Enhanced Degradation of Methyl Orange and Trichloroethylene with PNIPAm-PMMA-Fe/Pd-Functionalized Hollow Fiber Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
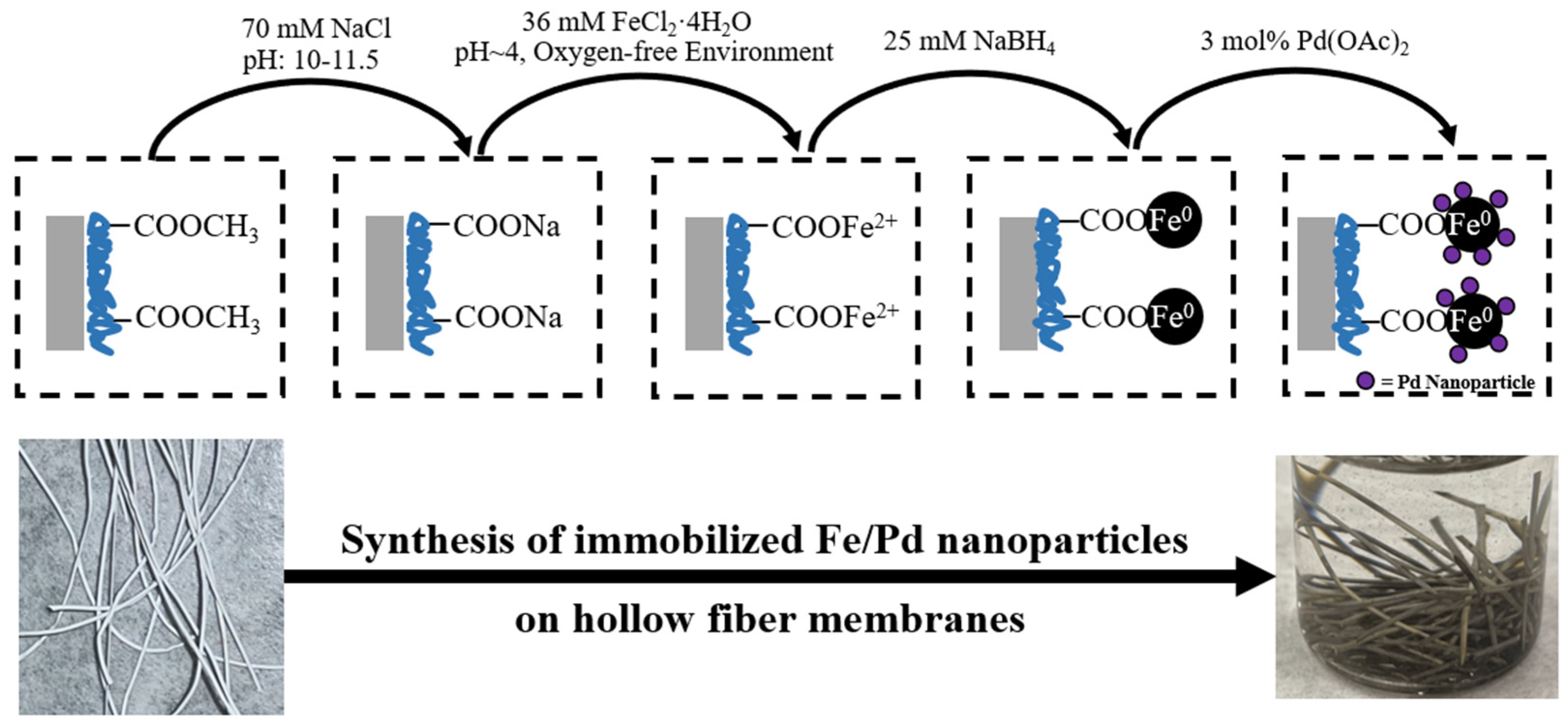
2.2. Hydrogel and Nanoparticle Synthesis
2.3. Hydrogel Functionalization of Membranes
2.4. Fe/Pd Nanoparticle Functionalization of Membrane
2.5. Responsive Flux Studies of Functionalized Membranes
2.6. Temperature-Responsive MO and TCE Adsorption/Desorption of Hydrogels
2.7. MO/TCE Degradation via Fe/Pd NPs and Catalytic Functionalized HFMs
2.8. Membrane-Air Stripping (MAS)
2.9. Gas Chromatography-Mass Spectroscopy (GCMS)
3. Results and Discussion
3.1. Membrane Functionalization and Nanoparticle Synthesis
3.1.1. Membranes Characteristics
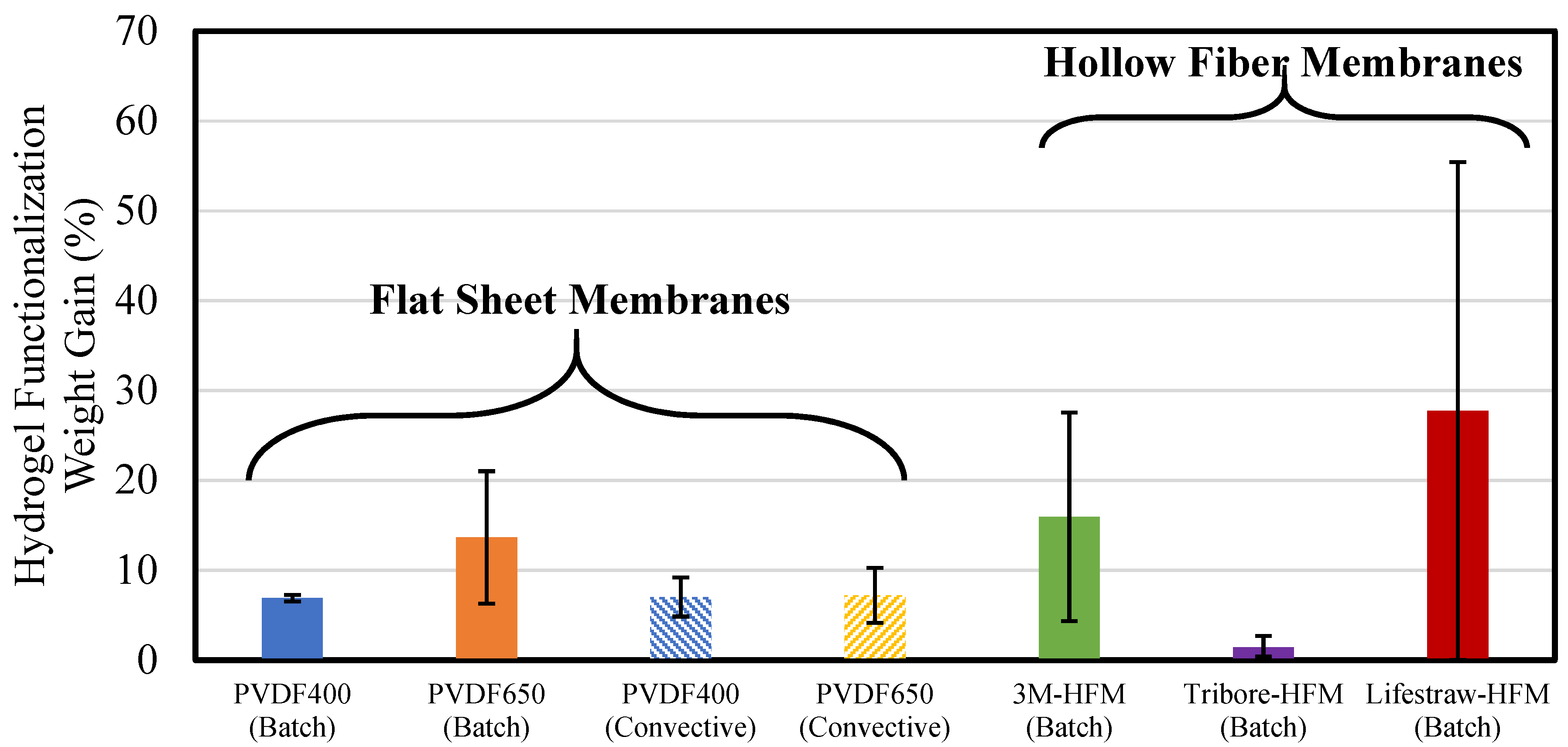
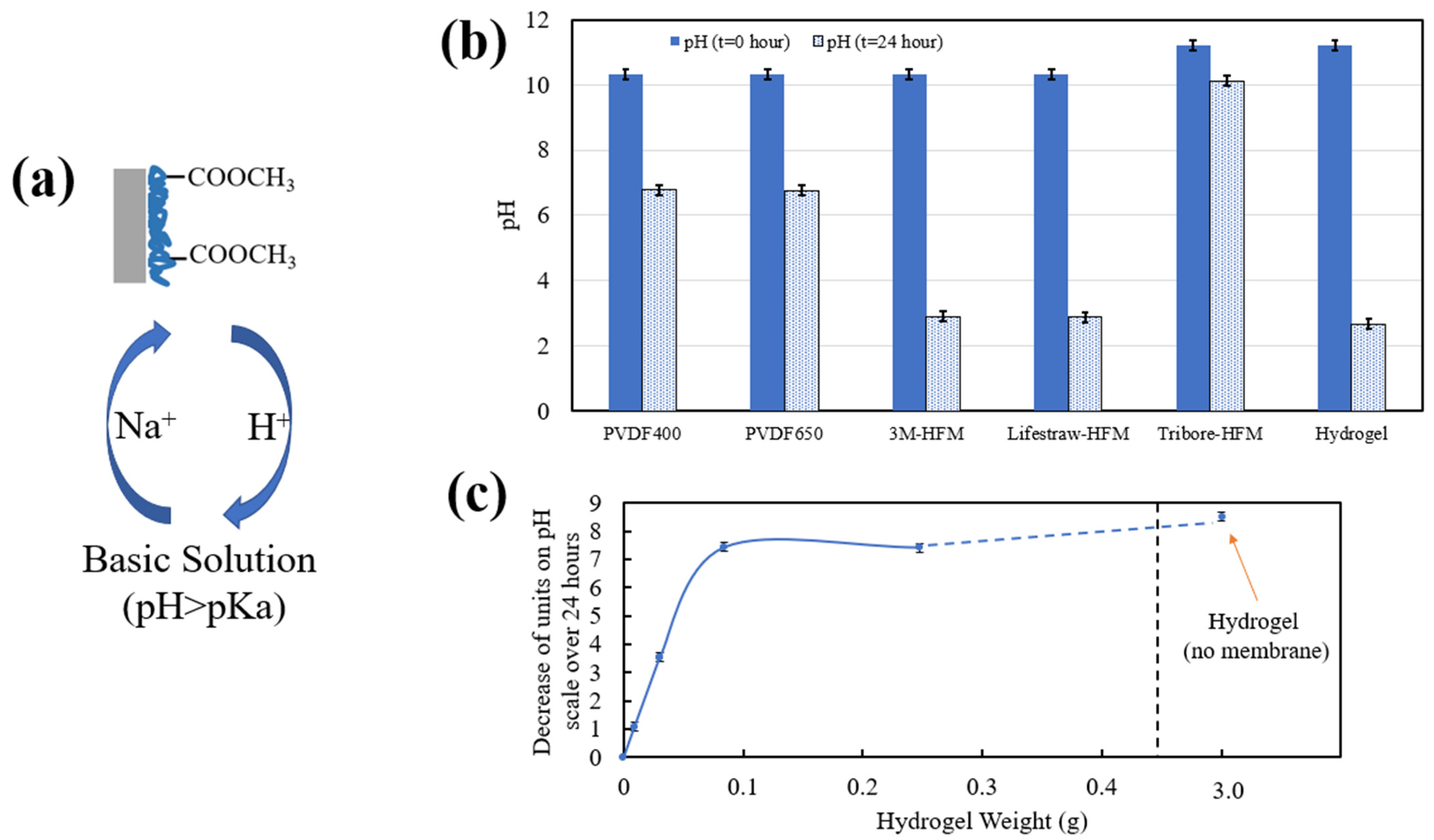
3.1.2. PNIPAm-PMMA Functionalization of Membranes
3.1.3. Fe/Pd Synthesis and Immobilization into the Membrane System
3.1.4. Functionalized Membrane Characterization via SEM and EDX
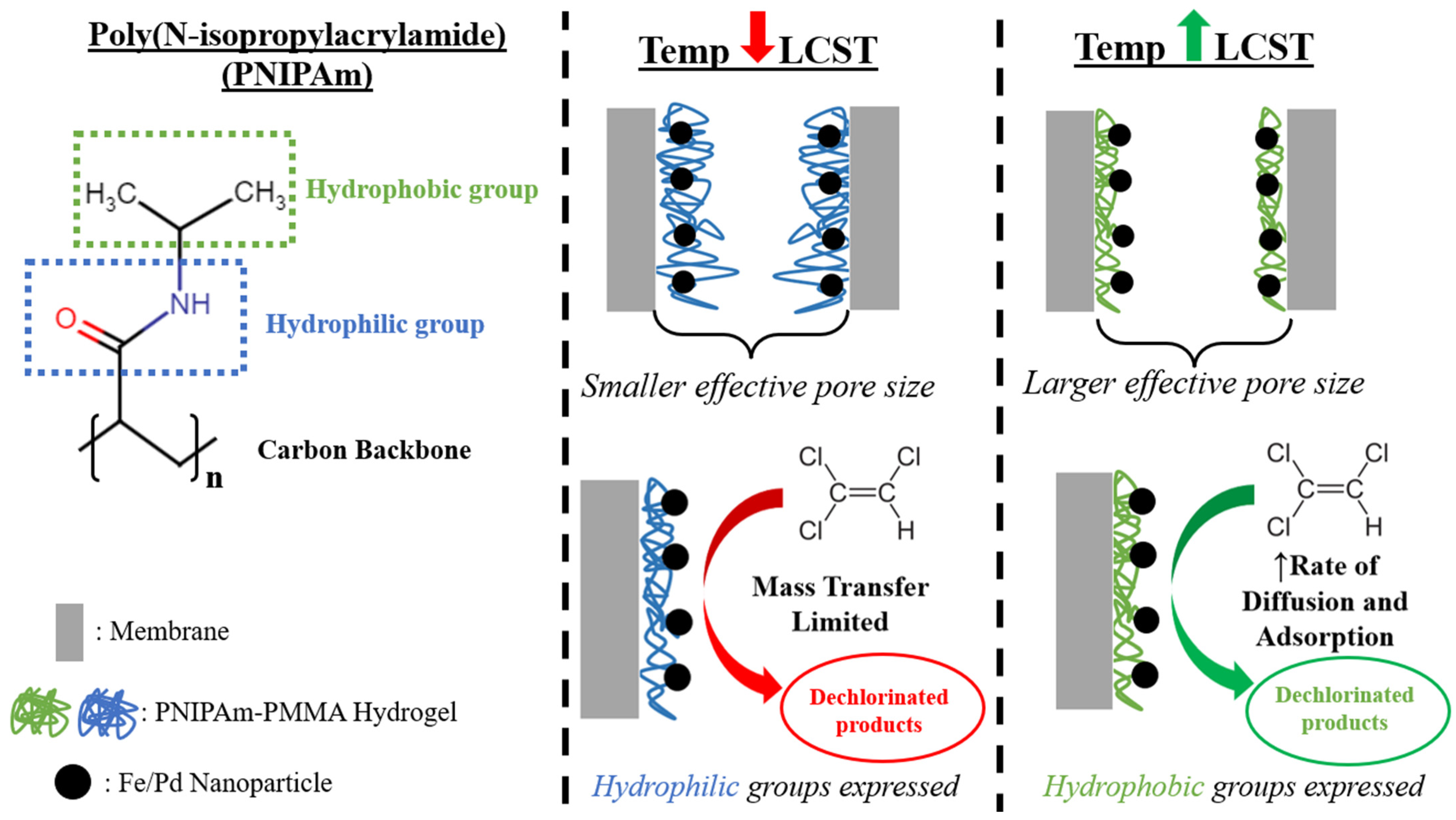
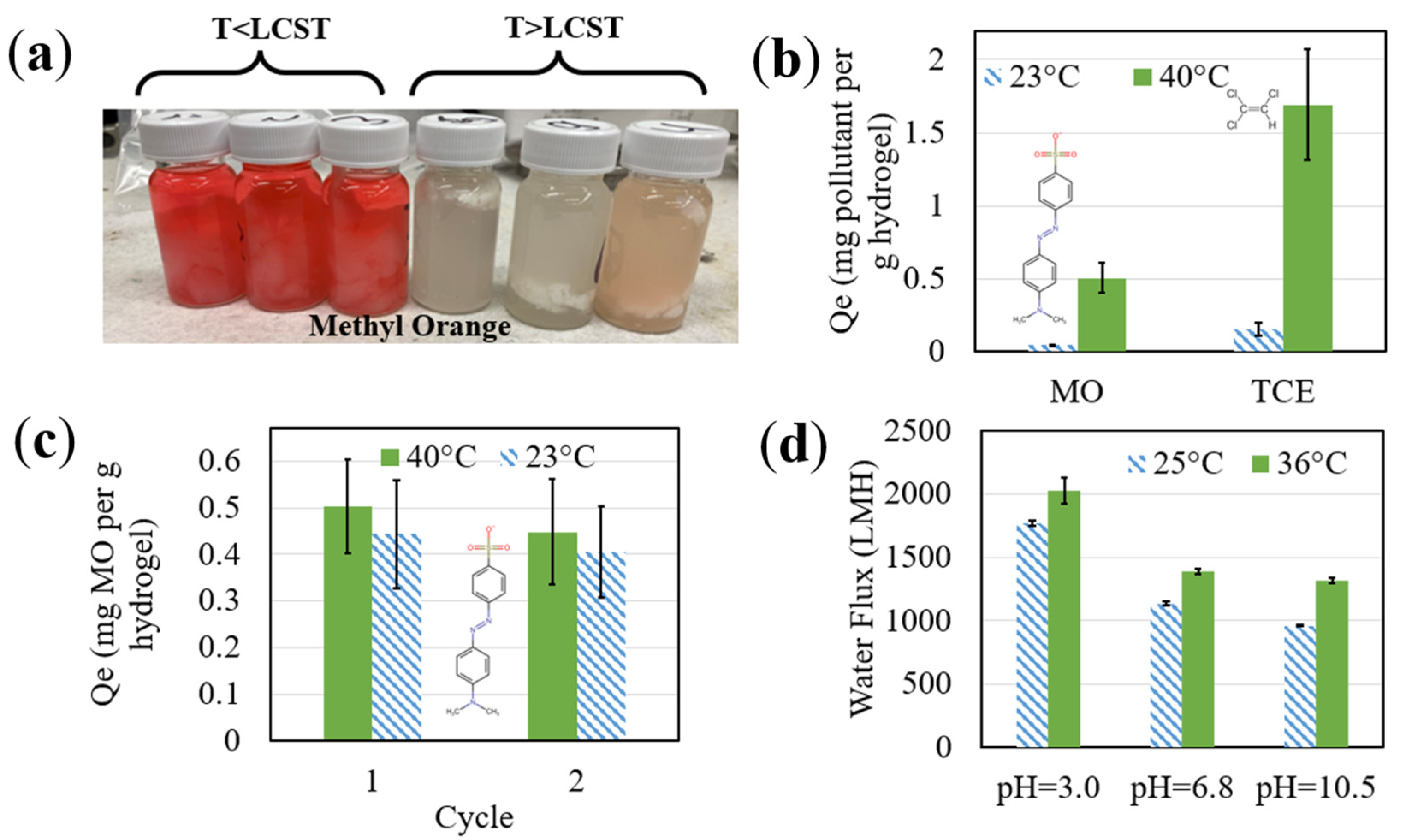
3.2. Temperature and pH Response of Functionalized Materials
3.3. Pollutant Degradation with Responsive Catalytic Membranes
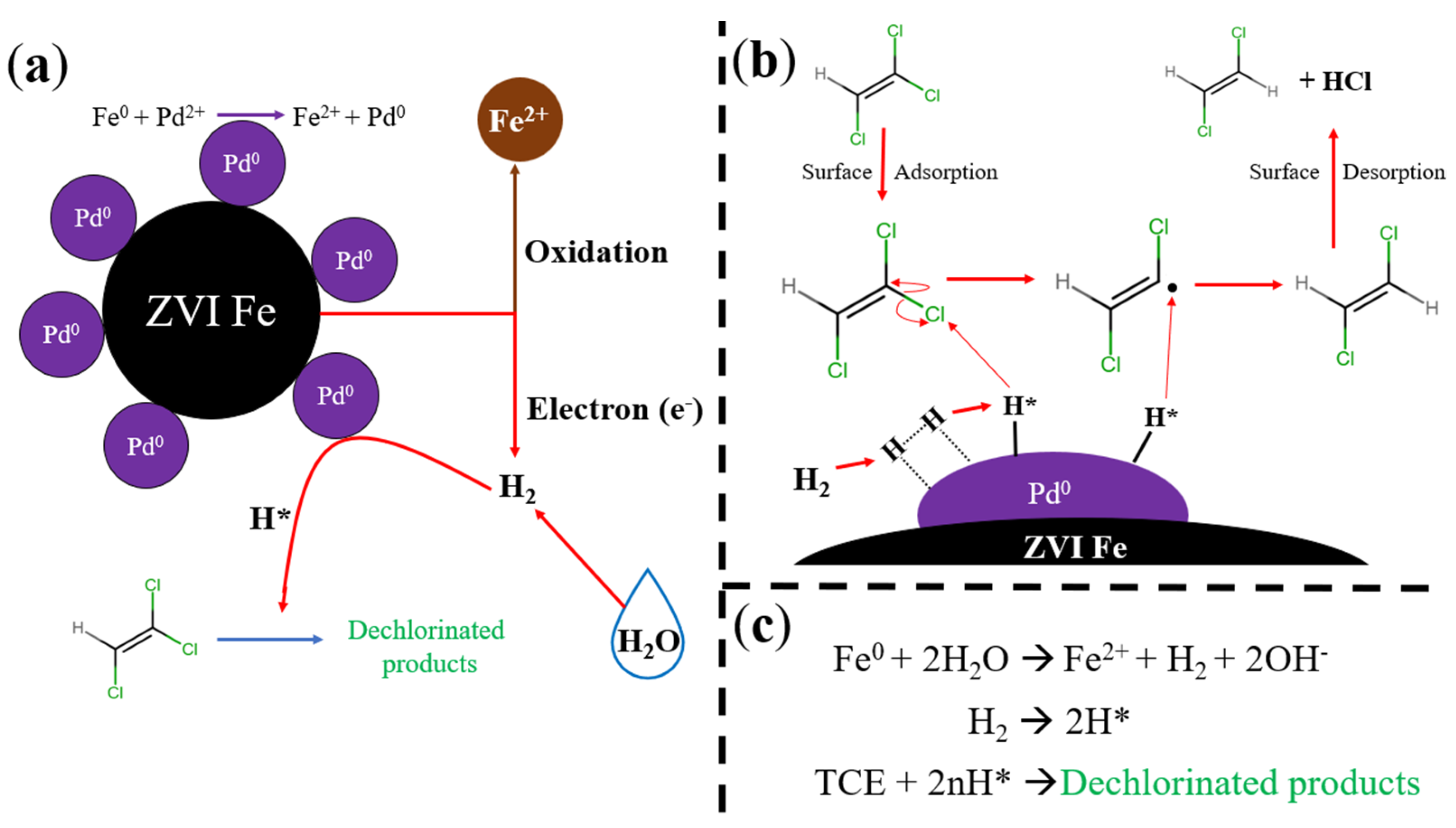
3.3.1. Steps of the Reductive Degradation Pathway with Corresponding Models
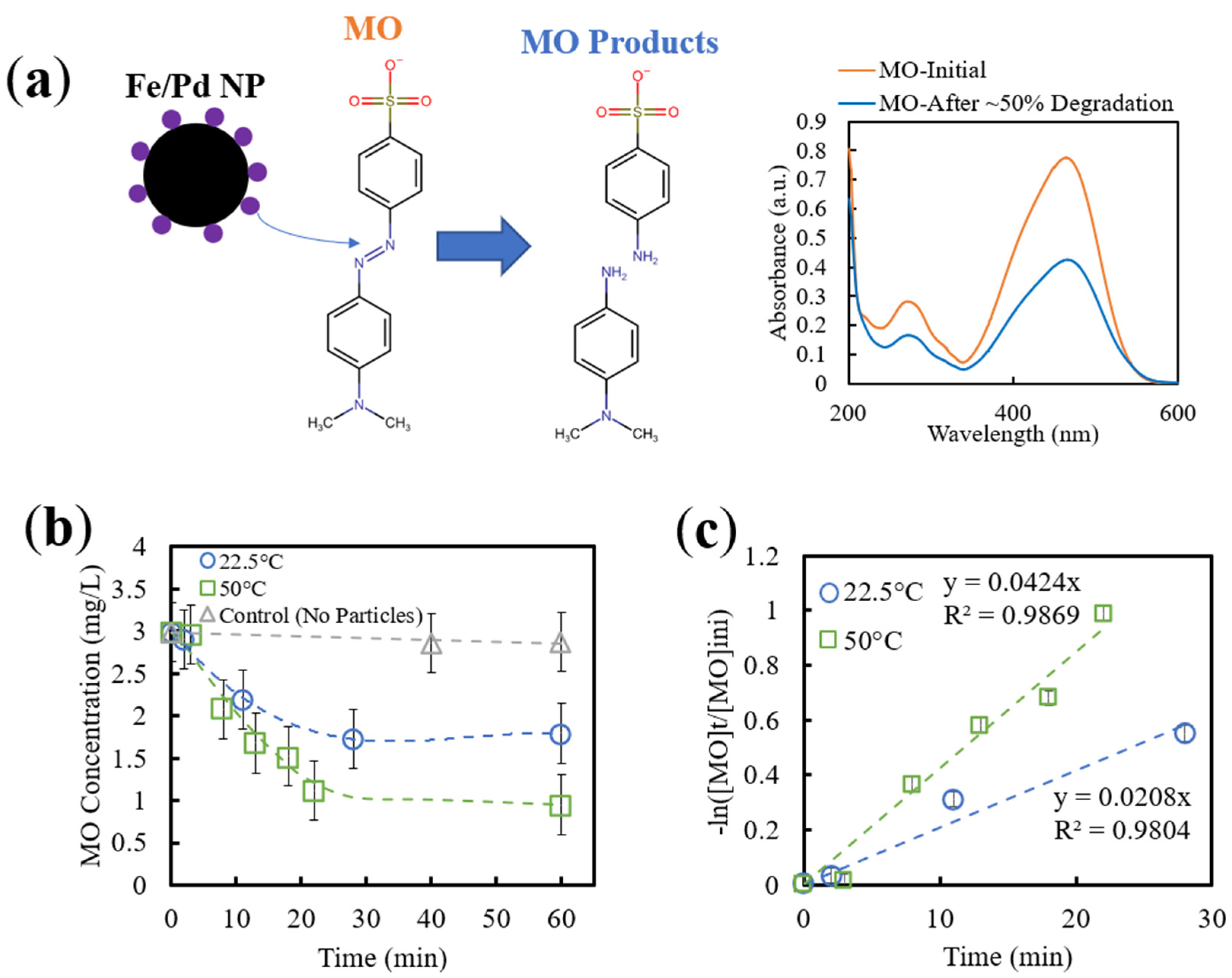
3.3.2. Effect of Temperature on MO Degradation via Fe/Pd Nanoparticles
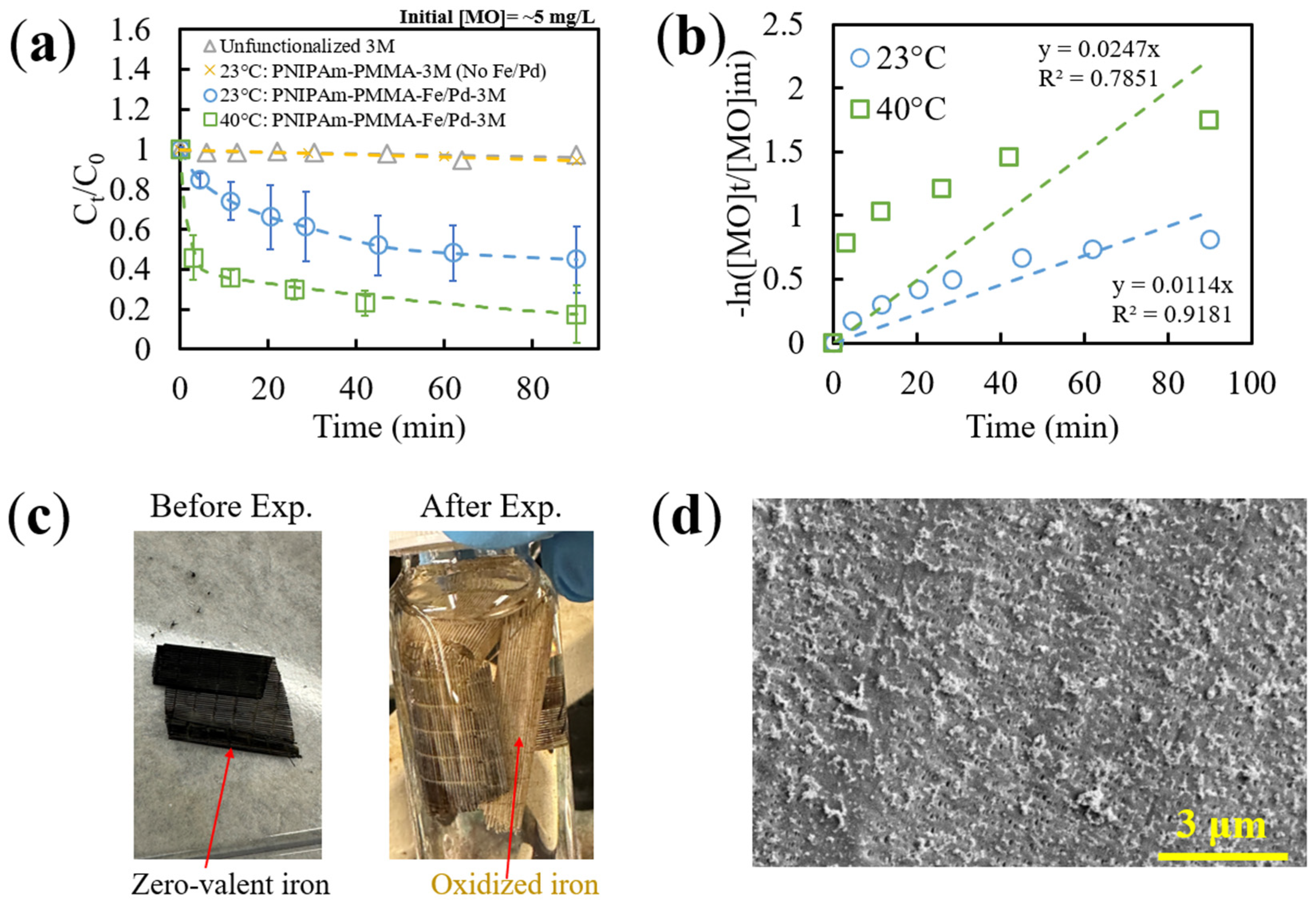
3.3.3. Thermo-Responsive Degradation of MO via Catalytic Membranes
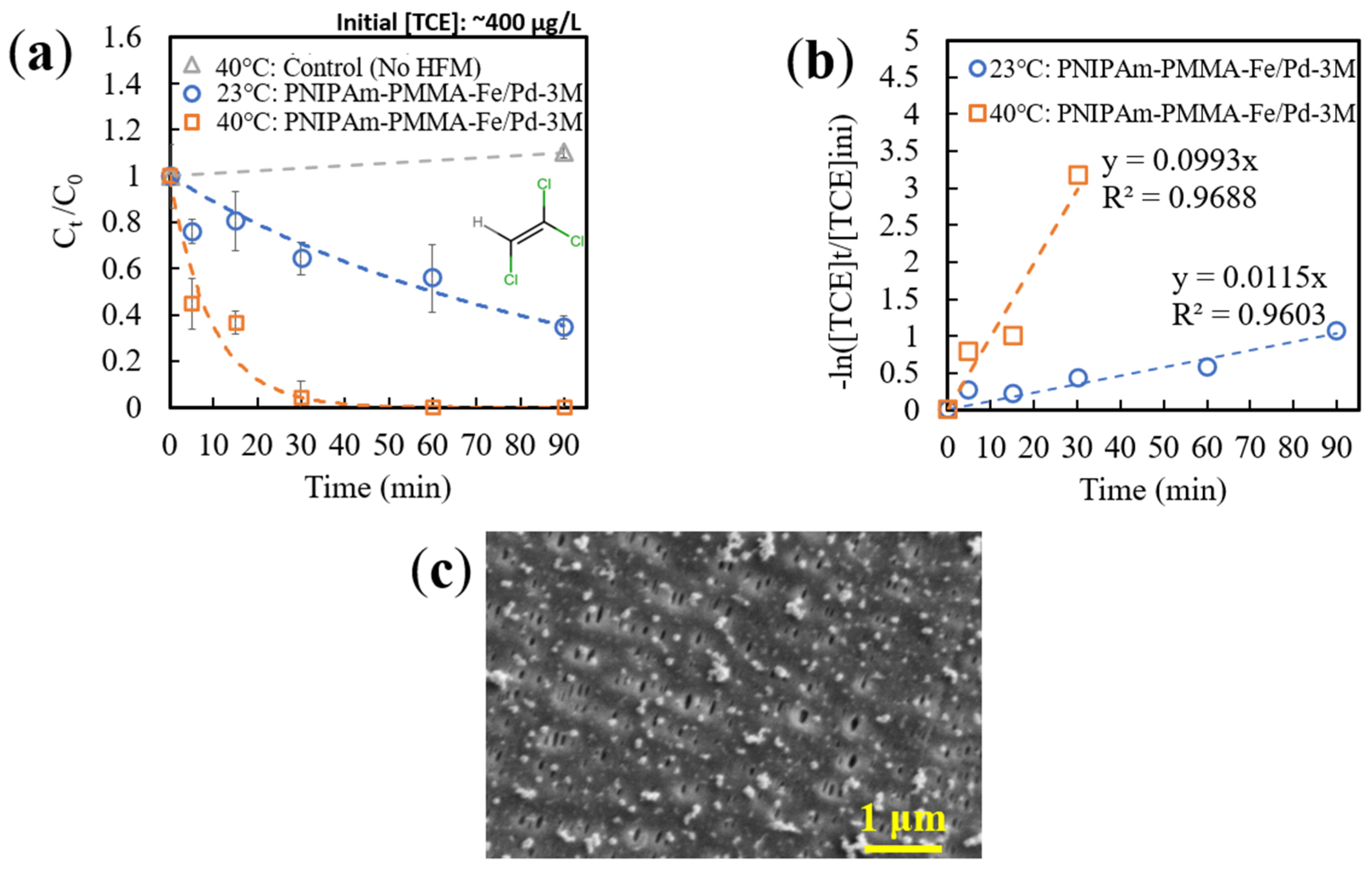
3.3.4. Thermoresponsive TCE Degradation via Catalytic Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, W.-K.; Park, K.-H. Heterogeneous photocatalysis of aromatic and chlorinated volatile organic compounds (VOCs) for non-occupational indoor air application. Chemosphere 2004, 57, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Schaum, J. Exposure assessment of trichloroethylene. Environ. Health Perspect. 2000, 108, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossett, J.M. Measurement of Henry’s law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 1987, 21, 202–208. [Google Scholar] [CrossRef]
