Green Synthesis and Characterization of Novel Silver Nanoparticles Using Achillea maritima subsp. maritima Aqueous Extract: Antioxidant and Antidiabetic Potential and Effect on Virulence Mechanisms of Bacterial and Fungal Pathogens
Abstract
:1. Introduction
2. Materials and Methods
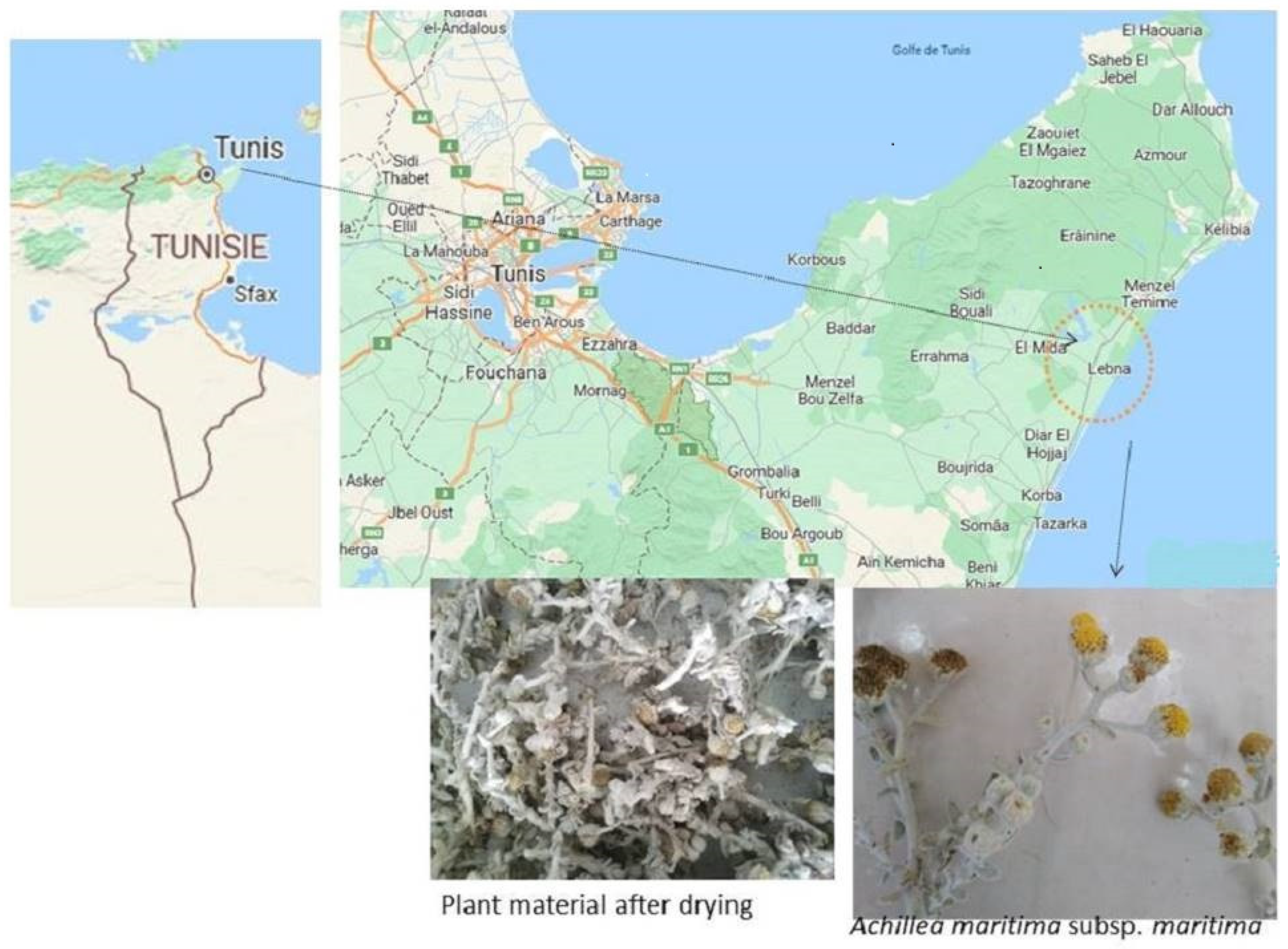
2.1. Preparation of Plant Extract and Biosynthesis of Silver Nanoparticles
2.2. Characterization of Silver Nanoparticles
2.3. Experimental Setup of Fluorescence Analysis
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity
2.4.2. Total Antioxidant Activity (TAA)
2.5. Antidiabetic Effect
2.5.1. α Amylase Inhibitory Assay
2.5.2. α Glucosidase Inhibitory Assay
2.6. Cytotoxic Effect on Human Cells
2.7. Antimicrobial Potential
2.7.1. Agar Well Diffusion Method
2.7.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration
2.7.3. In Vitro Silver Nanoparticles’ Effect on Lipopolysaccharides and Bacterial Biofilm
2.7.4. Silver Nanoparticles’ Effect on DNA Bacterial Genome
2.8. Lysozyme Activity
2.9. Silver Nanoparticles’ Effect on Candida Species Growth and Virulence
2.10. Statistical Analysis
3. Results
3.1. Characterization of Silver Nanoparticles
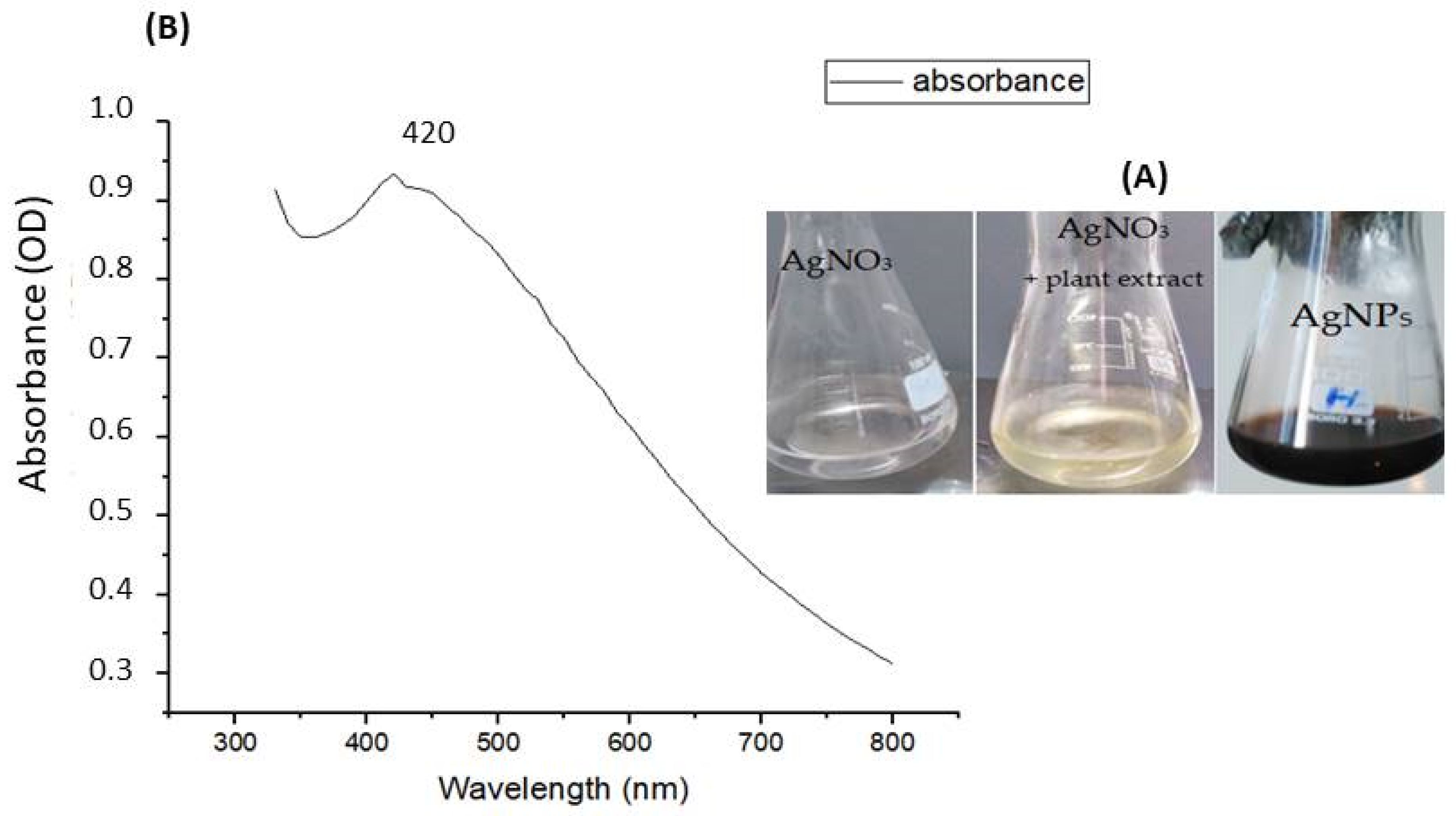
3.1.1. UV-Visible Spectroscopic Analysis
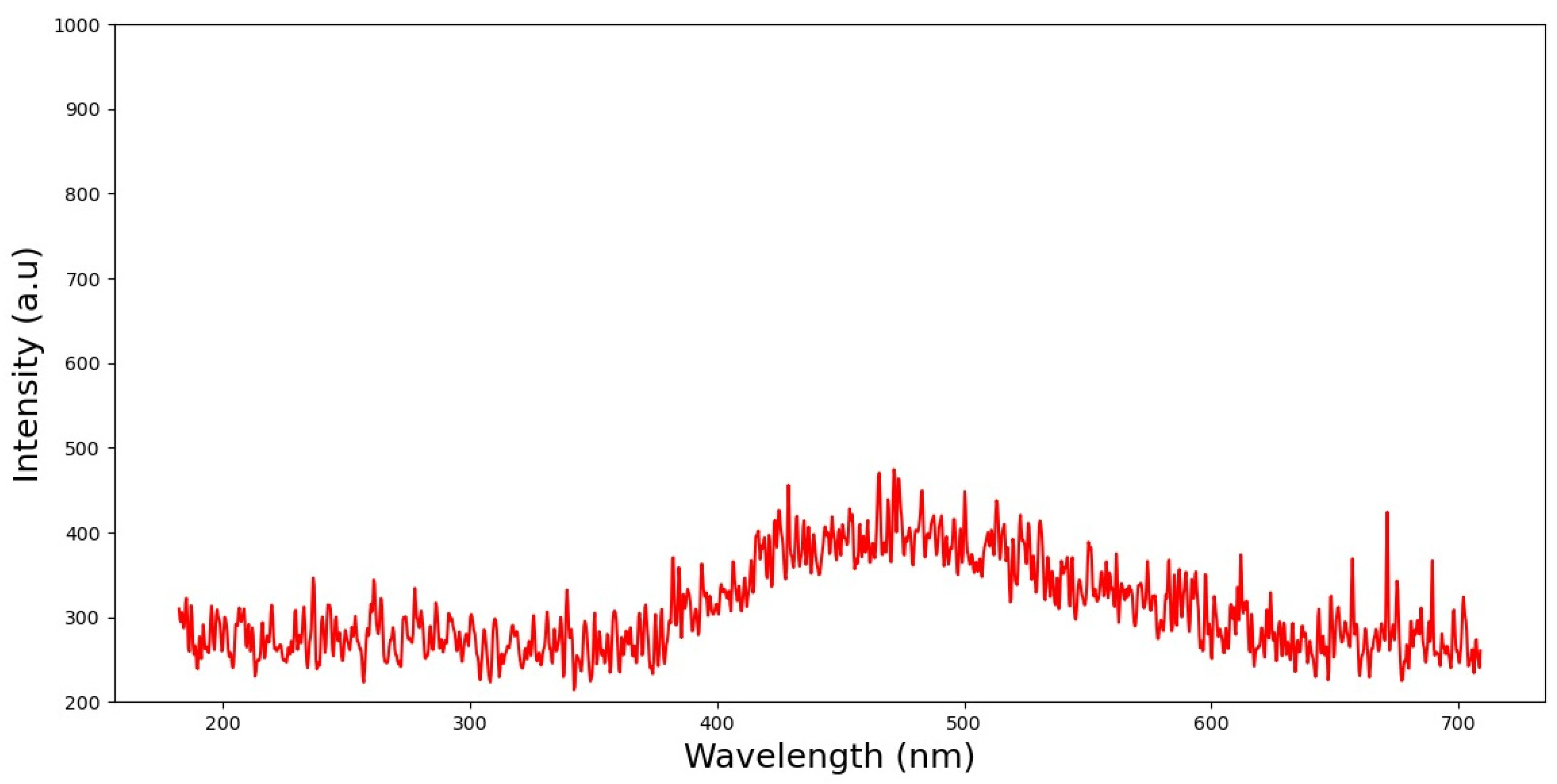
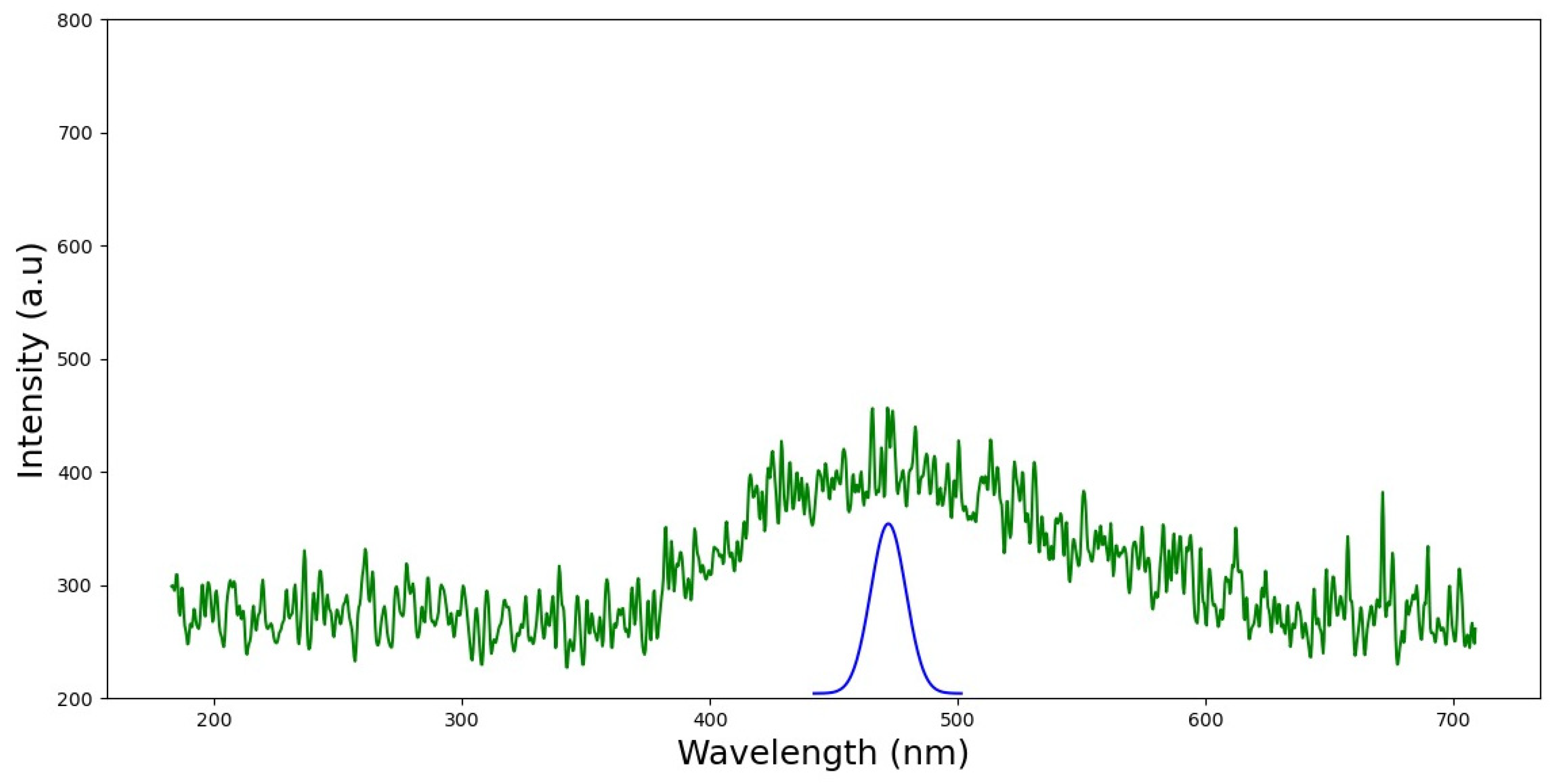
3.1.2. Fluorescence Analysis
3.1.3. FTIR Analysis
3.1.4. Structural Properties
3.2. Antioxidant Activity
3.3. Antidiabetic Effect
3.4. Cytotoxic Effect of Silver Nanoparticles
3.5. Antimicrobial Potential
3.5.1. Agar Well Diffusion Method
3.5.2. MIC, MBC and MFC Determinations
3.5.3. Silver Nanoparticles’ Effect on Bacterial Mechanism of Action
3.6. Silver Nanoparticles’ Effect on Candida albicans Growth and Virulence Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouafia, M.; Colak, N.; Faik, A.; Ayaz, F.A.; Benarfa, A.; Harrat, M.; Gourine, N.; Yousfi, M. The optimization of ultrasonic-asisted extraction of Centaurea sp. antioxidative phenolic compounds using response surface methodology. Appl. Res. Med. Aroamt. Plants 2021, 25, 100330. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 11, 8996. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; BenKhedher, G.; Hannachi, H.; Dridi, R.; Zid, M.F.; Chaffei, C. Green synthesis of silver nanoparticles using mixed leaves aqueous extract of wild olive and pistachio: Characterization, antioxidant, antimicrobial and effect on virulence factors of Candida. Arch. Microbiol. 2022, 204, 203. [Google Scholar] [CrossRef] [PubMed]
