Hollow Porous CoO@Reduced Graphene Oxide Self-Supporting Flexible Membrane for High Performance Lithium-Ion Storage
Abstract
1. Introduction
2. Material and Methods
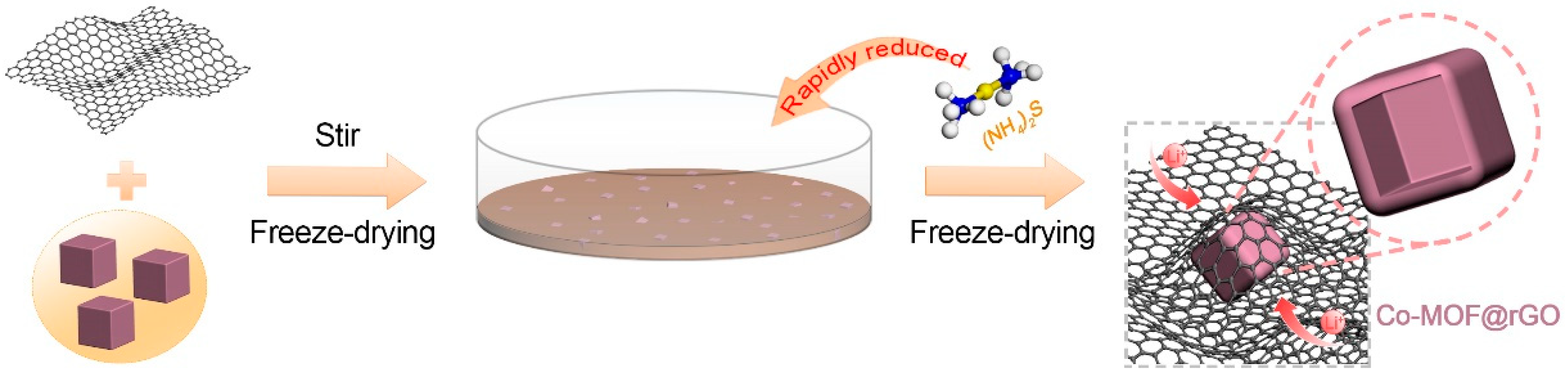
2.1. Synthesis of Co-MOF
2.2. Synthesis of Co-MOF@rGO and CoO@rGO Flexible Membranes
2.3. Material Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
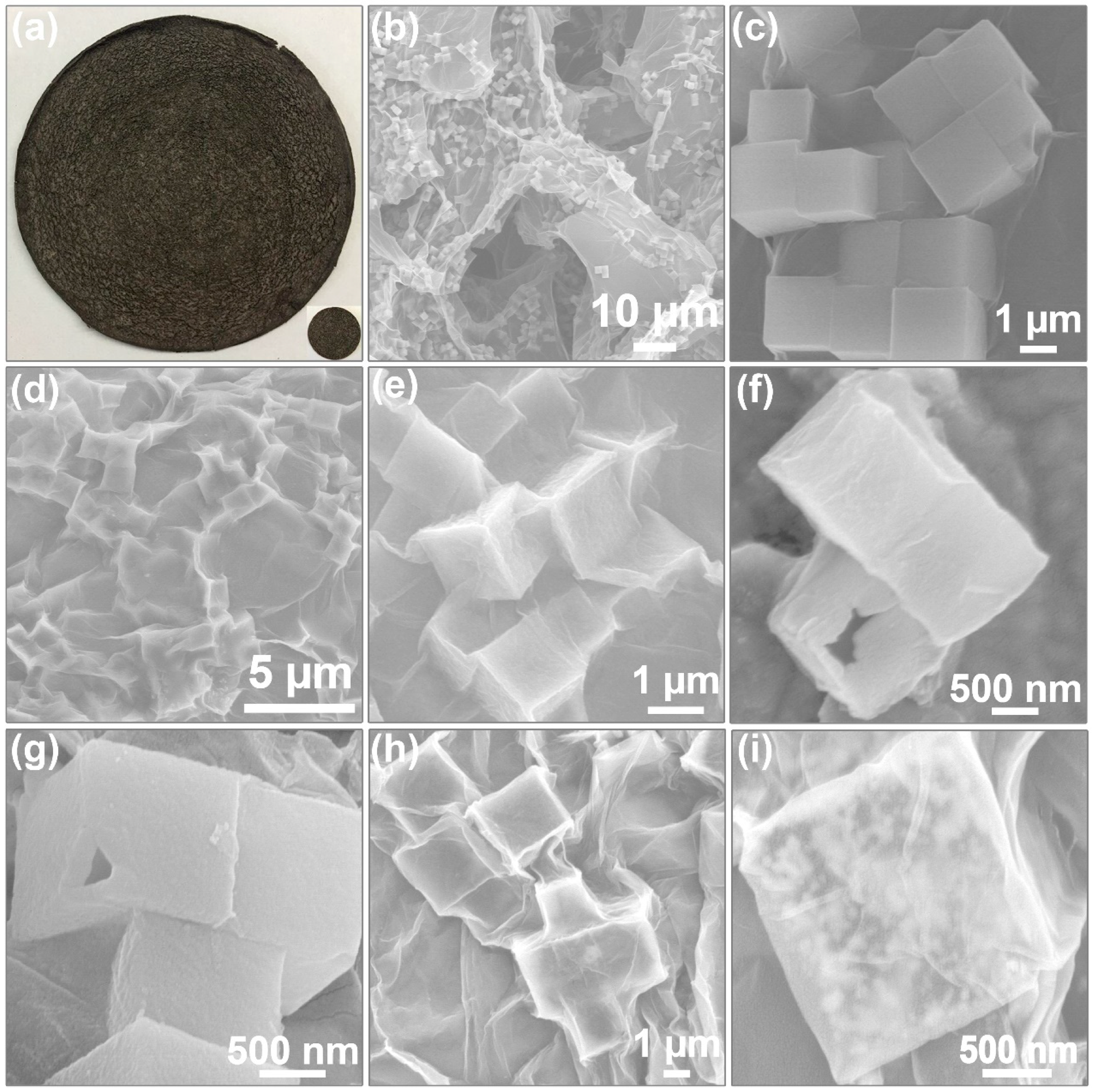
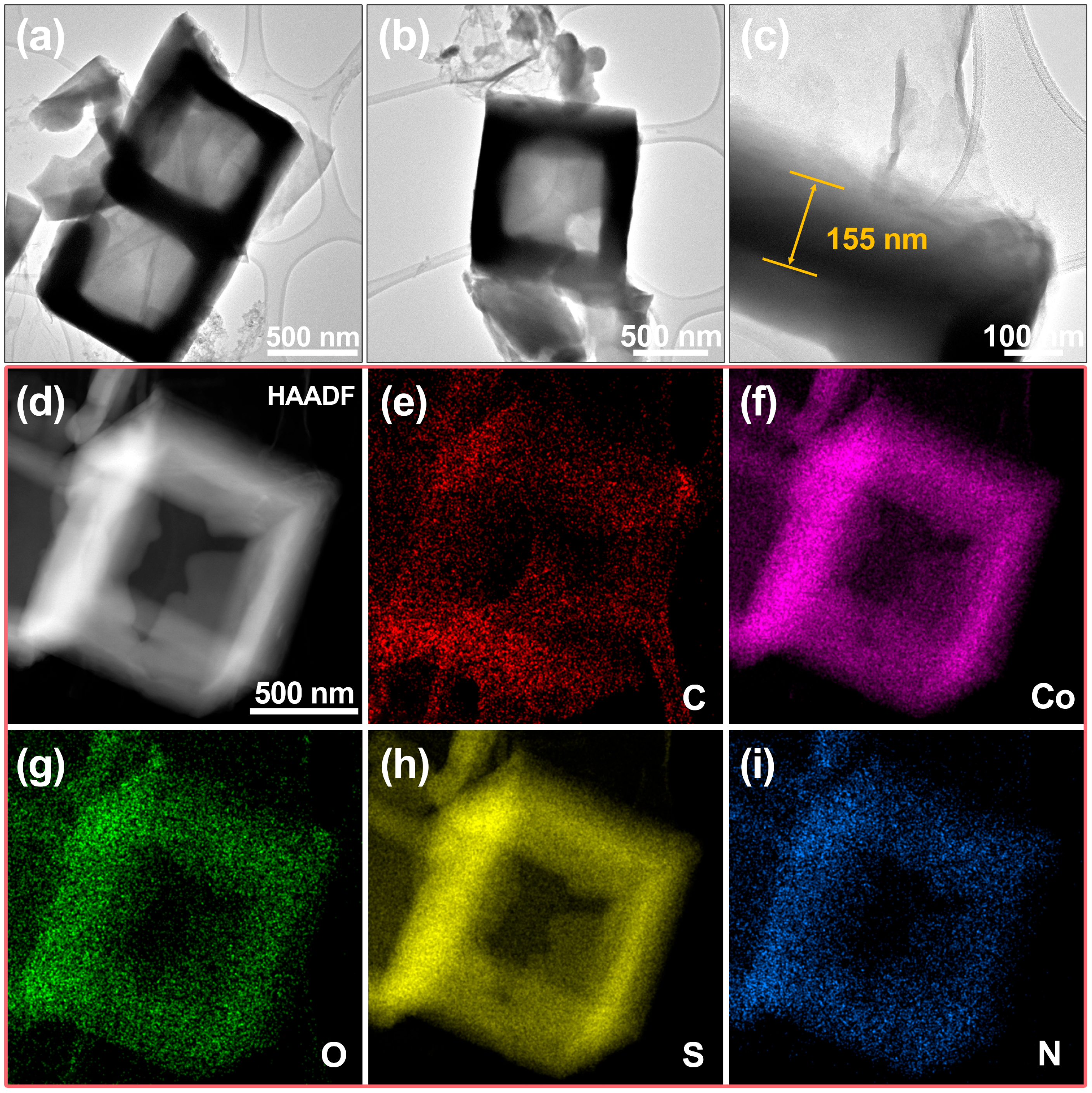
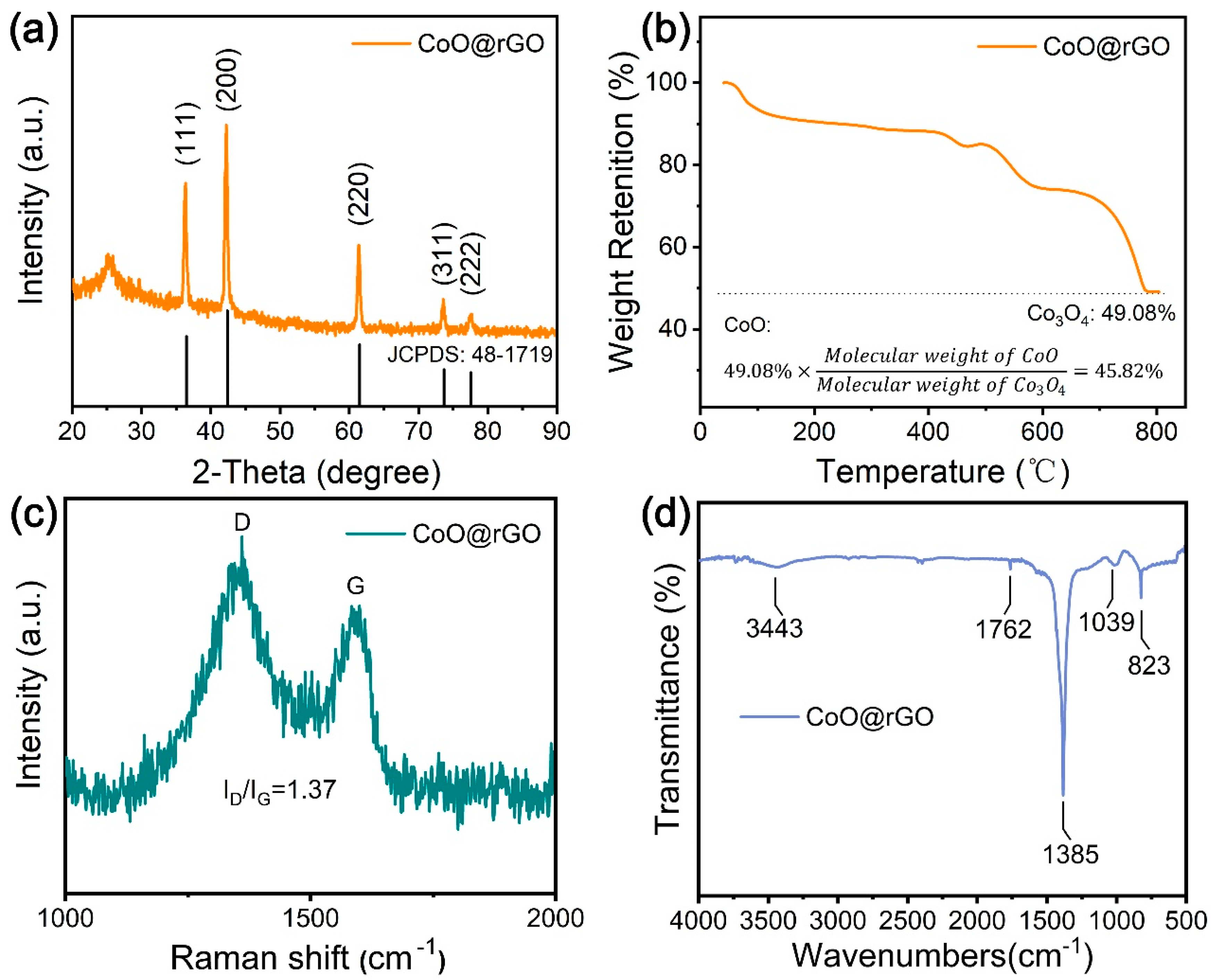
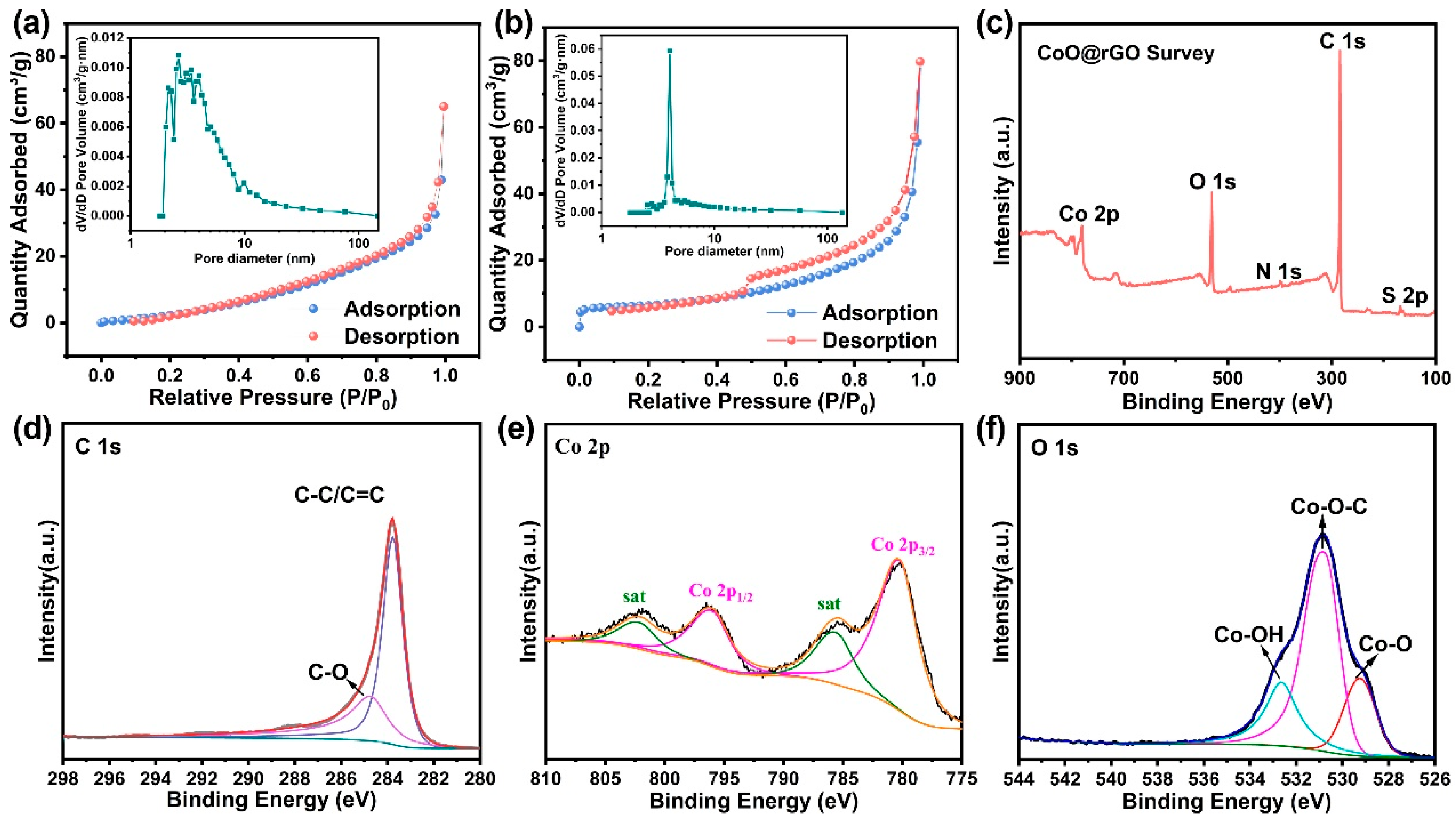
3.1. Characterization
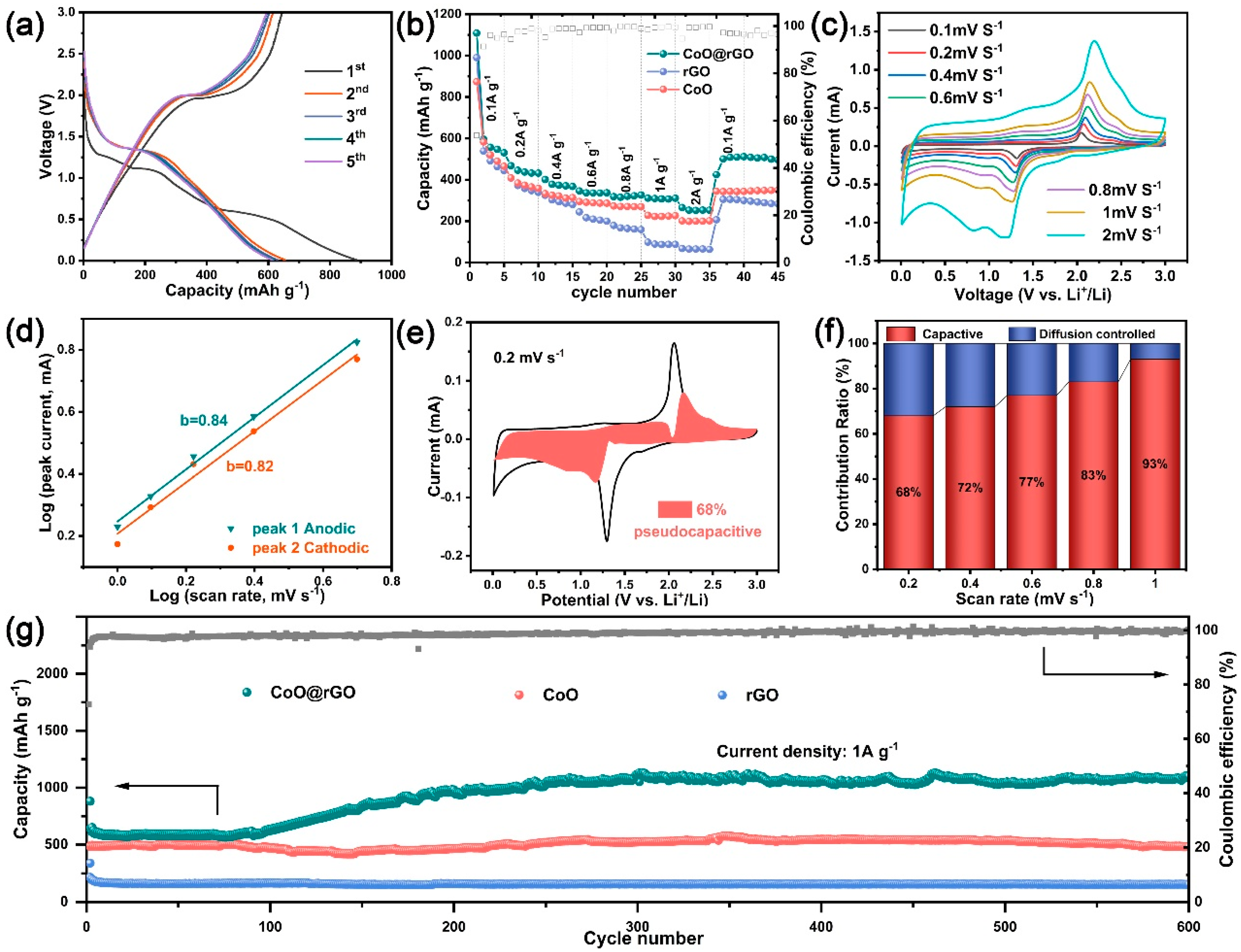
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tu, C.; Peng, A.; Zhang, Z.; Qi, X.; Zhang, D.; Wang, M.; Huang, Y.; Yang, Z. Surface-Seeding Secondary Growth for CoO@Co9S8 P-N Heterojunction Hollow Nanocube Encapsulated into Graphene as Superior Anode toward Lithium Ion Storage. Chem. Eng. J. 2021, 425, 130648. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Guan, X.; Nai, J.; Zhang, Y.; Wang, P.; Yang, J.; Zheng, L.; Zhang, J.; Guo, L. CoO Hollow Cube/Reduced Graphene Oxide Composites with Enhanced Lithium Storage Capability. Chem. Mater. 2014, 26, 5958–5964. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the Performances of Li-Ion Batteries by Carbon-Coating: Present and Future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on Advanced Materials for Li-Ion Batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.; Tu, J.; Xiong, Q.; Yu, Y.-X.; Wang, X.; Gu, C. Co3O4 –C Core–Shell Nanowire Array as an Advanced Anode Material for Lithium Ion Batteries. J. Mater. Chem. 2012, 22, 15056–15061. [Google Scholar] [CrossRef]
