Electronic Skin Based on Polydopamine-Modified Superelastic Fibers with Superior Conductivity and Durability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
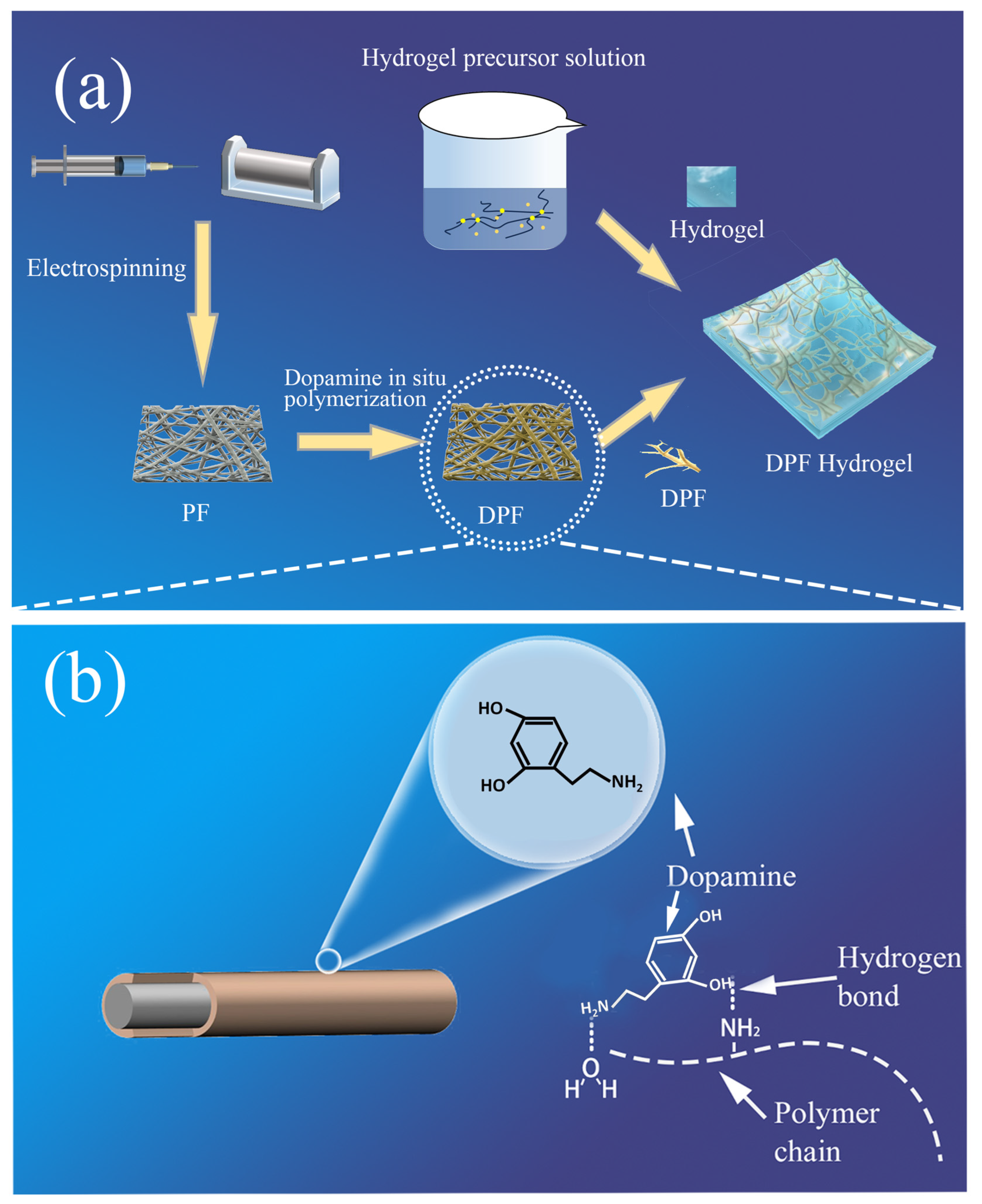
2.2.1. Preparation of Elastic Fibers
2.2.2. Preparation of Dopamine-Treated Fiber Mesh
2.2.3. Preparation of Hydrogel Precursor
2.2.4. Preparation of DPF Hydrogel
2.3. Characterization
3. Results
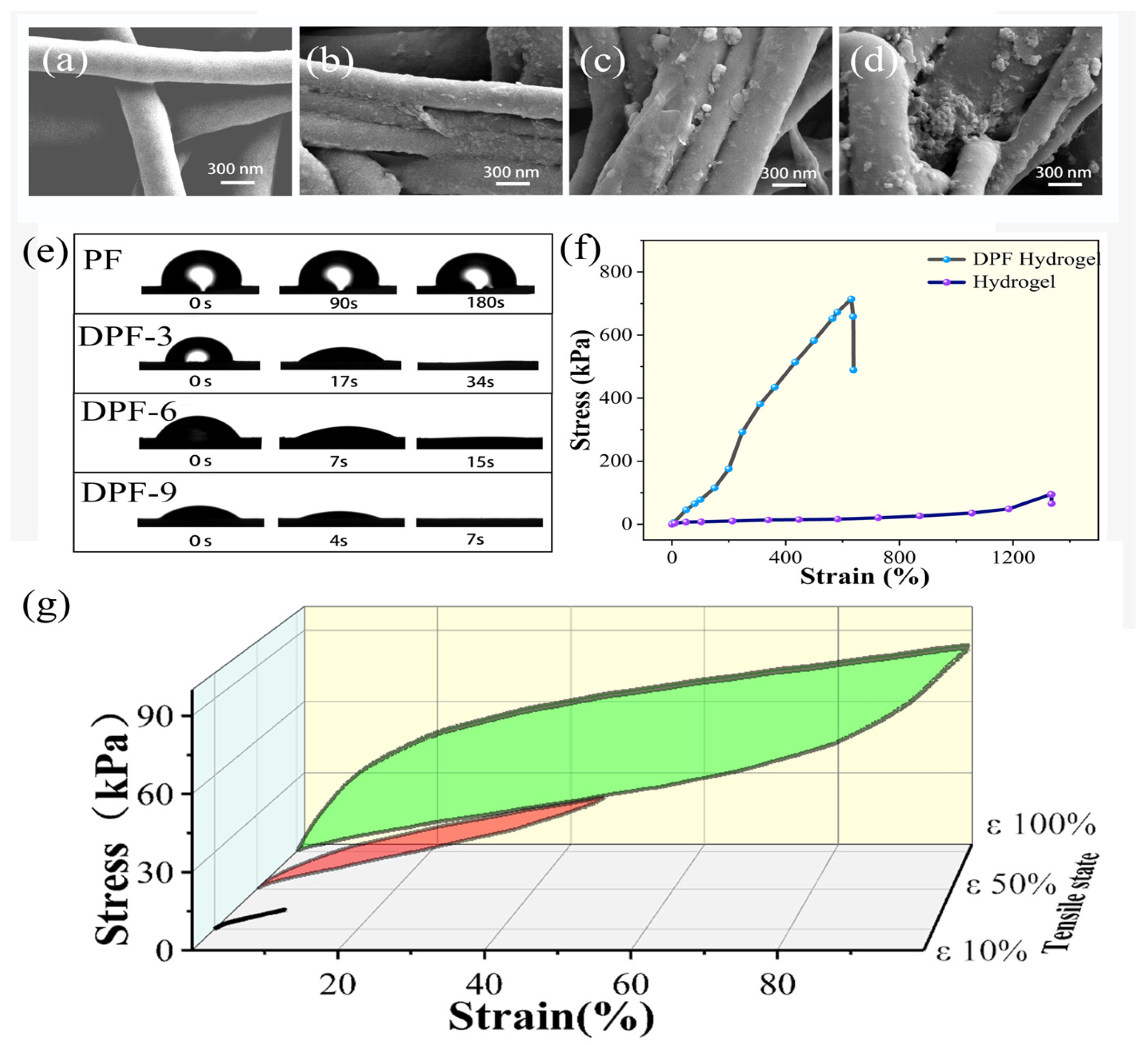
3.1. Fiber Modification
3.2. Mechanical Testing
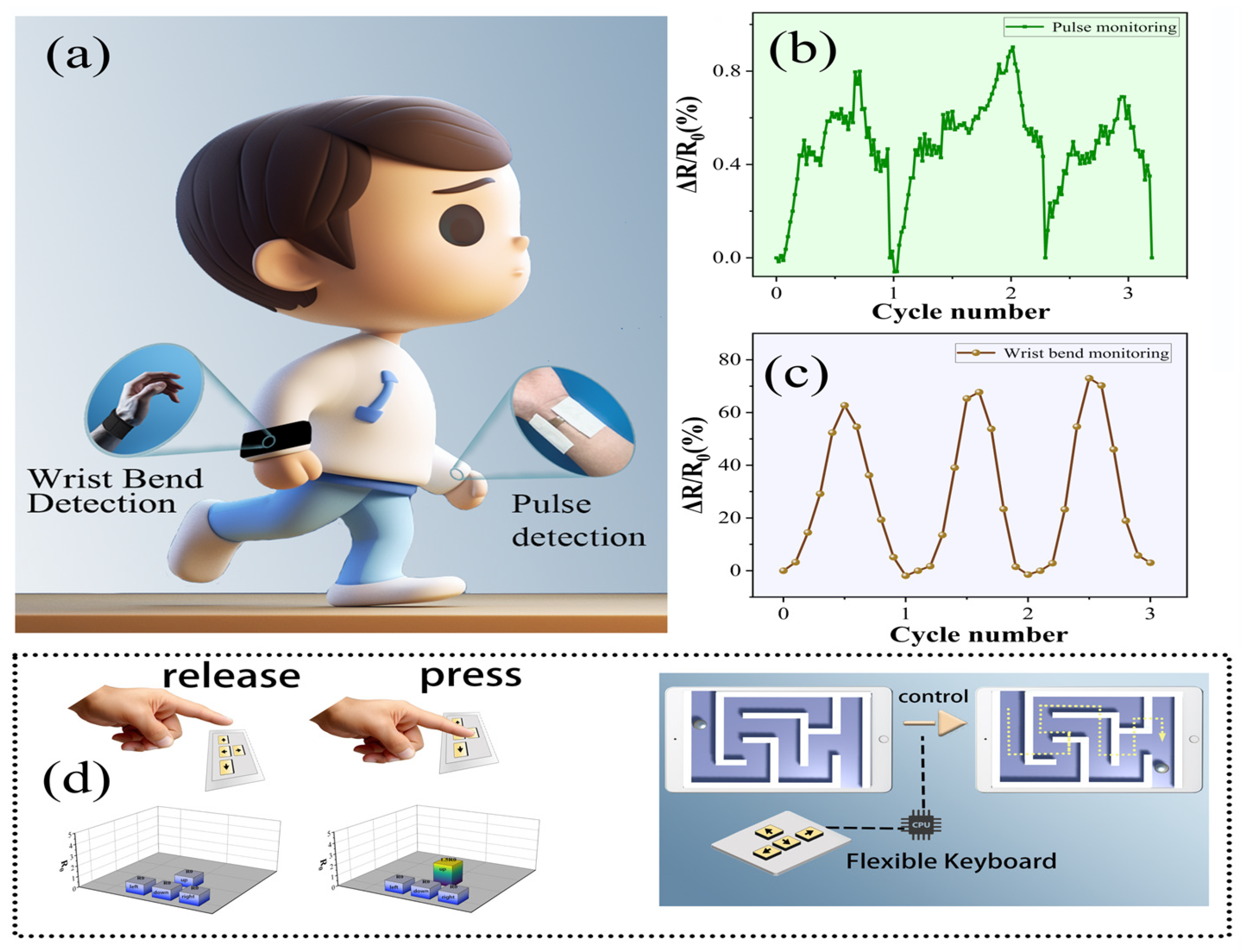
3.3. Sensing Performance
3.4. Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, K.; Wang, Z.; Zhao, T.; Zhang, Y.; Li, L.; Wang, L. A wearable flexible acceleration sensor for monitoring human motion. Biosensors 2022, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Research on application of flexible strain sensor in human motion monitoring. J. Ambient. Intell. Hum. Comput. 2022, 13, 97. [Google Scholar]
- Gong, X.; Huang, K.; Wu, Y.; Zhang, X. Recent progress on screen-printed flexible sensors for human health monitoring. Sens. Actuators A Phys. 2022, 345, 113821. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, S.; Ramadoss, A. Functionality of flexible pressure sensors in cardiovascular health monitoring: A Review. ACS Sens. 2022, 9, 2495–2520. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; He, H.; Zhang, L.; Dong, C.; Zeng, H.; Dai, Y.; Xing, L.; Zhang, Y.; Xue, X. A self-powered wearable noninvasive electronic-skin for perspiration analysis based on piezo-biosensing unit matrix of enzyme/ZnO nanoarrays. ACS Appl. Mater. Interfaces 2017, 35, 29526–29537. [Google Scholar] [CrossRef]