- Purcarea, C.; Necula-Petrareanu, G.; Vasilescu, A. Extremophile-assisted nanomaterial production and nanomaterial-based biosensing. In Micro and Nano Technologies Functional Nanostructured Interfaces for Environmental and Biomedical Applications; Dinca, V., Suchea, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–180. [Google Scholar] [CrossRef]
- Essghaier, B.; Toukabri, N.; Dridi, R.; Hannachi, H.; Limam, I.; Mottola, F.; Mokni, M.; Zid, M.F.; Rocco, L.; Abdelkarim, M. First report of biosynthesis andcharacterization of silver nanoparticles using Scabiosa atropurpurea subsp. maritima fruit extracts and their antioxidant, antimicrobial and cytotoxic properties. Nanomaterials 2022, 12, 1585. [Google Scholar] [CrossRef]
- Essghaier, B.; Dridi, R.; Mottola, F.; Rocco, L.; Zid, M.F.; Hannachi, H. Biosynthesis and Characterization of Silver Nanoparticles from the Extremophile Plant Aeonium haworthii and Their Antioxidant, Antimicrobial and Anti-Diabetic Capacities. Nanomaterials 2023, 13, 100. [Google Scholar] [CrossRef]
- Yener, I.; Yilmaz, M.A.; Olmez, O.; Akdeniz, M.; Tekin, F.; Hasimi, N.; Alkan, M.H.; Mehmet, O.; Ertas, A. A Detailed Biological and Chemical Investigation of Sixteen Achillea Species’Essential Oils via Chemometric Approach. Chem. Biodivers. 2020, 17, e1900484. [Google Scholar] [CrossRef]
- Kucukbay, Z.F.; Kuyumcu, E.; Bilenler, T.; Yildiz, B. Chemical composition and antimicrobial activity of essential oil of Achillea cretica L. (Asteraceae) from Turkey. Nat. Prod. Res. 2012, 26, 1668–1675. [Google Scholar] [CrossRef]
- Demirci, F.; Demirci, B.; Gurbuz, I.; Yesilada, E.; Baser, K.; Husnu, C. Characterization and Biological Activity of Achillea teretifolia Willd. and A. nobilis L. subsp. neilreichii (Kerner) Formanek Essential Oils. Turk. J. Biol. 2009, 33, 129–136. [Google Scholar] [CrossRef]
- Enas, M.A.; Heba, A. Antifungal Activity of Achillea santolina L. and Calendula officinalis L. Essential Oils and Their Constituents Against Fungal Infection of Liver as Complication of Cyclophosphamide Therapy. J. Essent. Oil Bear. Plants 2017, 20, 1030–1043. [Google Scholar] [CrossRef]
- Ozdemir, F.A. Potential Effects of Essential Oil Compositions on Antibacterial Activities of Achillea nobilis L. subsp. neilreichii. J. Essent. Oil Bear. Plants 2019, 22, 574–580. [Google Scholar] [CrossRef]
- Patocka, J.; Navratilova, Z. Achillea fragrantissima: Pharmacology Review. Clin. Oncol. 2019, 4, 1601. [Google Scholar]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H.; et al. GC-MS Analysis, Antioxidant and Antimicrobial Activities of Achillea Odorata Subsp. Pectinata and Ruta Montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 22, 668. [Google Scholar] [CrossRef]