- Chiu, W.A.; Jinot, J.; Scott, C.S.; Makris, S.L.; Cooper, G.S.; Dzubow, R.C.; Bale, A.S.; Evans, M.V.; Guyton, K.Z.; Keshava, N.; et al. Human health effects of trichloroethylene: Key findings and scientific issues. Environ. Health Perspect. 2013, 121, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Karami, S.; Lan, Q.; Rothman, N.; Stewart, P.A.; Lee, K.-M.; Vermeulen, R.; Moore, L.E. Occupational trichloroethylene exposure and kidney cancer risk: A meta-analysis. Occup. Environ. Med. 2012, 69, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, R.; Zhang, L.; Spierenburg, A.; Tang, X.; Bonventre, J.V.; Reiss, B.; Shen, M.; Smith, M.T.; Qiu, C.; Ge, Y.; et al. Elevated urinary levels of kidney injury molecule-1 among Chinese factory workers exposed to trichloroethylene. Carcinogenesis 2012, 33, 1538–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sohi, S.P.; Liu, S.; Guan, J.; Zhou, J.; Chen, J. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 2019, 235, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Hua, L.Q.; Nishijima, W.; Shoto, E.; Okada, M. Biodegradation of trichloroethylene (TCE) adsorbed on granular activated carbon (GAC). Water Res. 2000, 34, 4139–4142. [Google Scholar] [CrossRef]
- Liu, P.; Long, C.; Li, Q.; Qian, H.; Li, A.; Zhang, Q. Adsorption of trichloroethylene and benzene vapors onto hypercrosslinked polymeric resin. J. Hazard. Mater. 2009, 166, 46–51. [Google Scholar] [CrossRef]
- Erto, A.; Andreozzi, R.; Lancia, A.; Musmarra, D. Factors affecting the adsorption of trichloroethylene onto activated carbons. Appl. Surf. Sci. 2010, 256, 5237–5242. [Google Scholar] [CrossRef]
