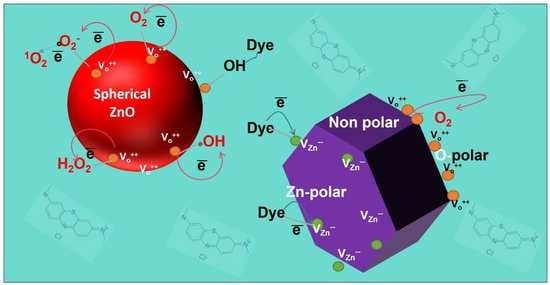
Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States
Abstract
:1. Introduction
2. Methods
2.1. Materials and Reagents
2.2. Synthesis of ZnO Nanoparticles
2.2.1. Synthesis of ZnO A
2.2.2. Synthesis of ZnO B
2.2.3. Synthesis of ZnO C
2.3. Characterization
2.4. Photocatalytic Degradation
2.4.1. Photocatalytic Activity under UV-Light
2.4.2. Photocatalytic Activity under Sunlight
2.4.3. Adsorption of Dye and Its Influence on the Photocatalytic Activity under UV-Light
3. Results
3.1. Crystal Structure and Morphology
3.2. Optical Characteristics
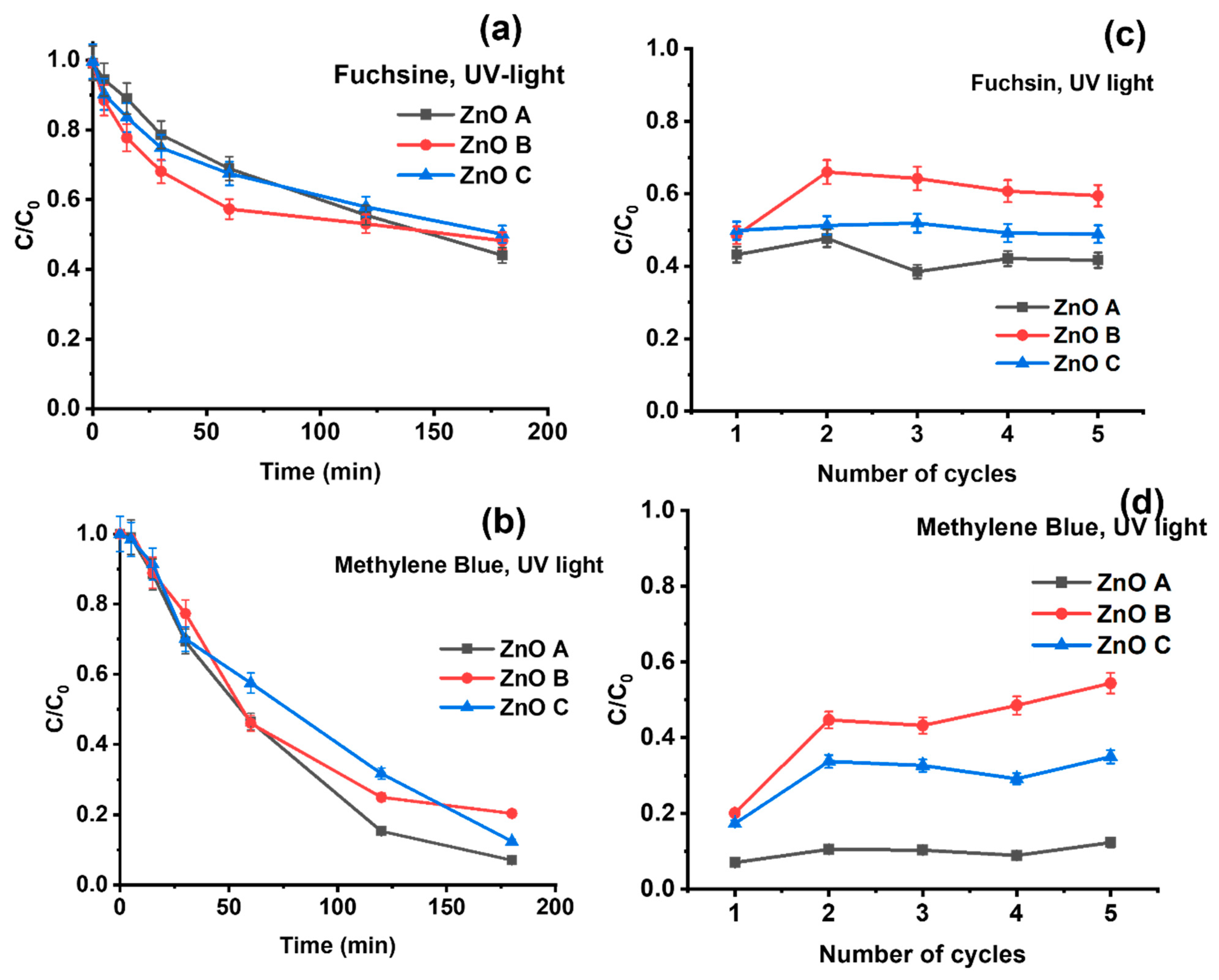
3.3. Photocatalytic Dyedegradation
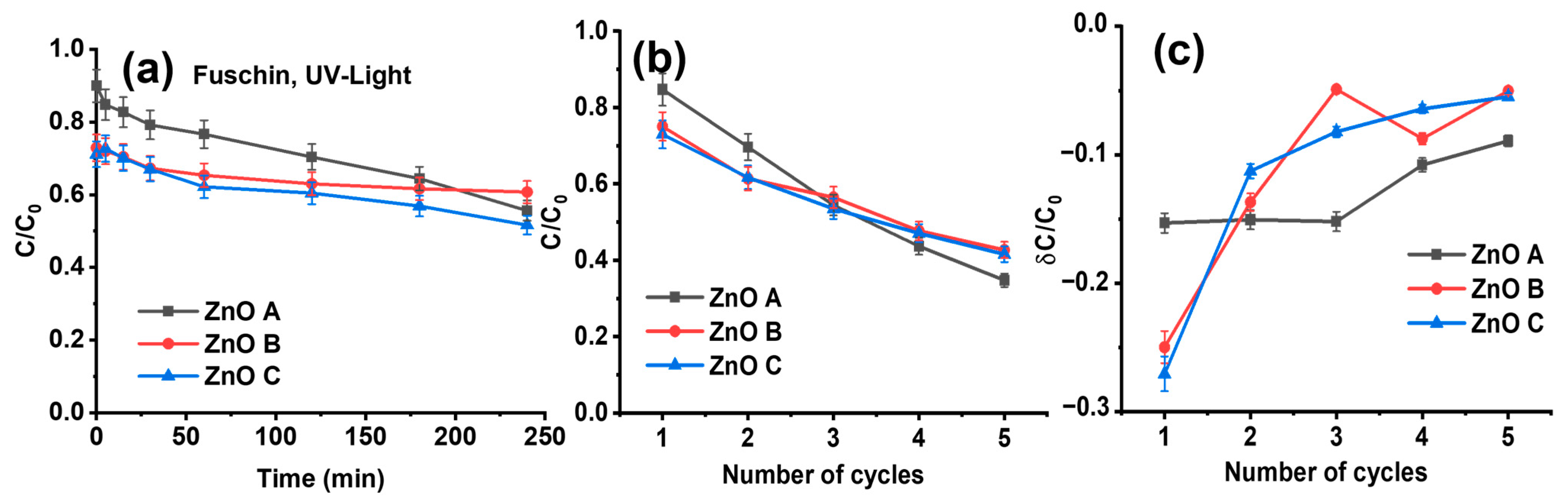
3.4. Adsorption Dependent Photocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, V.K.; Mittal, A.; Gajbe, V.; Mittal, J. Adsorption of basic fuchsin using waste materials—Bottom ash and deoiled soya—As adsorbents. J. Colloid Interface Sci. 2008, 319, 30–39. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Black, D.M. Bibliography of Work on the Photocatalytic Removal of Hazardous Compounds from Water and Air; National Renewable Energy Lab.: Golden, CO, USA, 1994. [Google Scholar]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Raghupathi, K.R.; St. Pierre, J.; Zhao, D.; Koodali, R.T. Tuning of the Crystallite and Particle Sizes of ZnO Nanocrystalline Materials in Solvothermal Synthesis and Their Photocatalytic Activity for Dye Degradation. J. Phys. Chem. C 2011, 115, 13844–13850. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Zhang, W.; Zhou, Q.; Shen, J.; Huang, Y. Stearic acid modified Co2+-doped ZnO: The construction of micro-nano structure for its superhydrophobic performance and visible-light photocatalytic degradation of methylene blue. J. Clean. Prod. 2023, 382, 135391. [Google Scholar] [CrossRef]