- Lee, H.J.; Sim, M.O.; Woo, K.W.; Jeong, D.-E.; Jung, H.K.; An, B.; Cho, H.W. Antioxidant and Antimelanogenic Activities of Compounds Isolated from the Aerial Parts of Achillea alpina L. Chem. Biodivers. 2019, 16, e1900033. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Mousavi, M.; Mohamad, R. Silver Nanoparticles Biosynthesized Using Achillea biebersteinii Flower Extract: Apoptosis Induction in MCF-7 Cells via Caspase Activation and Regulation of Bax and Bcl-2 Gene Expression. Molecules 2015, 20, 2693–2706. [Google Scholar] [CrossRef] [Green Version]
- Chahrdoli, A.; Qalekhani, F.; Ghowsi, M.; Nemati, H.; Shokoohinia, Y.; Fattahi, A. Achillea wilhelmsii C. Koch mediated blood compatible silver nanoparticles. Mater. Today Commun. 2020, 25, 101577. [Google Scholar] [CrossRef]
- Karimi, S.; Shahri, M.M. Medical and cytotoxicity effects of green synthesized silver nanoparticles using Achillea millefolium extract on MOLT-4 lymphoblastic leukemia cell line. J. Med. Virol. 2021, 93, 3899–3906. [Google Scholar] [CrossRef]
- Grigore, A.; Colceru-Mihul, S.; Bazdoaca, C.; Yuksel, R.; Ionita, C.; Glava, L. Antimicrobial Activity of an Achillea millefolium L. Proceedings 2020, 57, 34. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Gaboreanu, D.M.; Pircalabioru, G.G.; Buleandra, M.; Nagoda, E.; Badea, I.A.; Chifiriuc, M.C. Chemical and Biological Studies of Achillea setacea Herba Essential Oil—First Report on Some Antimicrobial and Antipathogenic Features. Antibiotics 2023, 12, 371. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, P.K.; Doble, M. Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Ther. Adv. Endocrinol. Metab. 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumalsamy, R.; Krishnadhas, L. Anti-Diabetic Activity of Silver Nanoparticles Synthesized from the Hydroethanolic Extract of Myristica fragrans Seeds. Appl. Biochem. Biotechnol. 2022, 194, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Jini, D.; Sharmila, S.; Anitha, A.; Pandian, M.; Rajapaksha, R.M.H. In vitro and in silico studies of silver nanoparticles (AgNPs) from Allium sativum against diabetes. Sci. Rep. 2022, 12, 22109. [Google Scholar] [CrossRef] [PubMed]
- Kajaria, D.; Tiwari, S.; Tripathi, J.; Tripathi, Y.; Ranjana, S. In-Vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: A green approach. J. Genet. Eng. Biotechnol. 2017, 15, 49–57. [Google Scholar] [CrossRef]
- Parang, Z.; Keshavarz, A.; Farahi, S.; Elahi, S.M.; Ghoranneviss, M.; Parhoodeh, S. Fluorescence emission spectra of silver and silver/cobalt nanoparticles. Sci. Iran. 2012, 19, 943–947. [Google Scholar] [CrossRef] [Green Version]
- Swetha, V.; Lavanya, S.; Sabeena, G.; Pushpalaksmi, E.; Jenson, S.J.; Annadurai, G. Synthesis and characterization of silver nanoparticles from Ashyranthus aspera extract for antimicrobial activity studies. J. Appl. Sci. Environ. Manag. 2020, 24, 1161–1167. [Google Scholar] [CrossRef]
