Fabrication and Characterization of Polylactic Acid Electrospun Wound Dressing Modified with Polyethylene Glycol, Rosmarinic Acid and Graphite Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
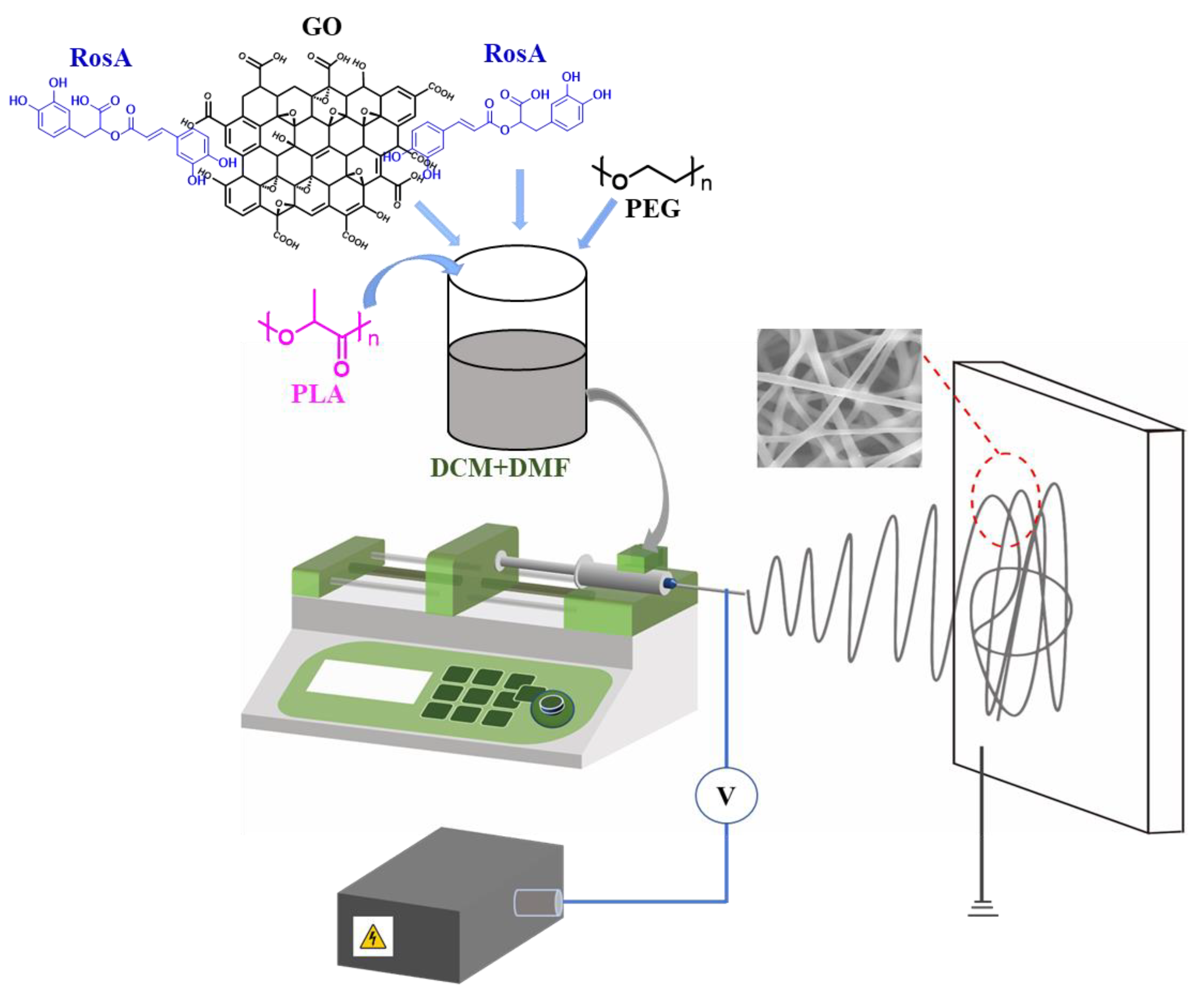
2.2. Preparation of e-Spinning Solutions
2.3. Preparation of e-Spinning Membranes
2.4. Characterizations
2.4.1. Morphology and Structure
2.4.2. Thermal and Mechanical Performance
2.4.3. Hydrophilicity
2.4.4. Porosity and Permeability
2.5. Antibacterial Activity
2.6. In Vivo Wound Healing Study
3. Results and Discussion
3.1. Characterization of e-Spun Nanofibers Morphology
3.2. Analysis of FT-IR and XRD for e-Spinning Fibers’ Structure
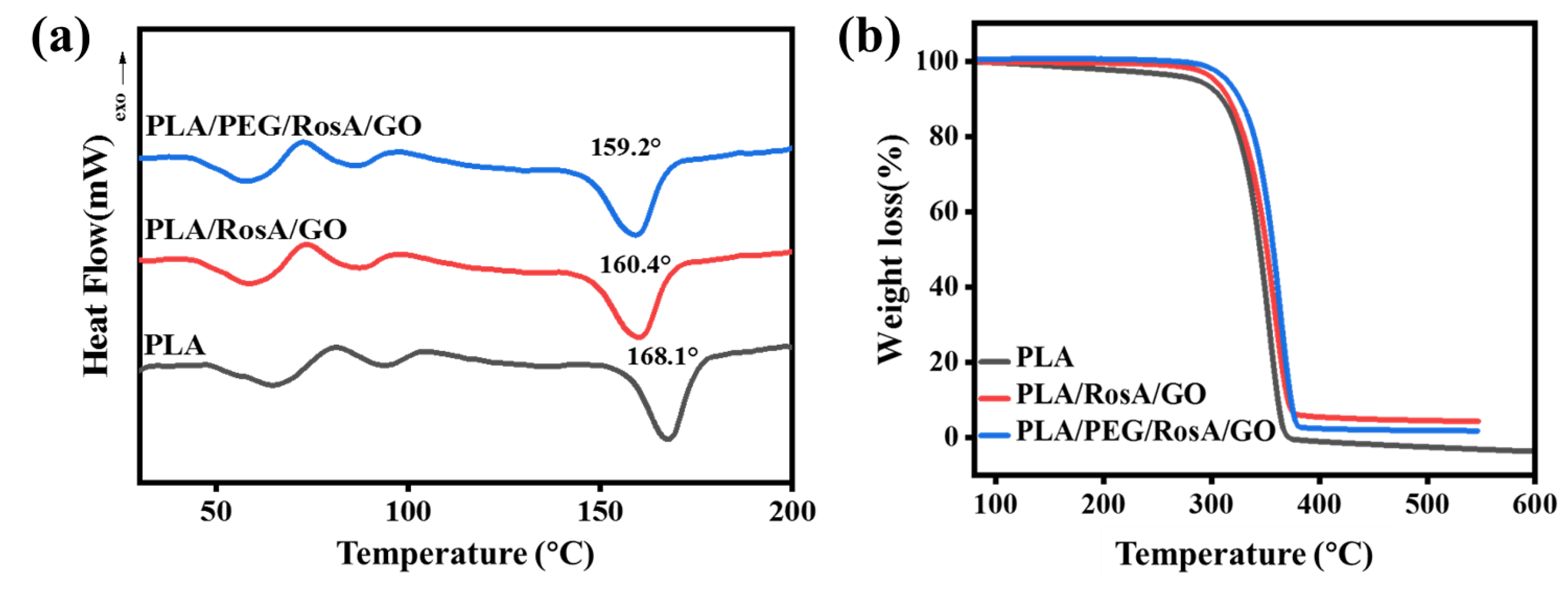
3.3. Thermal Properties
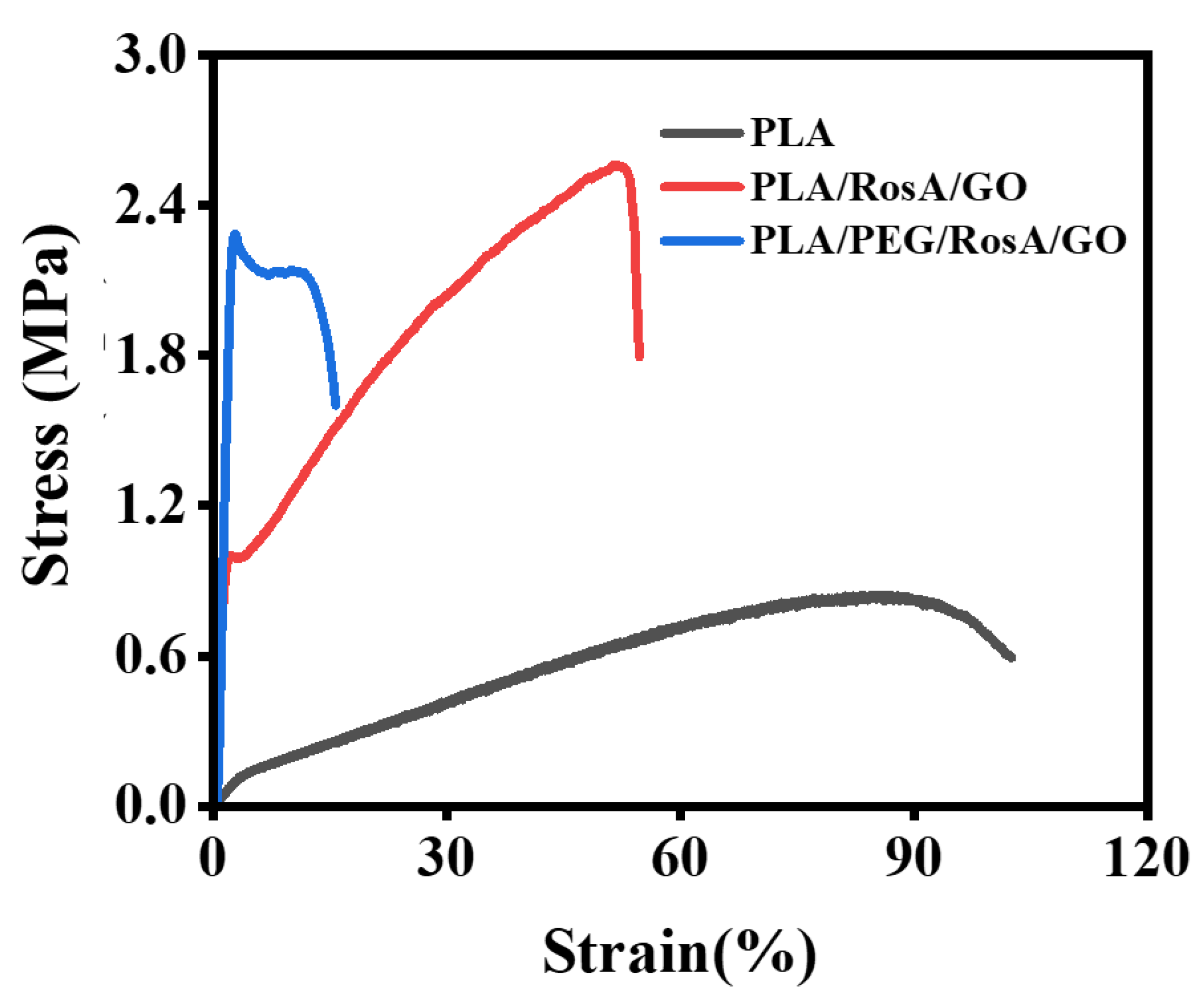
3.4. Mechanical Performance
3.5. Wettability
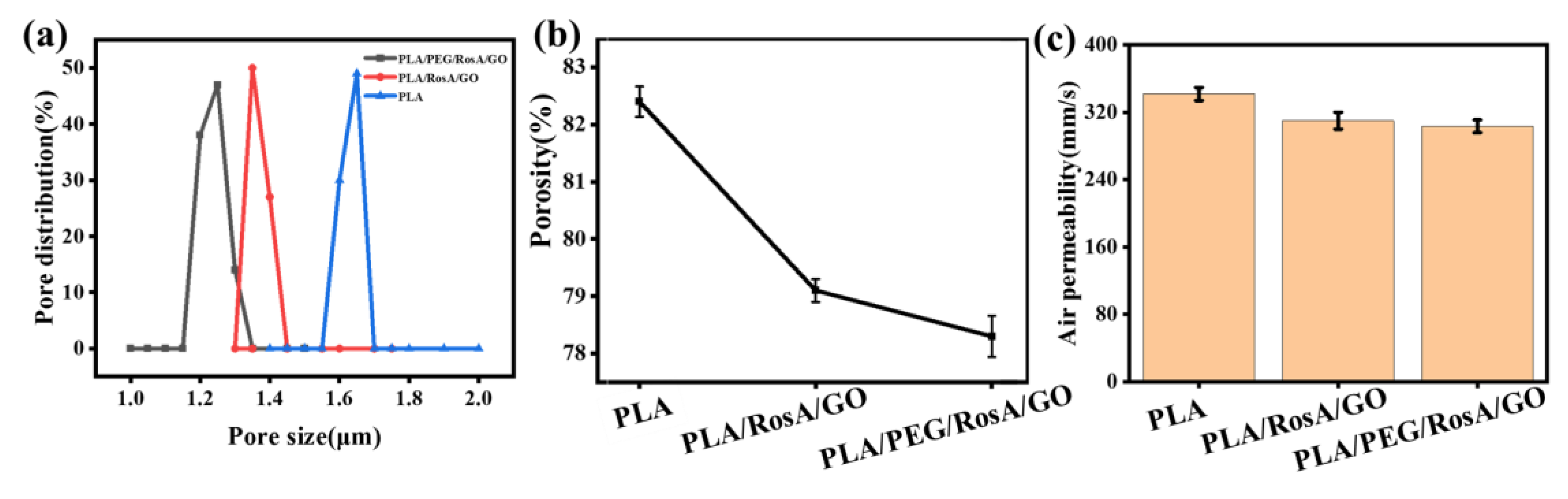
3.6. Porosity and Permeability
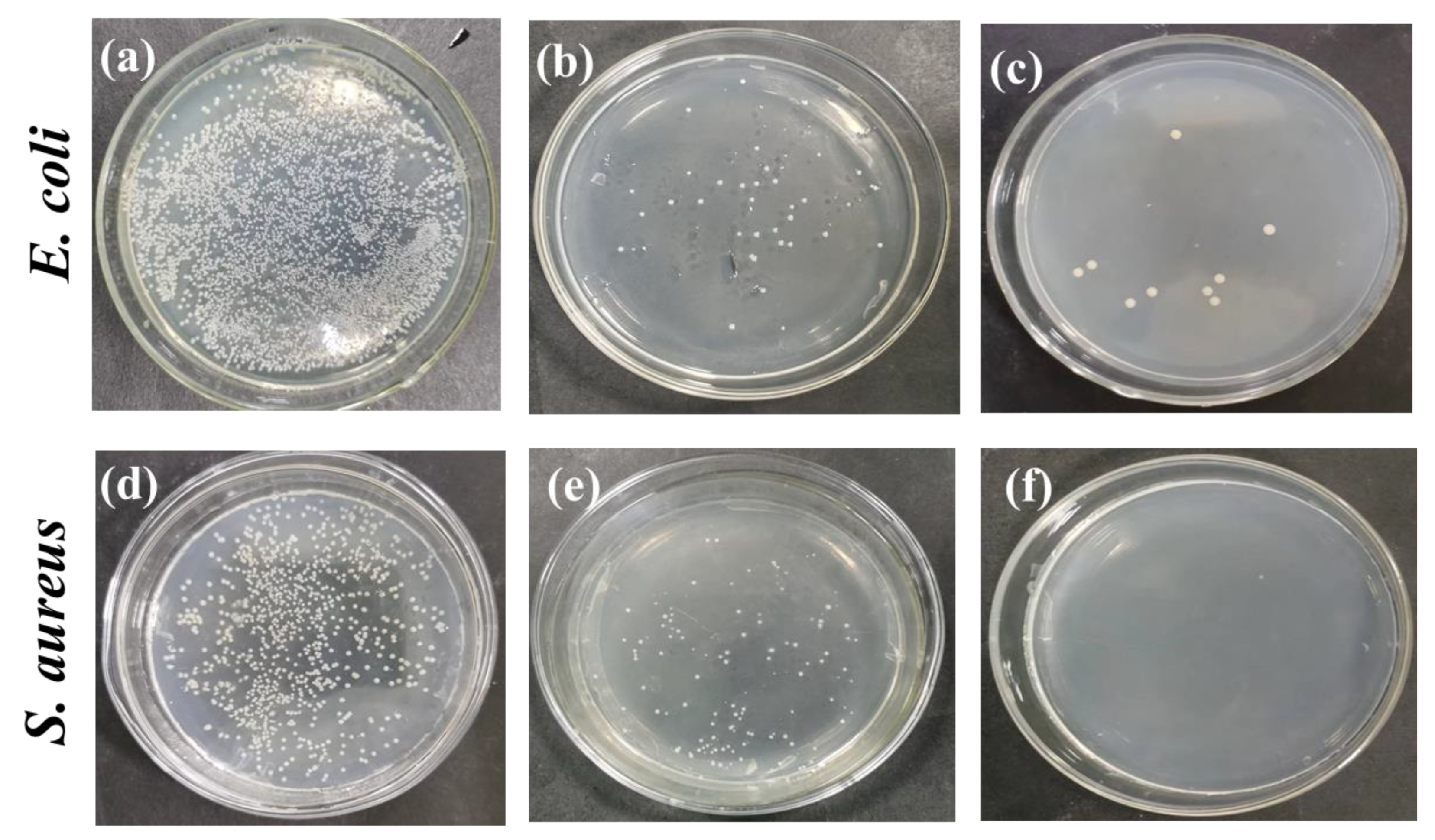
3.7. Evaluation of Antimicrobial Properties
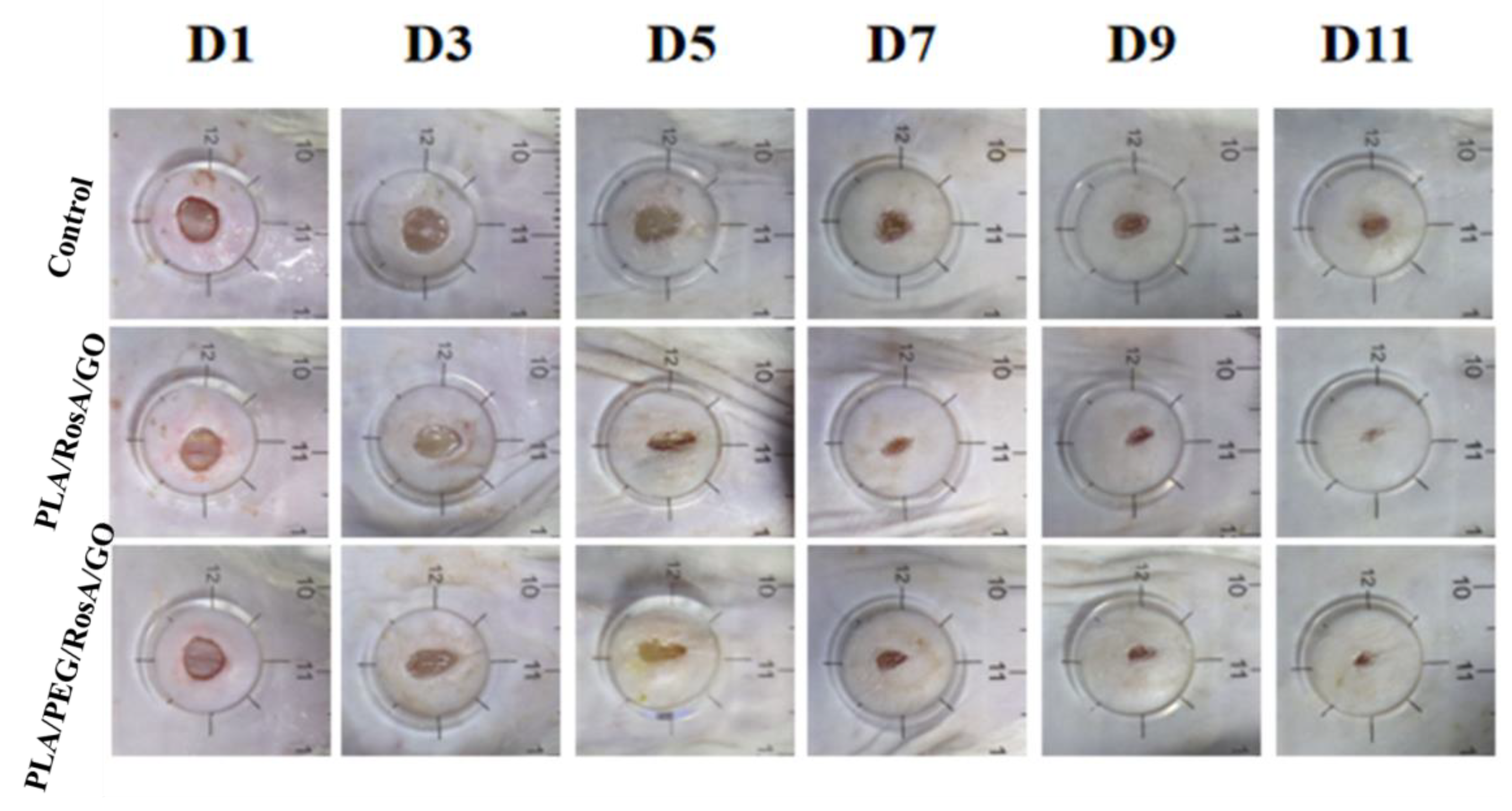
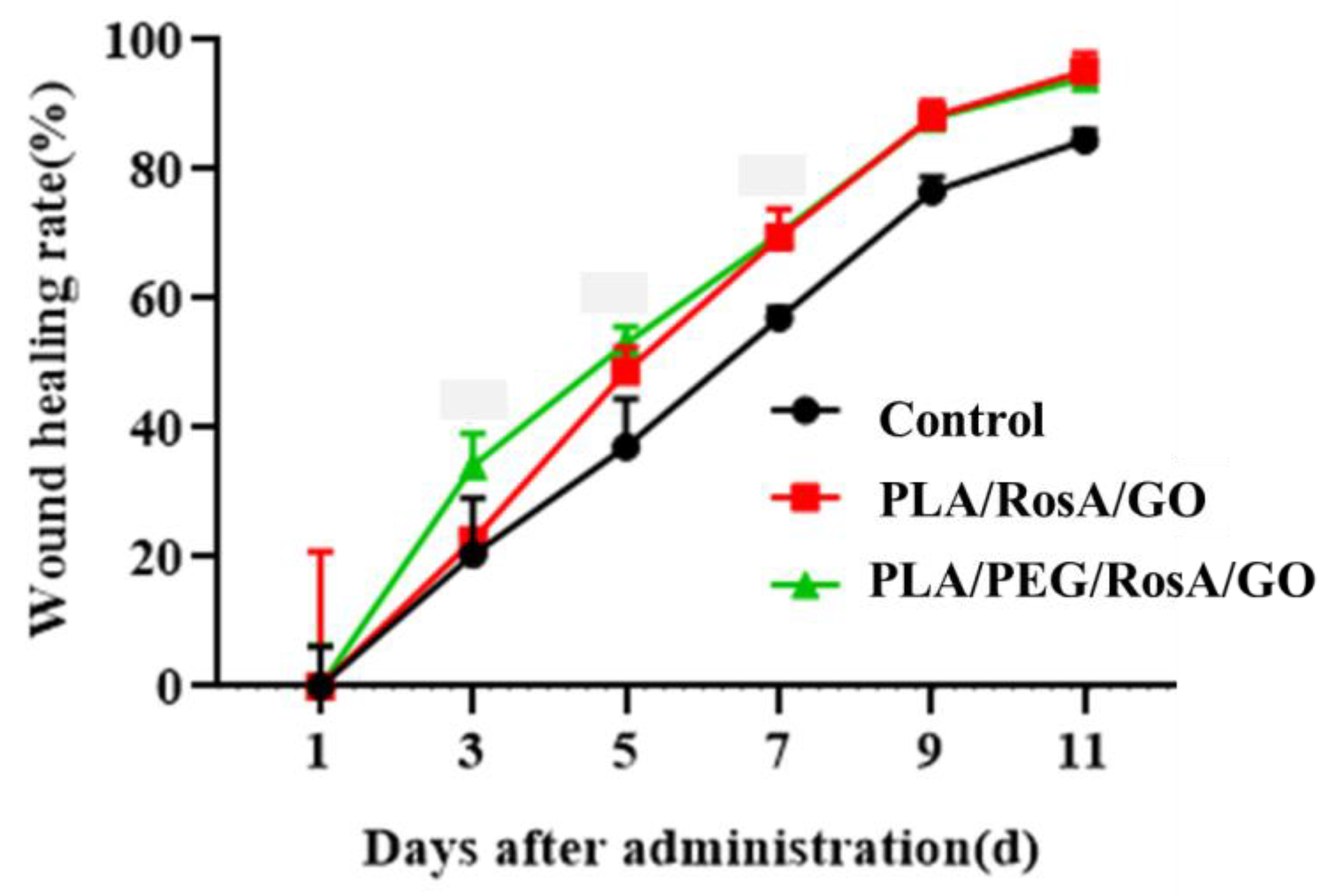
3.8. In Vivo Wound Healing Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Haalboom, M.; Blokhuis-Arkes, M.H.E.; Beuk, R.; Meerwaldt, R.J.; Klont, R.; Schijffelen, M.J.; Bowler, P.B.; Burnet, M.; Sigl, E.; Van Der Palen, J.A.M. Culture results from wound biopsy versus wound swab: Does it matter for the assessment of wound infection? Clin. Microbiol. Infect. 2019, 25, 629.e7–629.e12. [Google Scholar] [CrossRef]