- Peng, C.; Chen, B.; Qin, Y.; Yang, S.; Li, C.; Zuo, Y.; Liu, S.; Yang, J. Facile Ultrasonic Synthesis of Coo Quantum Dot/Graphene Nanosheet Composites with High Lithium Storage Capacity. ACS Nano 2012, 6, 1074–1081. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High Rate Capability of Graphite Negative Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A474. [Google Scholar] [CrossRef]
- Jeschull, F.; Brandell, D.; Edström, K.; Lacey, M.J. A Stable Graphite Negative Electrode for the Lithium–Sulfur Battery. Chem. Commun. 2015, 51, 17100–17103. [Google Scholar] [CrossRef]
- Park, S.-K.; Choi, J.H.; Kang, Y.C. Unique Hollow NiO Nanooctahedrons Fabricated through the Kirkendall Effect as Anodes for Enhanced Lithium-Ion Storage. Chem. Eng. J. 2018, 354, 327–334. [Google Scholar] [CrossRef]
- Li, L.; Seng, K.H.; Chen, Z.; Guo, Z.; Liu, H.K. Self-Assembly of Hierarchical Star-like Co3O4 Micro/Nanostructures and Their Application in Lithium Ion Batteries. Nanoscale 2013, 5, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, Q.; Xu, J.; Wang, X.; Zhao, G.; Liu, C.; Yan, X.; Long, Y.; Yan, S.; Li, S. CoO-Co Nanocomposite Anode with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Electrochim. Acta 2017, 224, 90–95. [Google Scholar] [CrossRef]
- Leng, X.; Ding, X.; Hu, J.; Wei, S.; Jiang, Z.; Lian, J.; Wang, G.; Jiang, Q.; Liu, J. In Situ Prepared Reduced Graphene Oxide/CoO Nanowires Mutually-Supporting Porous Structure with Enhanced Lithium Storage Performance. Electrochim. Acta 2016, 190, 276–284. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zheng, M.; Wang, T.; Yang, J.; Yuan, F.; Lu, X.; Liu, L.; Sun, D. Amorphous Fe2O3 Nanoshells Coated on Carbonized Bacterial Cellulose Nanofibers as a Flexible Anode for High-Performance Lithium Ion Batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, H.; Li, L.; Hu, Q.; Zhang, L.; Xue, H.; Pang, H. Hierarchically Nanostructured Transition Metal Oxides for Lithium-Ion Batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef]