- Abdullah, A.; Annapoorni, S. Fluorescent silver nanoparticles via exploding wire technique. Pramana 2005, 65, 815–819. [Google Scholar] [CrossRef]
- Alqudami, A.; Annapoorni, S. Fluorescence from metallic silver and iron nanoparticles prepared by exploding wire technique. Plasmonics 2007, 2, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef] [Green Version]
- Cherni, I.; Nouir, R.; Ghalila, H.; Somaï, M.; Daoued, F.; Aydi, Z.; Hamzaoui, S.; Boussema, F.; Jaïdane, N. Non-invasive and rapid diagnosis of type 2 diabetes mellitus based on the analysis of hair by front-face fluorescence spectroscopy. Appl. Opt. 2022, 61, 4022–4029. [Google Scholar] [CrossRef]
- Cherni, I.; Ghalila, H.; Hamzaoui, S.; Rachdi, I.; Daoued, F.; Jaidane, N. Diagnosis of osteoporosis by UV-visible fluorescence of hair in relation to calcium deficiency assessed by the LIBS technique. OSA Contin. 2021, 4, 2053–2059. [Google Scholar] [CrossRef]
- Nouir, R.; Cherni, I.; Ghalila, H.; Hamzaoui, S. Early diagnosis of dental pathologies by front face fluorescence (FFF) and laser-induced breakdown spectroscopy (LIBS) with principal component analysis (PCA). Instrum. Sci. Technol. 2022, 50, 465–480. [Google Scholar] [CrossRef]
- Cherni, I.; Ghalila, H.; Hamzaoui, S.; Rachdi, I.; Daoued, F. Simple and fast diagnosis of osteoporosis based on UV–visible hair fluorescence spectroscopy. Appl. Opt. 2020, 59, 6774–6780. [Google Scholar] [CrossRef]
- Vitthal, K.U.; Pillai, M.M.; Kininge, P. Study of solid lipid nanoparticles as a carrier for bacoside. Int. J. Pharm. Biol. Sci. 2013, 3, 414–426. [Google Scholar]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef]
- Thakur, S.; Barua, S.; Karak, N. Self-healable castor oil based tough smart hyper branched polyurethane nanocomposite with antimicrobial attributes. RSC Adv. 2015, 5, 2167–2176. [Google Scholar] [CrossRef]
- Essghaier, B.; Naouar, A.; Abdelhak, J.; Zid, M.F.; Sadfi-Zouaoui, N. Synthesis, crystal structure and potential antimicrobial activities of di (4-sulfamoyl-phenyl-ammonium) sulphate. Microbiol. Res. 2014, 169, 504–510. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 23, 105–109. [Google Scholar] [CrossRef]
- Okou, O.C.; Yapo, S.E.; Kporou, K.E.; Baibo, G.L.; Monthaut, S.; Djaman, A.J. Evaluation de l’activité antibactérienne des extraits de feuilles de Solanum torvum Swartz (Solanaceae) sur la croissance in vitro de 3 souches d’entérobactéries. J. Appl. Biosci. 2018, 122, 12287. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, D.; Ramanathan, S.K.; Arunachalam, K.; Jeyaraj, G.P.; Shunmugiah, K.P.; Arumugam, V.R. Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: An in vitro study. J. Appl. Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Zhao, A.; Wang, A.; Brown, Z.Z.; Muir, T.W.; Stone, H.A.; Bassler, B.L. Surface attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nat. Microbiol. 2017, 2, 17080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, W.S.; Atwan, Z.W.; Abdulhussein, Z.R.; Mahdi, M. Preparation of silver nanoparticles as antibacterial agents through DNA damage. Mater. Technol. 2019, 34, 867–873. [Google Scholar] [CrossRef]
- Sehimi, H.; Essghaier, B.; Barea, E.; Sadfi-Zouaoui, N.; Zid, M.F. Synthesis, structural study, magnetic susceptibility and antimicrobial activity of the first (m-oxo)-bis(oxalato)-vanadium(IV) 1D coordination polymer. J. Mol. Struct. 2019, 1175, 865–873. [Google Scholar] [CrossRef]
- Riazanova, L.P.; Ledova, L.A.; Tsurikova, N.V.; Stepnaia, O.A.; Sinitsyn, A.P.; Kulaev, I.S. Effect of the proteolytic enzymes of Bacillus licheniformis and the lysoamidase.sp. XL1 on Proteus vulgaris and Proteus mirabilis. Prikl. Biokhim. Mikrobiol. 2005, 41, 490–494. [Google Scholar]
- Jin, Y.; Samaranayake, Y.H.; Yip, H.K.; Samaranayke, L.P. Characterization of switch phenotypes in Candida albicans biofilms. Mycoâthologia 2005, 160, 191–200. [Google Scholar] [CrossRef]
- Mohandas, V.; Ballal, M. Distribution of Candida species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J. Glob. Infect. Dis. 2011, 3, 4–8. [Google Scholar] [CrossRef]
- Amirjani, A.; Firouzi, F.; Haghshenas Fatmehsari, D. Predicting the Size of Silver Nanoparticles from Their Optical Properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- Mittal, J.R.; Jain, R.; Sharma, M. Phytofabrication of silver nanoparticles using aqueous leaf extract of Xanthium strumerium L. and their bactericidal efficacy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025011. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dridi, R.; Essghaier, B.; Hannachi, H.; Ben Khedher, G.; Chaffei, C.; Zid, M.F. Biosynthesized silver nanoparticles using Anagallis monelli: Evaluation of antioxidant activity, antibacterial and antifungal effects. J. Mol. Struct. 2022, 1251, 132076. [Google Scholar] [CrossRef]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110901. [Google Scholar] [CrossRef]
- Jana, S.; Pal, T. Synthesis, characterization and catalytic application of silver nanoshell coated functionalized polystyrene beads. J. Nanosci. Nanotechnol. 2007, 7, 2151–2156. [Google Scholar] [CrossRef]
- Ganesan, S.; Palanichamy, P.M.; Selvam, G. Biosynthesis and characterization of silver nanoparticles using ginger spent and their antibacterial activity. Curr. Bot. 2021, 12, 174–179. [Google Scholar] [CrossRef]
- Öztürk Küp, F.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. 2020, 107, 110207. [Google Scholar] [CrossRef]