- Zhou, L.P.; Min, T.T.; Bian, X.C.; Dong, Y.Z.; Zhang, P.X.; Wen, Y.Q. Rational Design of Intelligent and Multifunctional Dressing to Promote Acute/Chronic Wound Healing. ACS Biomater. Sci. Eng. 2022, 5, 4055–4085. [Google Scholar] [CrossRef]
- Han, M.C.; Cai, S.Z.; Wang, J.; He, H.W. Single-Side Superhydrophobicity in Si3N4-Doped and SiO2-Treated Polypropylene Nonwoven Webs with Antibacterial Activity. Polymers 2022, 14, 2952. [Google Scholar] [CrossRef]
- Shi, S.; Si, Y.F.; Han, Y.T.; Wu, T.; Lqbal, M.I.; Fei, B.; Li, R.K.Y.; Hu, J.L.; Qu, J.P. Recent Progress in Protective Membranes Fabricated via Electrospinning: Advanced Materials, Biomimetic Structures, and Functional Applications. Adv. Mater. 2022, 34, 2107938. [Google Scholar] [CrossRef] [PubMed]
- Baskar, S.; Ratheesh, G.; Ramakrishna, S. An overview of electrospun nanofibers and their application in energy storage, sensors and wearable/flexible electronics. J. Mater. Chem. 2017, 5, 12657. [Google Scholar]
- Wang, X.M.; Sun, F.Z.; Yin, G.C.; Wang, Y.T.; Liu, B.; Dong, M.D. Tactile-Sensing Based on Flexible PVDF Nanofibers via Electrospinning: A Review. Sensors 2018, 18, 330. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.K.; Xu, H.X.; Zhang, M.X.; Yu, D.G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y.; Xu, L. Apparatus for preparing electrospun nanofibres: A comparative review. Mater. Sci. Technol. 2010, 26, 1275–1287. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Noordin, M.Y.; Idris, A.; Kurniawan, D. A review of evolution of electrospun tissue engineering scaffold: From two dimensions to three dimensions. Part H J. Eng. Med. 2017, 231, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. A 2017, 105, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Li, T.Y.; Han, Y.F.; Li, F.J.; Liu, Y. Recent development of electrospun wound dressing. Curr. Opin. Biomed. Eng. 2021, 17, 100247. [Google Scholar] [CrossRef]
- Jun, I.D.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Navare, K.J.; Colobani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, M.L.; Zhang, M.; Ren, X.H.; Kim, I.S. Development of Antibacterial and Hemostatic PCL/Zein/ZnO-Quaternary Ammonium Salts NPs Composite Mats as Wound Dressings. Macromol. Mater. Eng. 2021, 306, 2100587. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly (lactic acid) fiber: An overview. Prog. Polym. Sci. 2017, 32, 455–482. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mat. Sci. Eng. C-Mater. 2016, 70, 897–912. [Google Scholar] [CrossRef]
- Contreras-Caceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romer, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Park, M.; Kim, A.A. Electrospun Nanofibers for Dura Mater Regeneration: A Mini Review on Current Progress. Pharmaceutics 2023, 15, 1347. [Google Scholar] [CrossRef] [PubMed]