- Bhapkar, A.; Prasad, R.; Jaspal, D.; Shirolkar, M.; Gheisari, K.; Bhame, S. Visible light driven photocatalytic degradation of methylene blue by ZnO nanostructures synthesized by glycine nitrate auto combustion route. Inorg. Chem. Commun. 2023, 148, 110311. [Google Scholar] [CrossRef]
- Perez-Cuapio, R.; Alberto Alvarado, J.; Juarez, H.; Sue, H.J. Sun irradiated high efficient photocatalyst ZnO nanoparticles obtained by assisted microwave irradiation. Mater. Sci. Eng. B 2023, 289, 116263. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, S.K.; Das, N.C. Green synthesis of a reduced graphene oxide/silver nanoparticles-based catalyst for degradation of a wide range of organic pollutants. Nano-Struct. Nano-Objects 2023, 34, 100960. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Vandebriel, R.; de Jong, W. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Support for the Safe Use of Zinc Oxide Nanoparticle Sunscreens: Lack of Skin Penetration or Cellular Toxicity after Repeated Application in Volunteers. J. Investig. Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Leung, Y.H.; Djurišić, A.B.; Ng, A.M.C.; Chan, W.K. Native Defects in ZnO: Effect on Dye Adsorption and Photocatalytic Degradation. J. Phys. Chem. C 2013, 117, 12218–12228. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T.P. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Soares, M.R.; Rauwel, P. Influence of the Interface on the Photoluminescence Properties in ZnO Carbon-Based Nanohybrids. J. Phys. Chem. C 2017, 121, 14879–14887. [Google Scholar] [CrossRef]