- Li, X.; Dhanabalan, A.; Wang, C. Enhanced Electrochemical Performance of Porous NiO–Ni Nanocomposite Anode for Lithium Ion Batteries. J. Power Sources 2011, 196, 9625–9630. [Google Scholar] [CrossRef]
- Su, L.; Zhou, Z.; Shen, P. Ni/C Hierarchical Nanostructures with Ni Nanoparticles Highly Dispersed in N-Containing Carbon Nanosheets: Origin of Li Storage Capacity. J. Phys. Chem. C 2012, 116, 23974–23980. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, C.; Liu, D.; Yan, M. Metal-Organic Framework Derived Hierarchical Co/C@ V2O3 Hollow Spheres as a Thin, Lightweight, and High-Efficiency Electromagnetic Wave Absorber. Chem. –A Eur. J. 2019, 25, 2234–2241. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, X.; Wen, X.; Chen, X.; Chu, P.K.; Tang, T.; Mijowska, E. Interconnected Nanoporous Carbon Structure Delivering Enhanced Mass Transport and Conductivity toward Exceptional Performance in Supercapacitor. J. Power Sources 2019, 435, 226811. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W.D. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef]
- Sun, P.-P.; Zhang, Y.-H.; Shi, H.; Shi, F.-N. Study on the Properties of Cu Powder Modified 3-D Co-MOF in Electrode Materials of Lithium Ion Batteries. J. Solid State Chem. 2022, 307, 122740. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, C.; Ao, L.; Jiang, K.; Shang, L.; Li, Y.; Hu, Z.; Chu, J. Three-Dimensional Porous Co3O4–CoO@GO Composite Combined with N-Doped Carbon for Superior Lithium Storage. Nanotechnology 2019, 30, 425404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Jia, M. Three-Dimensional Reduced Graphene Oxides Hydrogel Anchored with Ultrafine CoO Nanoparticles as Anode for Lithium Ion Batteries. Electrochim. Acta 2014, 129, 425–432. [Google Scholar] [CrossRef]
- Liang, C.; Zhai, T.; Wang, W.; Chen, J.; Zhao, W.; Lu, X.; Tong, Y. Fe3O4/Reduced Graphene Oxide with Enhanced Electrochemical Performance towards Lithium Storage. J. Mater. Chem. A 2014, 2, 7214–7220. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Wang, K.; Li, H.H.; Wang, H.F.; Li, X.Y.; Wu, X.L.; Zhang, J.P.; Xie, H.M.; Su, Z.M.; Wang, J.W.; et al. Fe3O4 Nanoflakes-RGO Composites: A High Rate Anode Material for Lithium-Ion Batteries. Appl. Surf. Sci. 2020, 511, 145465. [Google Scholar] [CrossRef]
- Chen, T. Two-Dimensional MnO2/Reduced Graphene Oxide Nanosheet as a High-Capacity and High-Rate Cathode for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2018, 13, 8575–8588. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Sun, J.; Gao, L.; Lin, C. Flexible Free-Standing Hollow Fe3O4/Graphene Hybrid Films for Lithium-Ion Batteries. J. Mater. Chem. A 2013, 1, 1794–1800. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Y.; Wang, Z.; Lu, J.; Zhang, Q.; Zhou, X.; Xiong, Q.; Qiu, X.; Wei, T. MOF-Derived Co3O4@rGO Nanocomposites as Anodes for High-Performance Lithium-Ion Batteries. Ionics 2021, 27, 4197–4204. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Q.; Li, J.; Huang, J.; Cheng, Y. Assembly Control of CoO/Reduced Graphene Oxide Composites for Their Enhanced Lithium Storage Behavior. Appl. Surf. Sci. 2018, 455, 96–105. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, L.; Wu, F.; Li, J. Decreasing Co3O4 Particle Sizes by Ammonia-Etching and Catalytic Oxidation of Propane. Catal. Lett. 2017, 147, 407–415. [Google Scholar] [CrossRef]