- Blankenship, A.; Chang, D.P.Y.; Jones, A.D.; Kelly, P.B.; Kennedy, I.M.; Matsumura, F.; Pasek, R.; Yang, G. Toxic combustion by-products from the incineration of chlorinated hydrocarbons and plastics. Chemosphere 1994, 28, 183–196. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Wang, C.P.; Fan, J.Z.; Sun, H.W. Aerobic degradation of trichloroethylene by co-metabolism using phenol and gasoline as growth substrates. Int. J. Mol. Sci. 2014, 15, 9134–9148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Hong, H.J.; Jung, J.; Kim, S.H.; Yang, J.W. Degradation of trichloroethylene (TCE) by nanoscale zero-valent iron (nZVI) immobilized in alginate bead. J. Hazard. Mater. 2010, 176, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Wu, S.-C. The Enhancement Methods for the Degradation of TCE by Zero-valent Metals. Chemosphere 2000, 41, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Su, J.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Functional clay supported bimetallic nZVI/Pd nanoparticles used for removal of methyl orange from aqueous solution. J. Hazard. Mater. 2013, 262, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.; Mills, R.; Wan, H.; Mottaleb, M.A.; Ormsbee, L.; Bhattacharyya, D. Thermo-responsive adsorption-desorption of perfluoroorganics from water using PNIPAm hydrogels and pore functionalized membranes. J. Memb. Sci. 2020, 599, 117821. [Google Scholar] [CrossRef]
- Saad, A.; Mills, R.; Wan, H.; Ormsbee, L.; Bhattacharyya, D. Thermoresponsive PNIPAm–PMMA-Functionalized PVDF Membranes with Reactive Fe–Pd Nanoparticles for PCB Degradation. Ind. Eng. Chem. Res. 2020, 59, 16614–16625. [Google Scholar] [CrossRef]
- Xiao, L.; Davenport, D.M.; Ormsbee, L.; Bhattacharyya, D. Polymerization and Functionalization of Membrane Pores for Water Related Applications. Ind. Eng. Chem. Res. 2015, 54, 4174–4182. [Google Scholar] [CrossRef]
- Lewis, S.; Smuleac, V.; Montague, A.; Bachas, L.; Bhattacharyya, D. Iron-Functionalized Membranes for Nanoparticle Synthesis and Reactions. Sep. Sci. Technol. 2009, 44, 3289–3311. [Google Scholar] [CrossRef] [Green Version]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Jain, K.; Vedarajan, R.; Watanabe, M.; Ishikiriyama, M.; Matsumi, N. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym. Chem. 2015, 6, 6819–6825. [Google Scholar] [CrossRef]