- Shastri, J.; Rupani, M.; Kataria, R. Antimicrobial activity of nanosilver-coated socks fabrics against foot pathogens. J. Text. Inst. 2012, 103, 1234–1243. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Bhakya, S.; Kumar, T.S.; Rao, M.V. Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Ceropegia thwaitesii—An endemic species. Ind. Crops Prod. 2015, 63, 119–124. [Google Scholar] [CrossRef]
- Kanipandian, N.; Kannan, S.; Ramesh, R.; Subramanian, P.; Thirumurugan, R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater. Res. Bull. 2013, 49, 494–502. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Molecules 2014, 19, 4624. [Google Scholar] [CrossRef] [Green Version]
- Konappa, N.; Udayashankar, A.C.; Dhamodaran, N.; Krishnamurthy, S.; Jagannath, S.; Uzma, F.; Pradeep, C.K.; De Britto, S.; Chowdappa, S.; Jogaiah, S. Ameliorated Antibacterial and Antioxidant Properties by Trichoderma harzianum Mediated Green Synthesis of Silver Nanoparticles. Biomolecules 2021, 4, 535. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Thomas, J.; Vickram, A.; Anbarasu, K.; Karunakaran, R.; Palanivelu, J.; Srikumar, P. Efficacy of encapsulated biogenic silver nanoparticles and its disease resistance against Vibrio harveyi through oral administration in Macrobrachium rosenbergii. Saudi J. Biol. Sci. 2021, 28, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Nath, S.S.; Chakdar, D.; Gope, G.; Bhattacharjee, R. Synthesis of silver nanoparticles and their optical properties. J. Exp. Nanosci. 2010, 5, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Si, X.; Wu, T.; Wang, P. Silver nanoparticles enhanced fluorescence for sensitive determination of fluoroquinolones in water solutions. Open Chem. 2019, 17, 884–892. [Google Scholar] [CrossRef] [Green Version]
- Thangamani, N.; Bhuvaneshwari, N. Synthesis, characterization of Ag nanoparticles using the green approach towards degradation of environmental pollutant. J. Mater. Sci. Mater. Electron. 2022, 33, 9155–9162. [Google Scholar] [CrossRef]
- Masamu, N.; Shigeaki, A.; Yonezawa, T. Preparation of Ag nanoparticles using hydrogen peroxide as a reducing agent. New J. Chem. 2018, 42, 14493–14501. [Google Scholar]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro Evaluation of Antioxidant and Antibacterial Potential of Green Synthesized Silver Nanoparticles Using Prosopis farcta Fruit Extract. Iran J. Pharm. Res. 2019, 18, 430–445. [Google Scholar]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Padmavathi, R.; Kalaivanan, C.; Raja, R.; Kalaiselvan, S. Antioxidant and antimicrobial studies of silver nanoparticles synthesized via chemical reduction technique. Mater. Today Proc. 2022, 69 Pt 3, 1339. [Google Scholar] [CrossRef]