- Hazer, B. The Properties of PLA/Oxidized Soybean Oil Polymer Blends. J. Polym. Environ. 2014, 22, 200–208. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Lu, Q.Q.; Wu, J.Z.; Zhang, Y.H.; Guo, J.B.; Yu, J.J.; Shu, X.R.; Chen, Q. Flexible, robust and self-peeling PLA/AgNWs nanofiber membranes with photothermally antibacterial properties for wound dressing. Appl. Surf. Sci. 2023, 615, 156284. [Google Scholar] [CrossRef]
- Rashedi, S.M.; Khajavi, R.; Rashidi, A.; Rahimi, M.K.; Bahador, A. Novel PLA/ZnO Nanofibrous Nanocomposite Loaded with Tranexamic Acid as an Effective Wound Dressing: In Vitro and In Vivo Assessment. Iran. J. Biotechnol. 2021, 19, e2737. [Google Scholar]
- Fan, T.Y.; Daniels, R. Preparation and Characterization of Electrospun Polylactic Acid (PLA) Fiber Loaded with Birch Bark Triterpene Extract for Wound Dressing. AAPS PharmSciTech 2021, 22, 205. [Google Scholar] [CrossRef]
- Karami, Z.; Rezaeian, I.; Zehedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(epsilon-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Ploym. Sci. 2013, 129, 756–766. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Barbosa, W.S.; Rangel, W.S.P.; Monteiro, M.S.D.B. Polymeric membrane based on polyactic acid and babassu oil for wound healing. Mater. Today. Commun. 2021, 26, 102173. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Nouri, M. Production and in vitro analysis of catechin incorporated electrospun gelatin/poly (lactic acid) microfibers for wound dressing applications. J. Ind. Text. 2021, 51, 7529S–7544S. [Google Scholar] [CrossRef]