- Nagpal, K.; Rapenne, L.; Wragg, D.S.; Rauwel, E.; Rauwel, P. The role of CNT in surface defect passivation and UV emission intensification of ZnO nanoparticles. Nanomater. Nanotechnol. 2022, 12, 18479804221079419. [Google Scholar] [CrossRef]
- Nassar, S.J.M.; Wills, C.; Harriman, A. Inhibition of the Photobleaching of Methylene Blue by Association with Urea. ChemPhotoChem 2019, 3, 1042–1049. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, Z.; Yue, J.; Zakaria, Q.M.D.; Chen, C.; Jiang, X.; Yu, A. Crystal plane-dependent gas-sensing properties of zinc oxide nanostructures: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2014, 16, 11471–11480. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. Selective Adsorption to Particular Crystal Faces of ZnO. Langmuir 2012, 28, 7189–7196. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Wang, X. Face the Edges: Catalytic Active Sites of Nanomaterials. Adv. Sci. 2015, 2, 1500085. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Djurišić, A.B.; Hsu, Y.F.; Wong, K.S.; Brauer, G.; Ling, C.C.; Chan, W.K. Exceptionally Long Exciton Photoluminescence Lifetime in ZnO Tetrapods. J. Phys. Chem. C 2008, 112, 16286–16295. [Google Scholar] [CrossRef]
- Rao, F.; Zhu, G.; Zhang, W.; Xu, Y.; Cao, B.; Shi, X.; Gao, J.; Huang, Y.; Huang, Y.; Hojamberdiev, M. Maximizing the Formation of Reactive Oxygen Species for Deep Oxidation of NO via Manipulating the Oxygen-Vacancy Defect Position on (BiO)2CO3. ACS Catal. 2021, 11, 7735–7749. [Google Scholar] [CrossRef]