- Krzeminski, P.; Gil, J.A.; van Nieuwenhuijzen, A.F.; van der Graaf, J.H.J.M.; van Lier, J.B. Flat sheet or hollow fibre—comparison of full-scale membrane bio-reactor configurations. Desalination Water Treat. 2012, 42, 100–106. [Google Scholar] [CrossRef]
- Albu, P.C.; Ferencz, A.; Al-Ani, H.N.A.; Tanczos, S.-K.; Oprea, O.; Grosu, V.-A.; Nechifor, G.; Bungău, S.G.; Grosu, A.R.; Goran, A.; et al. Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes 2022, 12, 51. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, D.; Zhang, Z.; Yao, J.; Militky, J.; Wiener, J.; Zhu, G.; Zhang, G. Fabrication of Manganese Oxide/PTFE Hollow Fiber Membrane and Its Catalytic Degradation of Phenol. Materials 2021, 14, 3651. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef]
- Al-Qaradawi, S.; Salman, S.R. Photocatalytic degradation of methyl orange as a model compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.; Chen, J.; Phanichphant, S. Photocatalytic degradation of methyl orange by CeO2 and Fe-doped CeO2 films under visible light irradiation. Sci. Rep. 2014, 4, 5757. [Google Scholar] [CrossRef] [Green Version]
- Naik, A.P.; Mittal, H.; Wadi, V.S.; Sane, L.; Raj, A.; Alhassan, S.M.; Al Alili, A.; Bhosale, S.V.; Morajkar, P.P. Super porous TiO2 photocatalyst: Tailoring the agglomerate porosity into robust structural mesoporosity with enhanced surface area for efficient remediation of azo dye polluted waste water. J. Environ. Manag. 2020, 258, 110029. [Google Scholar] [CrossRef]
- Wang, J.; Qin, L.; Lin, J.; Zhu, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Enzymatic construction of antibacterial ultrathin membranes for dyes removal. Chem. Eng. J. 2017, 323, 56–63. [Google Scholar] [CrossRef]
