–11762. [Google Scholar] [CrossRef]
- Landaeta, E.; Kadosh, N.I.; Schultz, Z.D. Mechanistic Study of Plasmon-Assisted. ACS Catal. 2023, 13, 1638–1648. [Google Scholar] [CrossRef]
- Corson, E.R.; Subramani, A.; Cooper, J.K.; Kostecki, R.; Urban, J.J.; McCloskey, B.D. Reduction of Carbon Dioxide at a Plasmonically Active Copper–Silver Cathode. Chem. Commun. 2020, 56, 9970–9973. [Google Scholar] [CrossRef]
- Hou, W.; Hung, W.H.; Pavaskar, P.; Goeppert, A.; Aykol, M.; Cronin, S.B. Photocatalytic Conversion of CO2 to Hydrocarbon Fuels via Plasmon-Enhanced Absorption and Metallic Interband Transitions. ACS Catal. 2011, 1, 929–936. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Meng, D.; Song, B.; Liu, Z.; Hu, P.; Yang, H.; Ou, T.-H.; Liu, F.; Pi, H.; et al. Hot Electron-Driven Photocatalysis Using Sub-5 Nm Gap Plasmonic Nanofinger Arrays. Nanomaterials 2022, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lei, Z.; Li, Y.; Dong, L.; Zhao, Y.; Zhang, J. A Review on Modification of Facet-Engineered TiO2 for Photocatalytic CO2 Reduction. J. Photochem. Photobiol. C Photochem. Rev. 2018, 36, 24–47. [Google Scholar] [CrossRef]
- Song, B.; Yao, Y.; Groenewald, R.E.; Wang, Y.; Liu, H.; Wang, Y.; Li, Y.; Liu, F.; Cronin, S.B.; Schwartzberg, A.M.; et al. Probing Gap Plasmons Down to Sub-Nanometer Scales Using Collapsible Nano-Fingers. ACS Nano 2017, 11, 5836–5843. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Kim, G.H.; Lee, Y.; Lee, C.; Cha, S.; Nam, J.M. Toward Quantitative Surface-Enhanced Raman Scattering with Plasmonic Nanoparticles: Multiscale View on Heterogeneities in Particle Morphology, Surface Modification, Interface, and Analytical Protocols. J. Am. Chem. Soc. 2022, 144, 22337–22351. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, J.; Sun, K.; Tang, H.; Liu, Q. Enhancement in Photocatalytic Activity of CO2 Reduction to CH4 by 0D/2D Au/TiO2 Plasmon Heterojunction. Appl. Surf. Sci. 2019, 493, 1142–1149. [Google Scholar] [CrossRef]
- Khatun, F.; Abd Aziz, A.; Sim, L.C.; Monir, M.U. Plasmonic Enhanced Au Decorated TiO2 Nanotube Arrays as a Visible Light Active Catalyst towards Photocatalytic CO2 Conversion to CH4. J. Environ. Chem. Eng. 2019, 7, 103233. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constant of the Nobel Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Ou, F.S.; Hu, M.; Naumov, I.; Kim, A.; Wu, W.; Bratkovsky, A.M.; Li, X.; Williams, R.S.; Li, Z. Hot-Spot Engineering in Polygonal Nanofinger Assemblies for Surface Enhanced Raman Spectroscopy. Nano Lett. 2011, 11, 2538–2542. [Google Scholar] [CrossRef]
- Su, G.; Hu, P.; Hu, J.; Wang, X.; Wu, G.; Shang, Y.; Zhan, P.; Liu, F.; Wu, W. Collapsed Nanofingers by DNA Functionalization as SERS Platform for Mercury Ions Sensing. J. Raman Spectrosc. 2023, 54, 6–12. [Google Scholar] [CrossRef]
- DeVore, J.R. Refractive Indices of Rutile and Sphalerite. J. Opt. Soc. Am. 1951, 41, 416–419. [Google Scholar] [CrossRef]
- Hosoda, H.; Mori, H.; Sogoshi, N.; Nagasawa, A.; Nakabayashi, S. Refractive Indices of Water and Aqueous Electrolyte Solutions under High Magnetic Fields. J. Phys. Chem. A 2004, 108, 1461–1464. [Google Scholar] [CrossRef]
- Rumbach, P.; Xu, R.; Go, D.B. Electrochemical Production of Oxalate and Formate from CO2 by Solvated Electrons Produced Using an Atmospheric-Pressure Plasma. J. Electrochem. Soc. 2016, 163, F1157–F1161. [Google Scholar] [CrossRef]
- MacInnes, D.A.; Belcher, D. The Thermodynamic Ionization Constants of Carbonic Acid. J. Am. Chem. Soc. 1933, 55, 2630–2646. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 17 May 2023).