- Dong, B.; Qi, Y.; Cui, J.; Liu, B.; Xiong, F.; Jiang, X.; Li, Z.; Xiao, Y.; Zhang, F.; Li, C. Synthesis of BaTaO2N oxynitride from Ba-rich oxide precursor for construction of visible-light-driven Z-scheme overall water splitting. Dalton Trans. 2017, 46, 10707–10713. [Google Scholar] [CrossRef]
- Maeda, K.; Lu, D.; Domen, K. Solar-Driven Z-scheme Water Splitting Using Modified BaZrO3–BaTaO2N Solid Solutions as Photocatalysts. ACS Catal. 2013, 3, 1026–1033. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, J.; Vequizo, J.J.M.; Hisatomi, T.; Ma, Y.; Nakabayashi, M.; Takata, T.; Yamakata, A.; Shibata, N.; Domen, K. Overall Water Splitting by a SrTaO2N-Based Photocatalyst Decorated with an Ir-Promoted Ru-Based Cocatalyst. J. Am. Chem. Soc. 2023, 145, 3839–3843. [Google Scholar] [CrossRef]
- Nagpal, K.; Rauwel, E.; Estephan, E.; Soares, M.R.; Rauwel, P. Significance of Hydroxyl Groups on the Optical Properties of ZnO Nanoparticles Combined with CNT and PEDOT:PSS. Nanomaterials 2022, 12, 3546. [Google Scholar] [CrossRef]
- Rauwel, E.; Nilsen, O.; Rauwel, P.; Walmsley, J.C.; Frogner, H.B.; Rytter, E.; Fjellvåg, H. Oxide Coating of Alumina Nanoporous Structure Using ALD to Produce Highly Porous Spinel. Chem. Vap. Depos. 2012, 18, 315–325. [Google Scholar] [CrossRef]
- Khachadorian, S.; Gillen, R.; Choi, S.; Ton-That, C.; Kliem, A.; Maultzsch, J.; Phillips, M.R.; Hoffmann, A. Effects of annealing on optical and structural properties of zinc oxide nanocrystals. Phys. Status Solidi B 2015, 252, 2620–2625. [Google Scholar] [CrossRef]