- Chung, K.T. Azo dyes and human health: A review. J. Env. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Bishop, P.L.; Agha, A.M. Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag. 1994, 14, 127–137. [Google Scholar] [CrossRef]
- Hou, Y.; Mi, K.; Sun, L.; Zhou, K.; Wang, L.; Zhang, L.; Liu, Z.; Huang, L. The Application of Hollow Fiber Cartridge in Biomedicine. Pharmaceutics 2022, 14, 1485. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Ormsbee, L.E.; Bhattacharyya, D. Reactive Functionalized Membranes for Polychlorinated Biphenyl Degradation. Ind. Eng. Chem. Res. 2013, 52, 10430–10440. [Google Scholar] [CrossRef] [Green Version]
- Baldridge, K.C.; Edmonds, K.; Dziubla, T.; Hilt, J.Z.; Dutch, R.E.; Bhattacharyya, D. Demonstration of Hollow Fiber Membrane-Based Enclosed Space Air Remediation for Capture of an Aerosolized Synthetic SARS-CoV-2 Mimic and Pseudovirus Particles. ACS EST Eng. 2022, 2, 251–262. [Google Scholar] [CrossRef]
- Mills, R.; Vogler, R.J.; Bernard, M.; Concolino, J.; Hersh, L.B.; Wei, Y.; Hastings, J.T.; Dziubla, T.; Baldridge, K.C.; Bhattacharyya, D. Aerosol capture and coronavirus spike protein deactivation by enzyme functionalized antiviral membranes. Commun. Mater. 2022, 3, 34. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Zrimsek, A.B.; Bykov, S.V.; Jakubek, R.S.; Asher, S.A. Hydrophobic Collapse Initiates the Poly(N-isopropylacrylamide) Volume Phase Transition Reaction Coordinate. J. Phys. Chem. B 2018, 122, 3008–3014. [Google Scholar] [CrossRef]
- Sun, S.; Hu, J.; Tang, H.; Wu, P. Chain Collapse and Revival Thermodynamics of Poly(N-isopropylacrylamide) Hydrogel. J. Phys. Chem. B 2010, 114, 9761–9770. [Google Scholar] [CrossRef]
- Wan, H.; Islam, M.S.; Briot, N.J.; Schnobrich, M.; Pacholik, L.; Ormsbee, L.; Bhattacharyya, D. Pd/Fe nanoparticle integrated PMAA-PVDF membranes for chloro-organic remediation from synthetic and site groundwater. J. Membr. Sci. 2020, 594, 117454. [Google Scholar] [CrossRef]