- Campos-Delgado, J.; Romo-Herrera, J.M.; Jia, X.; Cullen, D.A.; Muramatsu, H.; Kim, Y.A.; Hayashi, T.; Ren, Z.; Smith, D.J.; Okuno, Y.; et al. Bulk Production of a New Form of Sp2 Carbon: Crystalline Graphene Nanoribbons. Nano Lett. 2008, 8, 2773–2778. [Google Scholar] [CrossRef]
- Zhang, M.; Uchaker, E.; Hu, S.; Zhang, Q.; Wang, T.; Cao, G.; Li, J. CoO–Carbon Nanofiber Networks Prepared by Electrospinning as Binder-Free Anode Materials for Lithium-Ion Batteries with Enhanced Properties. Nanoscale 2013, 5, 12342–12349. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-C.; Zhao, W. Zeolitic Imidazolate Frameworks Derived Co Nanoparticles Anchored on Graphene as Superior Anode Material for Lithium Ion Batteries. J. Alloys Compd. 2017, 716, 156–161. [Google Scholar] [CrossRef]
- Wan, B.; Guo, J.; Lai, W.-H.; Wang, Y.-X.; Liu, M.; Liu, H.-K.; Wang, J.-Z.; Chou, S.-L.; Dou, S.-X. Layered Mesoporous CoO/Reduced Graphene Oxide with Strong Interfacial Coupling as a High-Performance Anode for Lithium-Ion Batteries. J. Alloys Compd. 2020, 843, 156050. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.; Cheng, H.-M. Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Mohapatra, S.; Swain, N.; Tripathy, B.C.; Jiang, Z.-T.; Minakshi Sundaram, M. Role of Additives in Electrochemical Deposition of Ternary Metal Oxide Microspheres for Supercapacitor Applications. ACS Omega 2020, 5, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Samal, R.; Dash, B.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Senanayake, G.; Minakshi, M. Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications. Nanomaterials 2017, 7, 356. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Liu, W.; Song, Y.; Wang, L. Hollow Co-Co3O4@CNTs Derived from ZIF-67 for Lithium Ion Batteries. J. Alloys Compd. 2019, 784, 439–446. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Yu, Z.; Song, J.; Gordin, M.L.; Yi, R.; Tang, D.; Wang, D. Phosphorus-Graphene Nanosheet Hybrids as Lithium-Ion Anode with Exceptional High-Temperature Cycling Stability. Adv. Sci. 2015, 2, 1400020. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, K.; Minakshi, M. Status on Electrodeposited Manganese Dioxide and Biowaste Carbon for Hybrid Capacitors: The Case of High-Quality Oxide Composites, Mechanisms, and Prospects. J. Energy Storage 2022, 56, 106099. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D. Surfactant-Mediated Electrodeposition of a Pseudocapacitive Manganese Dioxide a Twofer. J. Energy Storage 2022, 55, 105403. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, D.; Madhavi, S.; Hu, Y.; Lou, X.W.D. Assembling Carbon-Coated α-Fe2O3 Hollow Nanohorns on the CNT Backbone for Superior Lithium Storage Capability. Energy Environ. Sci. 2012, 5, 5252–5256. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Consequences of Electrodeposition Parameters on the Microstructure and Electrochemical Behavior of Electrolytic Manganese Dioxide (EMD) for Supercapacitor. Ceram. Int. 2022, 48, 19913–19924. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef]
- Li, F.; Zou, Q.-Q.; Xia, Y.-Y. CoO-Loaded Graphitable Carbon Hollow Spheres as Anode Materials for Lithium-Ion Battery. J. Power Sources 2008, 177, 546–552. [Google Scholar] [CrossRef]