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- SpectraBase. Available online: https://spectrabase.com/spectrum/JDbmvpf0Uj1 (accessed on 13 May 2023).
- NMR Proton Shift for Residual Solvent Impurities. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/brochures-and-catalogs/posters/nmr-proton-shifts-for-residual-solvent-impurities-poster.pdf (accessed on 13 May 2023).
- Mota-Lima, A. The Electrified Plasma/Liquid Interface as a Platform for Highly Efficient CO2 Electroreduction to Oxalate. J. Phys. Chem. C 2020, 124, 10907–10915. [Google Scholar] [CrossRef]
- Flyunt, R.; Schuchmann, M.N.; Von Sonntag, C. A Common Carbanion Intermediate in the Recombination and Proton-Catalysed Disproportionation of the Carboxyl Radical Anion, CO2-, in Aqueous Solution. Chem. A Eur. J. 2001, 7, 796–799. [Google Scholar] [CrossRef]
- Sergio, T. The Absolute Electrode Potential: An Explanatory Note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar]
- Ola, O.; Maroto-Valer, M.M. Review of Material Design and Reactor Engineering on TiO2 Photocatalysis for CO2 Reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Fujisawa, J.I.; Eda, T.; Hanaya, M. Comparative Study of Conduction-Band and Valence-Band Edges of TiO2, SrTiO3, and BaTiO3 by Ionization Potential Measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Tran, P.D.; Wong, L.H.; Barber, J.; Loo, J.S.C. Recent Advances in Hybrid Photocatalysts for Solar Fuel Production. Energy Environ. Sci. 2012, 5, 5902–5918. [Google Scholar] [CrossRef]
- Beranek, R. (Photo)Electrochemical Methods for the Determination of the Band Edge Positions of TiO2-Based Nanomaterials. Adv. Phys. Chem. 2011, 2011, 80–83. [Google Scholar] [CrossRef]
- Corson, E.R.; Creel, E.B.; Kostecki, R.; McCloskey, B.D.; Urban, J.J. Important Considerations in Plasmon-Enhanced Electrochemical Conversion at Voltage-Biased Electrodes. iScience 2020, 23, 100911. [Google Scholar] [CrossRef] [PubMed]
- Corson, E.R.; Creel, E.B.; Kim, Y.; Urban, J.J.; Kostecki, R.; McCloskey, B.D. A Temperature-Controlled Photoelectrochemical Cell for Quantitative Product Analysis. Rev. Sci. Instrum. 2018, 89, 55112. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]


| CO2 Reduction Reactions | Standard Electrode Potentials vs. NHE (V) |
|---|---|
| −1.90 | |
| −0.61 | |
| −0.53 | |
| −0.48 | |
| −0.38 | |
| −0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, T.-H.; Hu, P.; Liu, Z.; Wang, Y.; Hossain, S.; Meng, D.; Shi, Y.; Zhang, S.; Zhang, B.; Song, B.; et al. Plasmon-Enhanced Photocatalytic CO2 Reduction for Higher-Order Hydrocarbon Generation Using Plasmonic Nano-Finger Arrays. Nanomaterials 2023, 13, 1753. https://doi.org/10.3390/nano13111753
Ou T-H, Hu P, Liu Z, Wang Y, Hossain S, Meng D, Shi Y, Zhang S, Zhang B, Song B, et al. Plasmon-Enhanced Photocatalytic CO2 Reduction for Higher-Order Hydrocarbon Generation Using Plasmonic Nano-Finger Arrays. Nanomaterials. 2023; 13(11):1753. https://doi.org/10.3390/nano13111753
Chicago/Turabian StyleOu, Tse-Hsien, Pan Hu, Zerui Liu, Yunxiang Wang, Sushmit Hossain, Deming Meng, Yudi Shi, Sonia Zhang, Boxin Zhang, Boxiang Song, and et al. 2023. "Plasmon-Enhanced Photocatalytic CO2 Reduction for Higher-Order Hydrocarbon Generation Using Plasmonic Nano-Finger Arrays" Nanomaterials 13, no. 11: 1753. https://doi.org/10.3390/nano13111753