- Sathya, S.; Murthy, P.S.; Das, A.; Gomathi Sankar, G.; Venkatnarayanan, S.; Pandian, R.; Sathyaseelan, V.S.; Pandiyan, V.; Doble, M.; Venugopalan, V.P. Marine antifouling property of PMMA nanocomposite films: Results of laboratory and field assessment. Int. Biodeterior. Biodegrad. 2016, 114, 57–66. [Google Scholar] [CrossRef]
- Mills, R.; Baldridge, K.C.; Bernard, M.; Bhattacharyya, D. Recent advances in responsive membrane functionalization approaches and applications. Sep. Sci. Technol. 2022, 58, 1202–1236. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Yap Ang, M.B.M.; Patra, T.; Castilla-Casadiego, D.A.; Fan, R.; Almodovar, J.; Hung, W.-S.; Wickramasinghe, S.R. High-Performance Polyacrylic Acid-Grafted PVDF Nanofiltration Membrane with Good Antifouling Property for the Textile Industry. Polymers 2020, 12, 2443. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.C.; Arning, A. Performance Comparison between Polyvinylidene Fluoride and Polytetrafluoroethylene Hollow Fiber Membranes for Direct Contact Membrane Distillation. Membranes 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Li, S.; Liang, X. High-Performance Catalytic Four-Channel Hollow Fibers with Highly Dispersed Nickel Nanoparticles Prepared by Atomic Layer Deposition for Dry Reforming of Methane. Ind. Eng. Chem. Res. 2022, 61, 10377–10386. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.; Choi, W. Effect of magnetic field on the zero valent iron induced oxidation reaction. J. Hazard. Mater. 2011, 192, 928–931. [Google Scholar] [CrossRef]
- Wan, H.; Briot, N.J.; Saad, A.; Ormsbee, L.; Bhattacharyya, D. Pore Functionalized PVDF Membranes with In-Situ Synthesized Metal Nanoparticles: Material Characterization, and Toxic Organic Degradation. J. Memb. Sci. 2017, 530, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Bhattacharyya, D. Modeling of Fe/Pd Nanoparticle-Based Functionalized Membrane Reactor for PCB Dechlorination at Room Temperature. J. Phys. Chem. C Nanomater. Interfaces 2008, 112, 9133–9144. [Google Scholar] [CrossRef]