- Wang, J.B.; Huang, G.J.; Zhong, X.L.; Sun, L.Z.; Zhou, Y.C.; Liu, E.H. Raman scattering and high temperature ferromagnetism of Mn-doped ZnO nanoparticles. Appl. Phys. Lett. 2006, 88, 252502. [Google Scholar] [CrossRef]
- Gluba, M.A.; Nickel, N.H.; Karpensky, N. Interstitial zinc clusters in zinc oxide. Phys. Rev. B 2013, 88, 245201. [Google Scholar] [CrossRef]
- Zeferino, R.S.; Flores, M.B.; Pal, U. Photoluminescence and Raman Scattering in Ag-doped ZnO Nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Hejral, U.; Franz, D.; Volkov, S.; Francoual, S.; Strempfer, J.; Stierle, A. Identification of a Catalytically Highly Active Surface Phase for CO Oxidation over PtRh Nanoparticles under Operando Reaction Conditions. Phys. Rev. Lett. 2018, 120, 126101. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Dai, Y.; Li, C.; Yang, L.; Li, X.; Cui, C. Correlation between oxygen vacancies and dopant concentration in Mn-doped ZnO nanoparticles synthesized by co-precipitation technique. J. Alloys Compd. 2016, 684, 669–676. [Google Scholar] [CrossRef]
- Souissi, A.; Amlouk, M.; Khemakhem, H.; Guermazi, S. Deep analysis of Raman spectra of ZnO:Mo and ZnO:In sprayed thin films along with LO and TA+LO bands investigation. Superlattices Microstruct. 2016, 92, 294–302. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Andelman, T.; Neumark, G.F.; O’Brien, S.; Kuskovsky, I.L. Origin of defect-related green emission from ZnO nanoparticles: Effect of surface modification. Nanoscale Res. Lett. 2007, 2, 297. [Google Scholar] [CrossRef] [Green Version]
- Rauwel, E.; Galeckas, A.; Rauwel, P.; Sunding, M.F.; Fjellvåg, H. Precursor-Dependent Blue-Green Photoluminescence Emission of ZnO Nanoparticles. J. Phys. Chem. C 2011, 115, 25227–25233. [Google Scholar] [CrossRef]