- Shanker, K.; Mohan, G.K.; Hussain, A.; Jayarambabu, N.; Pravallika, P.L. Green biosynthesis, characterization, in vitro antidiabetic activity, and investigational acute toxicity studies of some herbal-mediated silver nanoparticles on animal models. Pharmacogn. Mag. 2017, 13, 188. [Google Scholar]
- Prabhu, S.; Vinodhini, S.; Elanchezhiyan, C.; Rajeswari, D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 2018, 10, 28–42. [Google Scholar] [CrossRef]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 2020, 10, 16781. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, B.; Aziz, T. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. [Google Scholar] [CrossRef]
- Anish, S.; Mahesh, K.D. Evaluation of the In Vitro Cytotoxicity of Silver Nanoparticles on PBMC Cells Using MTT Assay. Ann. Int. Med. Dent. Res. E 2022, 8, 185–191. [Google Scholar] [CrossRef]
- Blinov, A.V.; Nagdalian, A.A.; Povetkin, S.N.; Gvozdenko, A.A.; Verevkina, M.N.; Rzhepakovsky, I.V.; Lopteva, M.S.; Maglakelidze, D.G.; Kataeva, T.S.; Blinova, A.A.; et al. Surface-Oxidized Polymer-Stabilized Silver Nanoparticles as a Covering Component of Suture Materials. Micromachines 2022, 13, 1105. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative Bacteria: A preliminary study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, D.M. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Ferreyra Maillard, A.P.V.; Dalmasso, P.R.; de Mishima, B.A.L.; Hollmann, A. Interaction of green silver nanoparticles with model membranes: Possible role in the antibacterial activity. Colloids Surf. B Biointerfaces 2018, 171, 320–326. [Google Scholar] [CrossRef]
- Yang, K.; Swanson, K.; Jin, W.; Coley, C.; Eiden, P.; Gao, H.; Guzman-Perez, A.; Hopper, T.; Kelley, B.; Mathea, M.; et al. Analyzing Learned Molecular Representations for Property Prediction. J. Chem. Inf. Model. 2019, 59, 3370–3388. [Google Scholar] [CrossRef] [Green Version]
- Wieder, O.; Kuenemann, M.; Wieder, M.; Seidel, T.; Meyer, C.; Bryant, S.D.; Langer, T. Improved Lipophilicity and Aqueous Solubility Prediction with Composite Graphc Neural Networks. Molecules 2021, 26, 6185. [Google Scholar] [CrossRef]
- Xie, Y.; Gong, M.; Gao, Y.; Qin, A.K.; Fan, X. A Multi-Task Representation Learning Architecture for Enhanced Graph Classification. Front. Neurosci. 2020, 13, 1395. [Google Scholar] [CrossRef] [Green Version]





| DPPH Free Radical Scavenging Activity IC50 (µg/mL) | |
| AgNPs | 89.46 ± 2.48 a |
| Ascorbic acid | 22.54 ± 0 b |
| Total Antioxidant Activity (mg AAE/g DM) | |
| AgNPs | 1.88 ± 0.01 a |
| Ascorbic acid | 0.80 ± 0.01 b |
| Clinical Pathogens | AgNPs | Standards | |
|---|---|---|---|