- Xiao, L.; Isner, A.B.; Hilt, J.Z.; Bhattacharyya, D. Temperature responsive hydrogel with reactive nanoparticles. J. Appl. Polym. Sci. 2013, 128, 1804–1814. [Google Scholar] [CrossRef]
- Sinek, A.; Kupczak, M.; Mielańczyk, A.; Lemanowicz, M.; Yusa, S.I.; Neugebauer, D.; Gierczycki, A. Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions. Polymers 2020, 12, 486. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, D.; Köber, R.; Dahmke, A. Competing TCE and cis-DCE degradation kinetics by zero-valent iron—Experimental results and numerical simulation. J. Contam. Hydrol. 2003, 65, 183–202. [Google Scholar] [CrossRef]
- Tee, Y.-H.; Bachas, L.; Bhattacharyya, D. Degradation of Trichloroethylene by Iron-Based Bimetallic Nanoparticles. J. Phys. Chem. C 2009, 113, 9454–9464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubendiran, H.; Hui, D.; Pulimi, M.; Chandrasekaran, N.; Murthy, P.S.; Mukherjee, A. Removal of methyl orange from aqueous solution using SRB supported Bio-Pd/Fe NPs. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100561. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Santhosh, S.; Sudakaran, S.V.; Nancharaiah, Y.V.; Mrudula, P.; Chandrasekaran, N.; Mukherjee, A. Biogenic nano zero valent iron (Bio-nZVI) anaerobic granules for textile dye removal. J. Environ. Chem. Eng. 2018, 6, 1683–1689. [Google Scholar] [CrossRef]
- Arshadi, M.; SalimiVahid, F.; Salvacion, J.W.L.; Soleymanzadeh, M. Adsorption studies of methyl orange on an immobilized Mn-nanoparticle: Kinetic and thermodynamic. RSC Adv. 2014, 4, 16005–16017. [Google Scholar] [CrossRef]
- Shih, Y.-h.; Tso, C.-P.; Tung, L.-Y. Rapid degradation of methyl orange with nanoscale zerovalent iron particles. Sustain. Environ. Res. 2010, 20, 137–143. [Google Scholar]
- Yang, C.; Nuxoll, E.E.; Cussler, E.L. Reactive barrier films. AIChE J. 2001, 47, 295–302. [Google Scholar] [CrossRef]