- Guan, H.; Wang, X.; Li, H.; Zhi, C.; Zhai, T.; Bando, Y.; Golberg, D. CoO Octahedral Nanocages for High-Performance Lithium Ion Batteries. Chem. Commun. 2012, 48, 4878–4880. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, P.; Zhao, X.; Tian, R.; Zou, R.; Xia, D. Controllable Synthesis of Core–Shell Co@CoO Nanocomposites with a Superior Performance as an Anode Material for Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 18279–18283. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Hu, L.; Li, Y.; Wang, Y.; Zhong, H.; Hu, X.; Kong, X.; Chen, Q. Co3O4 Nanocages for High-Performance Anode Material in Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 7227–7235. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Shao, J.; Li, G.; Qu, M.; Yin, G. Co3O4@graphene Composites as Anode Materials for High-Performance Lithium Ion Batteries. Inorg. Chem. 2011, 50, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, F.; Du, X.; Qin, Y.; Yin, D.; Wang, L. Metal Organic Frameworks Route to in Situ Insertion of Multiwalled Carbon Nanotubes in Co3O4 Polyhedra as Anode Materials for Lithium-Ion Batteries. ACS Nano 2015, 9, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Chen, G.; Zhou, X.; Sun, J.; Lv, C. Template-Based Engineering of Carbon-Doped Co3O4 Hollow Nanofibers as Anode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 1428–1436. [Google Scholar] [CrossRef]
- Zhu, J.; Tu, W.; Pan, H.; Zhang, H.; Liu, B.; Cheng, Y.; Deng, Z.; Zhang, H. Self-Templating Synthesis of Hollow Co3O4 Nanoparticles Embedded in N,S-Dual-Doped Reduced Graphene Oxide for Lithium Ion Batteries. ACS Nano 2020, 14, 5780–5787. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Li, X.; Xu, D.; Wang, Z.; Guo, H.; Zhang, K. Three-Dimensional Hierarchical Co3O4/CuO Nanowire Heterostructure Arrays on Nickel Foam for High-Performance Lithium Ion Batteries. Nano Energy 2014, 6, 19–26. [Google Scholar] [CrossRef]