- Wang, X.; Yin, L.; Liu, G.; Wang, L.; Saito, R.; Lu, G.Q.; Cheng, H.-M. Polar interface-induced improvement in high photocatalytic hydrogen evolution over ZnO–CdS heterostructures. Energy Environ. Sci. 2011, 4, 3976–3979. [Google Scholar] [CrossRef]
- Wöll, C. The chemistry and physics of zinc oxide surfaces. Prog. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- Diaspro, A.; Chirico, G.; Usai, C.; Ramoino, P.; Dobrucki, J. Photobleaching. In Handbook of Biological Confocal Microscopy; Pawley, J.B., Ed.; Springer: Boston, MA, USA, 2006; pp. 690–702. [Google Scholar] [CrossRef]
- Pena Luengas, S.L.; Marin, G.H.; Aviles, K.; Cruz Acuña, R.; Roque, G.; Rodríguez Nieto, F.; Sanchez, F.; Tarditi, A.; Rivera, L.; Mansilla, E. Enhanced singlet oxygen production by photodynamic therapy and a novel method for its intracellular measurement. Cancer Biother. Radiopharm. 2014, 29, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Shunji, K.; Masaharu, M.; Masao, K. Studies of the Transient Intermediates in the Photoreduction of Methylene Blue. Bull. Chem. Soc. Jpn. 1964, 37, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [Green Version]
- Shvalagin, V.V.; Stroyuk, A.L.; Kuchmii, S.Y. Photochemical synthesis of ZnO/Ag nanocomposites. J. Nanoparticle Res. 2007, 9, 427–440. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Pala, A.; Kurşun, G. The effect of different nanocatalysts for photocatalytic degradation of Methylene blue. Environ. Eng. 2019, 6, 79–83. [Google Scholar] [CrossRef]