| Bacteria | Ceftazidime CAZ30 | Tobramycin | |
| Escherchia coli | 15.8 b ± 0 | 15 e ± 0 | 25 a ± 0 |
| Salmonella typhi | 14.5 c ± 0 | 26 b ± 0.5 | 18 d ± 0.5 |
| Pseudomonas aeruginosa | 12.5 d | 17 d ± 0 | 22 c ± 0.5 |
| Staphylococcus aureus | 0 e | 18 c ± 0 | 14.5 e ± 0 |
| Yeasts | Fluconazole 25 | Amphotericin B | |
| Candida albicans | 16.5 a | 13.5 f ± 0 | 23 b ± 0 |
| Candida tropicalis | 0 e | 35 a ± 0.5 | 22 c ± 0 |
| Bacterial Strains | MIC | MBC | Ratio MBC/MIC |
|---|---|---|---|
| Escherchia coli | 6.75 b | 12.5 b | 2 b |
| Pseudomonas aeruginosa | 6.75 b | 12.5 b | 2 b |
| Salmonella typhi | 6.75 b | 25 a | 4 a |
| Fungal Strains | MIC | MFC | Ratio MFC/MIC |
| Candida albicans | 12.5 a | 25 a | 2 b |
| Bacterial Species | Biofilm Eradication (I%) | Lipopolysaccharide Degradation (I%) | DNA Damage |
|---|---|---|---|
| Pseudomonas aeruginosa | 79 | 47 | + |
| Escherchia coli | 62 | 65 | + |
| Salmonella typhi | 82 | 58 | + |
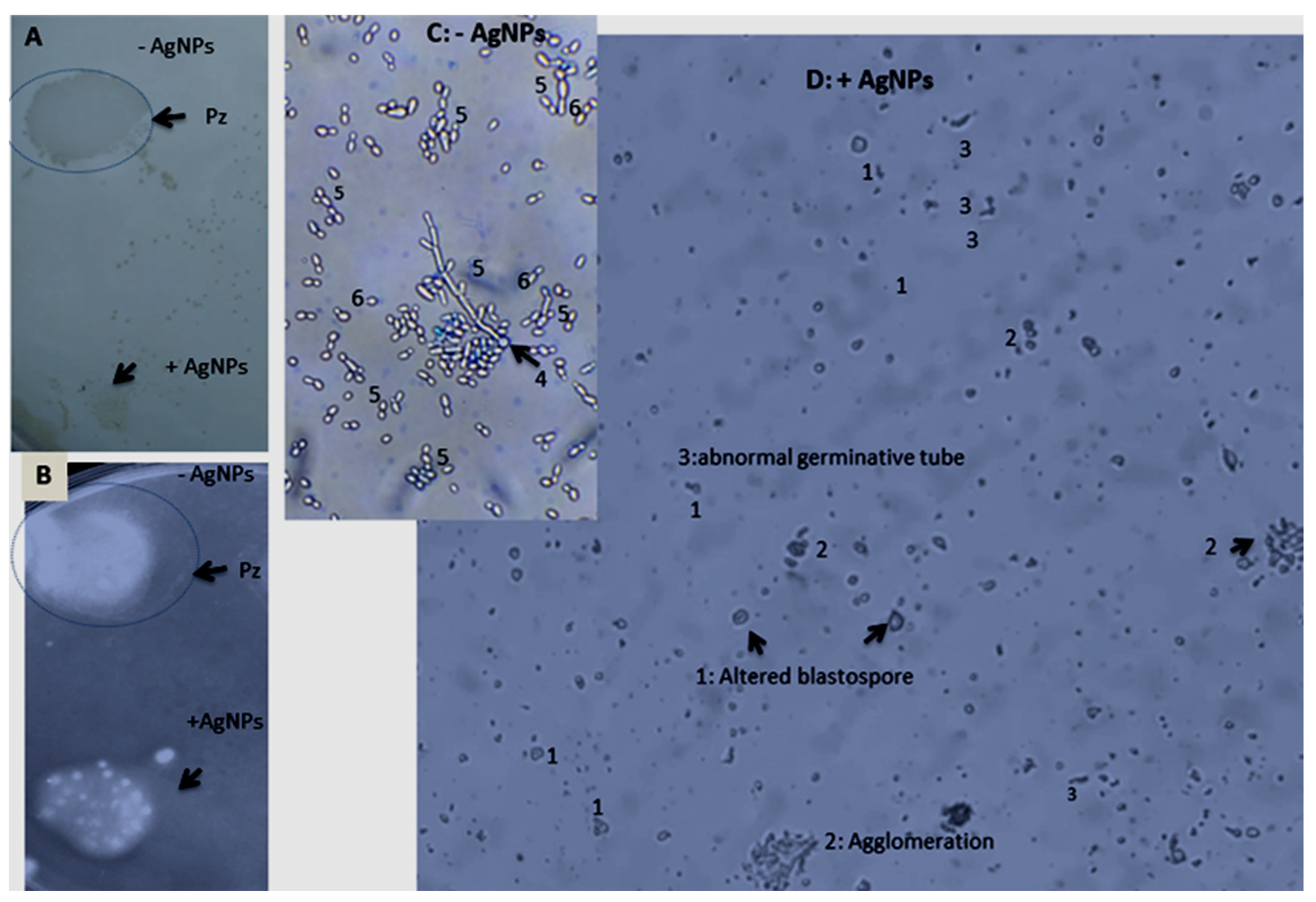
| Culture Conditions of C. albicans | Cell Growth DO600 nm, 48 h (%) | Biofilm Formation and Morphogenesis | Hydrolytic Enzyme Production (Pz in mm) ±SD | |
|---|---|---|---|---|
| Lipase Pz | Proteinase Pz | |||
| Untreated C. albicans | 0.174 ± 0.04 | +++ Morphogenesis change: germ tube; chlamydospores; pseudofilament | Pz = 0.6 ± 0 | Pz = 0.54 ± 0.1 |
| C. albicans treated with AgNPs at 50 µg/mL | 0.166 ± 0.03 | (-) Altered blastospores (1), abnormal germinative tube (2), agglomeration (3) | Pz = 1 negative | Pz = 1 negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essghaier, B.; Hannachi, H.; Nouir, R.; Mottola, F.; Rocco, L. Green Synthesis and Characterization of Novel Silver Nanoparticles Using Achillea maritima subsp. maritima Aqueous Extract: Antioxidant and Antidiabetic Potential and Effect on Virulence Mechanisms of Bacterial and Fungal Pathogens. Nanomaterials 2023, 13, 1964. https://doi.org/10.3390/nano13131964
Essghaier B, Hannachi H, Nouir R, Mottola F, Rocco L. Green Synthesis and Characterization of Novel Silver Nanoparticles Using Achillea maritima subsp. maritima Aqueous Extract: Antioxidant and Antidiabetic Potential and Effect on Virulence Mechanisms of Bacterial and Fungal Pathogens. Nanomaterials. 2023; 13(13):1964. https://doi.org/10.3390/nano13131964
Chicago/Turabian StyleEssghaier, Badiaa, Hédia Hannachi, Rihem Nouir, Filomena Mottola, and Lucia Rocco. 2023. "Green Synthesis and Characterization of Novel Silver Nanoparticles Using Achillea maritima subsp. maritima Aqueous Extract: Antioxidant and Antidiabetic Potential and Effect on Virulence Mechanisms of Bacterial and Fungal Pathogens" Nanomaterials 13, no. 13: 1964. https://doi.org/10.3390/nano13131964