- Li, M.; Yin, Y.-X.; Li, C.; Zhang, F.; Wan, L.-J.; Xu, S.; Evans, D.G. Well-Dispersed Bi-Component-Active CoO/CoFe2O4 Nanocomposites with Tunable Performances as Anode Materials for Lithium-Ion Batteries. Chem. Commun. 2012, 48, 410–412. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Liu, H.; Gao, Z.-W.; Hu, J.; Yin, W.-J.; Ke, Q.; Lu Zhu, H. Strategic Synthesis of Sponge-like Structured SiOx@C@CoO Multifunctional Composites for High-Performance and Stable Lithium-Ion Batteries. J. Mater. Chem. A 2021, 9, 18440–18453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; You, J.; Wei, Q.; Han, J.-I.; Liu, Z. Hollow Porous CoO@Reduced Graphene Oxide Self-Supporting Flexible Membrane for High Performance Lithium-Ion Storage. Nanomaterials 2023, 13, 1986. https://doi.org/10.3390/nano13131986
Zhang J, You J, Wei Q, Han J-I, Liu Z. Hollow Porous CoO@Reduced Graphene Oxide Self-Supporting Flexible Membrane for High Performance Lithium-Ion Storage. Nanomaterials. 2023; 13(13):1986. https://doi.org/10.3390/nano13131986
Chicago/Turabian StyleZhang, Junxuan, Jie You, Qing Wei, Jeong-In Han, and Zhiming Liu. 2023. "Hollow Porous CoO@Reduced Graphene Oxide Self-Supporting Flexible Membrane for High Performance Lithium-Ion Storage" Nanomaterials 13, no. 13: 1986. https://doi.org/10.3390/nano13131986
APA StyleZhang, J., You, J., Wei, Q., Han, J.-I., & Liu, Z. (2023). Hollow Porous CoO@Reduced Graphene Oxide Self-Supporting Flexible Membrane for High Performance Lithium-Ion Storage. Nanomaterials, 13(13), 1986. https://doi.org/10.3390/nano13131986