- Nnodim, U.J.; Adogwa, A.A.; Akpan, U.G.; Ani, I.J. Photocatalytic Degradation of Methylene Blue Dye with Green Zinc Oxide Doped with Nitrogen. J. Clin. Rheumatol. Res. 2022, 2, 59. [Google Scholar]
- Chekir, N.; Benhabiles, O.; Tassalit, D.; Laoufi, N.A.; Bentahar, F. Photocatalytic degradation of methylene blue in aqueous suspensions using TiO2 and ZnO. Desalination Water Treat. 2016, 57, 6141–6147. [Google Scholar] [CrossRef]
- Ranjbari, A.; Kim, J.; Kim, J.H.; Yu, J.; Demeestere, K.; Heynderickx, P.M. Enhancement of commercial ZnO adsorption and photocatalytic degradation capacity of methylene blue by oxygen vacancy modification: Kinetic study. Catal. Today 2023, 413, 113976. [Google Scholar] [CrossRef]
- Priyadharshini, S.S.; Shubha, J.P.; Shivalingappa, J.; Adil, S.F.; Kuniyil, M.; Hatshan, M.R.; Shaik, B.; Kavalli, K. Photocatalytic Degradation of Methylene Blue and Metanil Yellow Dyes Using Green Synthesized Zinc Oxide (ZnO) Nanocrystals. Crystals 2022, 12, 22. [Google Scholar] [CrossRef]

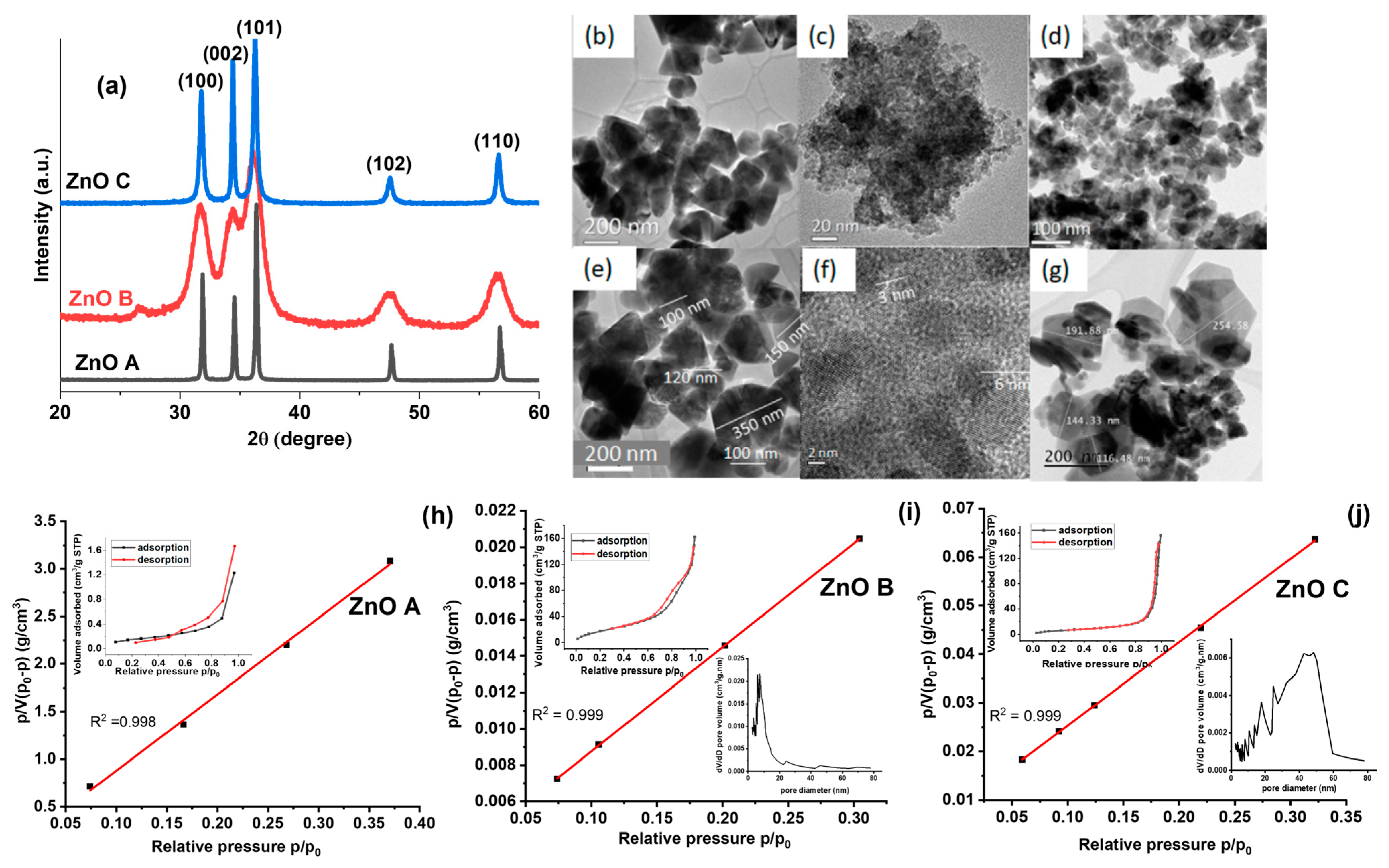
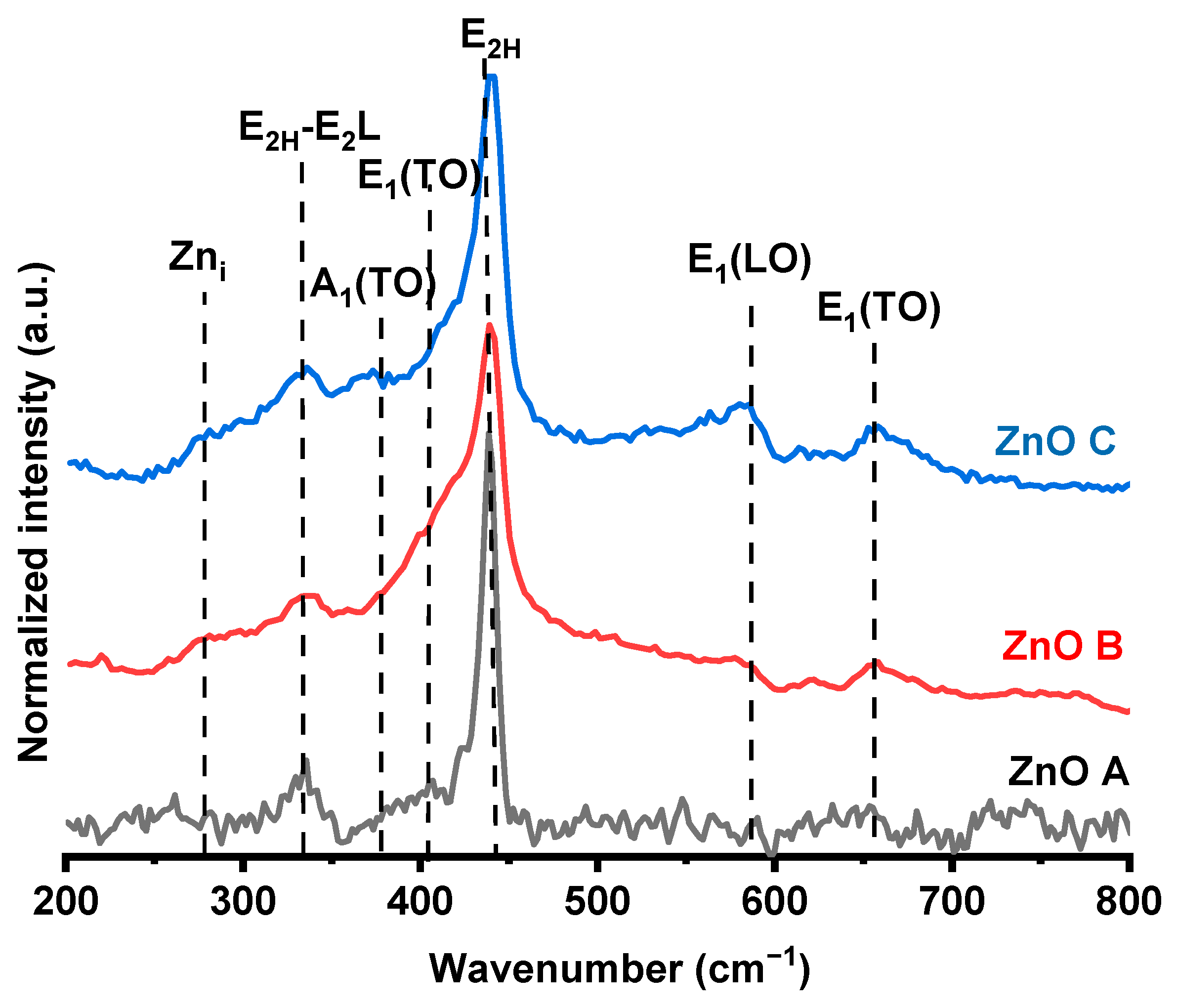
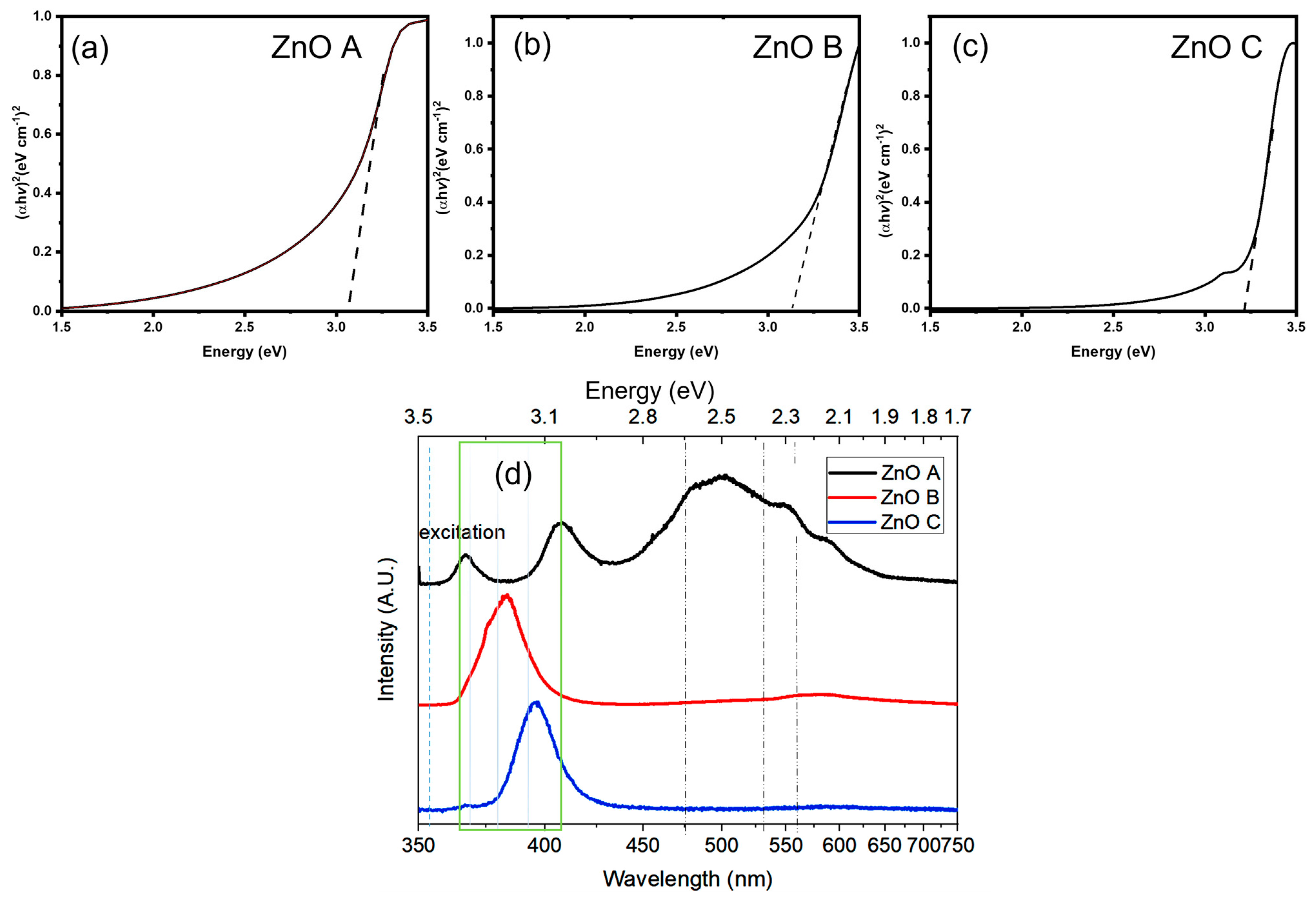


| Sample | SBET (m²/g) | CBET | (nm) | dTEM (nm) | Vm (cm3/g) | Vp (cm3/g) |
|---|---|---|---|---|---|---|
| ZnO A | 0.5 | 74 | >50 | 114 | 1.2 × 10−7 | 0 |
| ZnO B | 72 | 20 | 14.8 | 5 | 1.66 × 10−5 | 0.23 |
| ZnO C | 24 | 22 | 44.2 | 47 | 5.5 × 10−6 | 0.22 |
| Sample | Diameter (nm) | Shape | Synthesis Route (Solvent) | Temp (°C) | Reaction Time (h) |
|---|---|---|---|---|---|
| ZnO A | 80–140 | Hexagon, tetrahedron, octahedron with sharp edges and facets. | Non-aqueous (Benzylamine) | 300 | 48 |
| ZnO B | 5 | Spheres | Non-aqueous (Ethanol) | 65 | 2 |