- Zhang, X.M.; Lv, R.J.; Chen, L.X.; Sun, R.M.; Zhang, Y.; Sheng, R.T.; Du, T.; Li, Y.H.; Qi, Y.F. A Multifunctional Janus Electrospun Nanofiber Dressing with Biofluid Draining, Monitoring, and Antibacterial Properties for Wound Healing. ACS Appl. Mater. Inter. 2022, 14, 12984–13000. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L.; Ahmed, A. Batch Preparation and Characterization of Electrospun Porous Polylactic Acid-Based Nanofiber Membranes for Antibacterial Wound Dressing. Adv. Fiber. Mater. 2022, 4, 832–844. [Google Scholar] [CrossRef]
- Yeniay, E.; Ocal, L.; Altun, E.; Giray, B.; Oktar, F.N.; Inan, A.T.; Ekren, N.; Kilic, O.; Gunduz, O. Nanofibrous wound dressing material by electrospinning method. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 11–18. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Nandkumar, A.M.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA-Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mat. Sci. Eng. C-Mater. 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liang, X.; Zhang, R.; Lan, W.T.; Qin, W. Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/beta-Cyclodextrin Fibers as an Antimicrobial Wound Dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj. A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Feng, L.Z.; Liu, Z.A. Graphene in biomedicine: Opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zhang, J.T.; Xiong, Z.G.; Yong, Y.; Zhao, X.S. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Ciftci, F.; Ayan, S.; Duygulu, N.; Yilmazer, Y.; Karavelioglu, Z.; Vehapi, M.; Koc, R.C.; Sengor, M.; Yılmazer, H.; Ozcimen, D.; et al. Selenium and clarithromycin loaded PLA-GO composite wound dressings by electrospinning method. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 898–909. [Google Scholar] [CrossRef]
- Qian, W.H.; Wang, Z.L.; He, D.N.; Huang, X.Y.; Su, J.S. Ornidazole-loaded graphene paper for combined antibacterial materials. J. Saudi Chem. Soc. 2018, 22, 581–587. [Google Scholar] [CrossRef]
- Azimi, S.; Behin, J.; Abiri, R.; Rajabi, L.; Derakhshan, A.A.; Karimnezhad, H. Synthesis, Characterization and Antibacterial Activity of Chlorophyllin Functionalized Graphene Oxide Nanostructures. Sci. Adv. Mater. 2014, 6, 771–781. [Google Scholar] [CrossRef]
- Budhiraja, A.; Dhingra, G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015, 22, 723–730. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Li, J.; Chen, M.; Zhang, S.; Cheng, Y.; Ning, X.; Wang, N. Tailoring moisture electroactive Ag/Zn@ cotton coupled with electrospun PVDF/PS nanofibers for antimicrobial face masks. J. Hazard. Mater. 2022, 428, 128239. [Google Scholar] [CrossRef]
- Hajikhani, M.; Emam-Djomeh, Z.; Askari, G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021, 172, 143–153. [Google Scholar] [CrossRef]
- Zhong, G.F.; Qiu, M.Y.; Zhang, J.B.; Jiang, F.C.; Yue, X.; Huang, C.; Zhao, S.Y.; Zeng, R.; Zhang, C.; Qu, Y. Fabrication and characterization of PVA@PLA electrospinning nanofibers embedded with Bletilla striata polysaccharide and Rosmarinic acid to promote wound healing. Int. J. Biol. Macromol. 2023, 234, 123693. [Google Scholar] [CrossRef]