- Shi, S.; Ming, Y.; Wu, H.; Zhi, C.; Yang, L.; Meng, S.; Si, Y.; Wang, D.; Fei, B.; Hu, J. A bionic skin for health management: Excellent breathability, in situ sensing, and big data analysis. Adv. Mater. 2023, e2306435. [Google Scholar] [CrossRef]
- Sahu, S.K.; Tamadon, I.; Rosa, B.; Renaud, P.; Menciassi, A. A spring-based inductive sensor for soft and flexible robots. IEEE Sens. J. 2022, 22, 19931–19940. [Google Scholar] [CrossRef]
- Liu, W.; Li, F.; Stefanini, C.; Chen, D.; Dario, P. Biomimetic flexible/compliant sensors for a soft-body lamprey-like robot. Robot. Auton. Syst. 2010, 58, 1138–1148. [Google Scholar] [CrossRef]
- Chen, S.; Luo, T.; Geng, D.; Shen, Z.; Zhou, W. Facile fabrication of a fast-response flexible temperature sensor via laser reduced graphene oxide for contactless human-machine interface. Carbon 2022, 187, 35–46. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Chen, T.; Song, L.; Zhang, Y.; Lin, Q.; Wang, M.; Wang, F.; Ma, N.; Su, L. Wearable human-machine interface based on the self-healing strain sensors array for control interface of unmanned aerial vehicle. Sens. Actuators A Phys. 2021, 321, 112583. [Google Scholar] [CrossRef]