| ZnO C | 27 | Spheres, facetted nanoparticles | Aqueous (Ethanol (70%) and water (30%)) | 60 | 2 |
| Photocatalyst | Illumination | C/C0 | Crystal Size (nm) | Time (min) | C0 | Mass | Ref |
|---|---|---|---|---|---|---|---|
| ZnO (commercial Nanograph, Chicago, IL, USA) | UV lamp | 0.3 | 30–50 | - | 5 | 4 g | [53] |
| ZnO (green synthesized) | sunlight | 0 | 200 | 90 | 5 | 2 g | [54] |
| ZnO (commercial Aldrich) | sunlight | 0.03 | 50 | 140 | 10 | 25 mg | [55] |
| ZnO (commercial Sigma Aldrich, St. Louis, MO, USA) | Xenon lamp | 0.03 | 50 | 150 | 10 | 25 mg | [56] |
| ZnO (green synthesized) | UV lamp | 0.03 | 50 | 100 | 5 | 15 mg | [57] |
| ZnO A (this work) | Sunlight/UV light | 0/0.08 | 100–300 | 180 | 5 | 1 mg | |
| ZnO B (this work) | Sunlight/UV light | 0/0.15 | 5 | 180 | 5 | 1 mg | |
| ZnO C (this work) | Sunlight/UV light | 0/0.22 | 50–200 | 180 | 5 | 1 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrix, Y.; Rauwel, E.; Nagpal, K.; Haddad, R.; Estephan, E.; Boissière, C.; Rauwel, P. Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States. Nanomaterials 2023, 13, 1998. https://doi.org/10.3390/nano13131998
Hendrix Y, Rauwel E, Nagpal K, Haddad R, Estephan E, Boissière C, Rauwel P. Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States. Nanomaterials. 2023; 13(13):1998. https://doi.org/10.3390/nano13131998
Chicago/Turabian StyleHendrix, Yuri, Erwan Rauwel, Keshav Nagpal, Ryma Haddad, Elias Estephan, Cédric Boissière, and Protima Rauwel. 2023. "Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States" Nanomaterials 13, no. 13: 1998. https://doi.org/10.3390/nano13131998