- Moreira, J.B.; Terra, A.L.M.; Costa, J.A.V.; de Morais, M.G. Development of pH indicator from PLA/PEO ultrafine fibers containing pigment of microalgae origin. Int. J. Biol. Macromol. 2018, 118, 1855–1862. [Google Scholar] [CrossRef]
- Zhao, N.Q.; Shi, S.X.; Lu, G.; Wei, M. Polylactide (PLA)/layered double hydroxides composite fibers by electrospinning method. J. Phys. Chem. Solids 2008, 69, 1564–1568. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, L.; Li, J.S.; Chen, L.H.; Zheng, Q.; Chen, T.; Chen, Z.P.; Fu, T.M.; Di, L.Q. Enhanced oral bioavailability and prophylactic effects on oxidative stress and hepatic damage of an oil solution containing a rosmarinic acid-phospholipid complex. J. Funct. Foods 2015, 19, 63–73. [Google Scholar] [CrossRef]
- Huang, J.H.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the Phospholipid Effect on the Bioaccessibility of Rosmarinic Acid-Phospholipid Complex through a Dynamic Gastrointestinal in Vitro Model. Pharmaceutics 2019, 11, 156. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Zhao, J.; Gao, X. Microstructures and mechanical properties of electrospun PLA fiber composite nonwoven mat. J. Text. Res. 2007, 28, 4–8. [Google Scholar]
- Xiang, C.; Joo, Y.; Frey, M. Nanocomposite Fibers Electrospun from Poly(lactic acid)/Cellulose Nanocrystals. J. Biobased Mater. Bioenerny 2009, 3, 147–155. [Google Scholar] [CrossRef]