- Wang, X.; Dong, L.; Zhang, H.; Yu, R.; Pan, C.; Wang, Z.L. Recent progress in electronic skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.; Tok, J.B.; Bao, Z. 25th Anniversary article: The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Wang, Z. Electronic skin for closed-loop systems. ACS Nano 2019, 13, 12287–12293. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shi, J.M.; Chi, Y.H. Tannic acid physically cross-linked responsive hydrogel. Macromol. Chem. Phys. 2018, 219, 1800234. [Google Scholar] [CrossRef]
- Laquerbe, S.; Sayed, J.; Lorthioir, C.; Meyer, C.; Narita, T.; Ducouret, G.; Perrin, P.; Sanson, N. Supramolecular crosslinked hydrogels: Similarities and differences with chemically crosslinked hydrogels. Macromolecules 2023, 56, 7406–7418. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, J.; Tang, J.; Hu, J.; Lu, T.; Suo, Z. Fatigue of double-network hydrogels. Eng. Fract. Mech. 2018, 187, 74–93. [Google Scholar] [CrossRef]
- Ahmed, S.; Nakajima, T.; Kurokawa, T.; Haque, M.; Gong, J. Brittleeductile transition of double network hydrogels: Mechanical balance of two networks as the key factor. Polymer 2014, 55, 914–923. [Google Scholar] [CrossRef]
- Song, B.; Fan, X.; Gu, H. Chestnut-tannin-crosslinked, antibacterial, antifreezing, conductive organohydrogel as a strain sensor for motion monitoring, flexible keyboards, and velocity monitoring. ACS Appl. Mater. Interfaces 2023, 15, 2147–2162. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Liu, Z. Deformation behavior of fiber-reinforced hydrogel structures. Int. J. Struct. Stab. Dyn. 2019, 19, 1950032. [Google Scholar] [CrossRef]
- Wei, J.; Wang, J.; Shao, Z. Bioinspired cellulose nanofibrils and NaCl composited polyacrylamide hydrogels with improved toughness, resilience, and strain-sensitive conductivity. J. Appl. Polym. Sci. 2022, 139, e53188. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, W. Highly stretchable and transparent double-network hydrogel ionic conductors as flexible thermal-mechanical dual sensors and electroluminescent devices. ACS Appl. Mater. Interfaces 2019, 11, 16765–16775. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Wei, P.; Sun, S.; Wu, P. Fatigue-free artificial ionic skin toughened by self-healable elastic nanomesh. Nat. Commun. 2022, 13, 4411. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wang, R.; Che, Y.; Han, C.; Feng, W.; Wang, C.; Zhao, W. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J. Mater. Sci. Technol. 2023, 162, 157–178. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, Y.; Wang, H.; Wang, X.; Tian, M. Electronic Skin Based on Polydopamine-Modified Superelastic Fibers with Superior Conductivity and Durability. Nanomaterials 2024, 14, 438. https://doi.org/10.3390/nano14050438
Chen C, Wang Y, Wang H, Wang X, Tian M. Electronic Skin Based on Polydopamine-Modified Superelastic Fibers with Superior Conductivity and Durability. Nanomaterials. 2024; 14(5):438. https://doi.org/10.3390/nano14050438
Chicago/Turabian StyleChen, Chengfeng, Yimiao Wang, Hang Wang, Xinqing Wang, and Mingwei Tian. 2024. "Electronic Skin Based on Polydopamine-Modified Superelastic Fibers with Superior Conductivity and Durability" Nanomaterials 14, no. 5: 438. https://doi.org/10.3390/nano14050438