- Sinsup, P.; Teeranachaideekul, V.; Makarasen, A.; Chuenchom, L.; Prajongtat, P.; Techasakul, S.; Yingyuad, P.; Dechtrirat, D. Zingiber cassumunar Roxb. Essential Oil-Loaded Electrospun Poly(lactic acid)/Poly(ethylene oxide) Fiber Blend Membrane for Antibacterial Wound Dressing Application. Membranes 2021, 11, 648. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]



| Run. | DCM/DMF, mL/mL | PLA, g | PEG, g | RosA, g | GO, mL |
|---|---|---|---|---|---|
| PLA | 8/2 | 1 | 0 | 0 | 0 |
| PLA/RosA/GO | 8/2 | 1 | 0 | 0.1 | 2 |
| PLA/PEG/RosA/GO | 8/2 | 0.95 | 0.05 | 0.1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Du, G.; Guo, Q.; Li, R.; Li, C.; He, H. Fabrication and Characterization of Polylactic Acid Electrospun Wound Dressing Modified with Polyethylene Glycol, Rosmarinic Acid and Graphite Oxide. Nanomaterials 2023, 13, 2000. https://doi.org/10.3390/nano13132000
Liu C, Du G, Guo Q, Li R, Li C, He H. Fabrication and Characterization of Polylactic Acid Electrospun Wound Dressing Modified with Polyethylene Glycol, Rosmarinic Acid and Graphite Oxide. Nanomaterials. 2023; 13(13):2000. https://doi.org/10.3390/nano13132000
Chicago/Turabian StyleLiu, Chengyi, Guicai Du, Qunqun Guo, Ronggui Li, Changming Li, and Hongwei He. 2023. "Fabrication and Characterization of Polylactic Acid Electrospun Wound Dressing Modified with Polyethylene Glycol, Rosmarinic Acid and Graphite Oxide" Nanomaterials 13, no. 13: 2000. https://doi.org/10.3390/nano13132000