- Xu, J.; Bhattacharyya, D. Fe/Pd Nanoparticle Immobilization in Microfiltration Membrane Pores: Synthesis, Characterization, and Application in the Dechlorination of Polychlorinated Biphenyls. Ind. Eng. Chem. Res. 2007, 46, 2348–2359. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Ren, B.; Huang, Y.; Wang, D.; Liao, Z.; Li, Q.; Wang, L.; Dionysiou, D.D. Polymeric ultrafiltration membrane with in situ formed nano-silver within the inner pores for simultaneous separation and catalysis. J. Membr. Sci. 2019, 579, 190–198. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hernández, S.; Lei, S.; Rong, W.; Ormsbee, L.; Bhattacharyya, D. Functionalization of flat sheet and hollow fiber microfiltration membranes for water applications. ACS Sustain. Chem. Eng. 2016, 4, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Niño de Guzmán, G.T.; Hapeman, C.J.; Millner, P.D.; Torrents, A.; Jackson, D.; Kjellerup, B.V. Using adaptive management to guide multiple partners in TCE remediation using a permeable reactive barrier. Environ. Res. Commun. 2019, 1, 075001. [Google Scholar] [CrossRef]
- Khan, M.A.; Lipscomb, G.; Lin, A.; Baldridge, K.C.; Petersen, E.M.; Steele, J.; Abney, M.B.; Bhattacharyya, D. Performance evaluation and model of spacesuit cooling by hydrophobic hollow fiber-membrane based water evaporation through pores. J. Membr. Sci. 2023, 673, 121497. [Google Scholar] [CrossRef]
- Mahmud, H.; Kumar, A.; Narbaitz, R.M.; Matsuura, T. Membrane Air Stripping: A Process for Removal of Organics from Aqueous Solutions. Sep. Sci. Technol. 1998, 33, 2241–2255. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Arnold, R.G.; Sáez, A.E.; Betterton, E.A.; Ela, W.P. Removal of Aqueous Phase Trichloroethylene Using Membrane Air Stripping Contactors. J. Environ. Eng. 2004, 130, 1232–1241. [Google Scholar] [CrossRef]
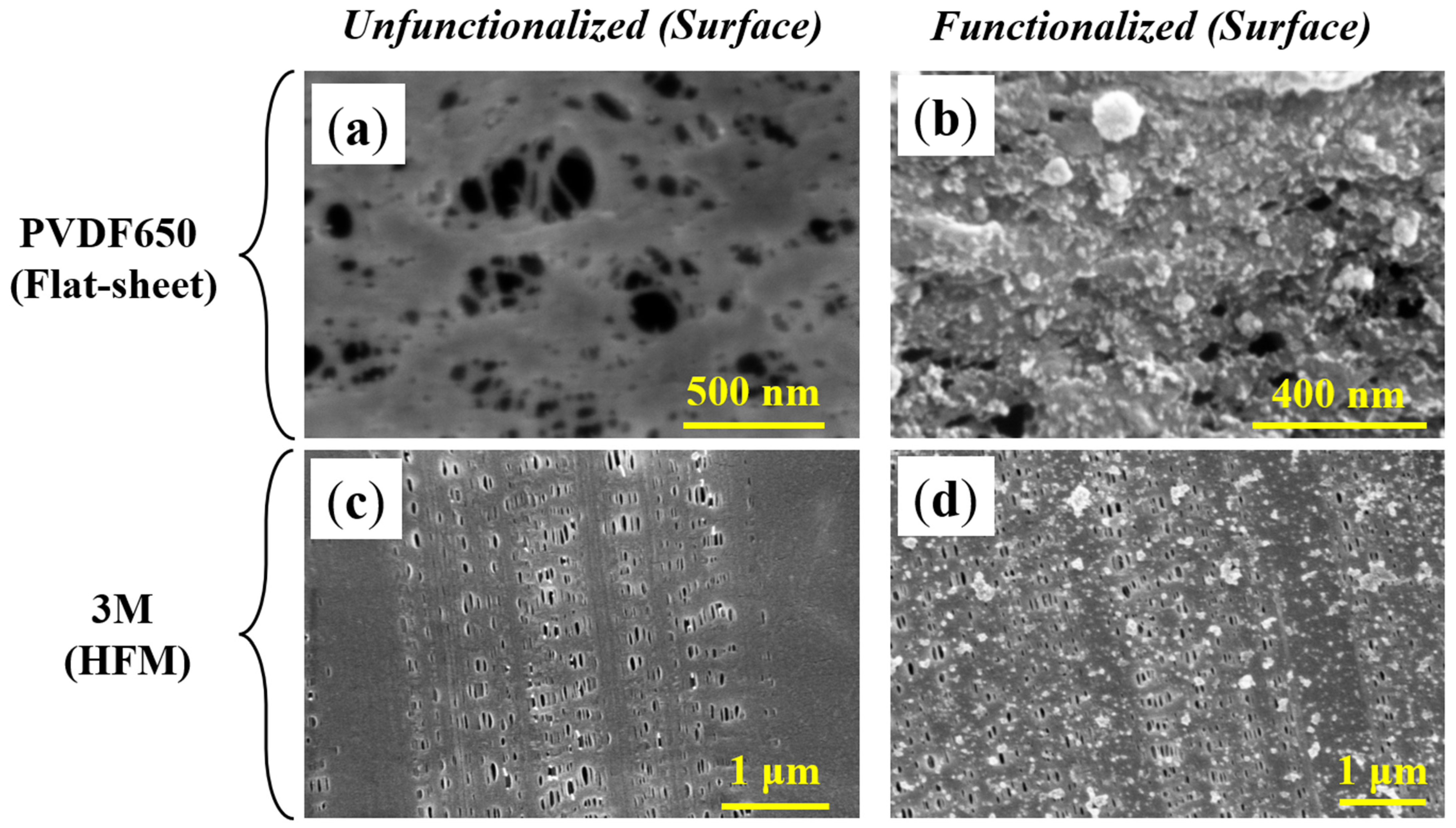
| PVDF400-Flat Sheet | PVDF650-Flat Sheet | Tribore-HFM | 3M-HFM | Lifestraw-HFM | |
|---|---|---|---|---|---|
| Material | Hydrophilized polyvinylidene fluoride (PVDF) | Hydrophilized polyvinylidene fluoride (PVDF) | Hydrophobic polyvinylidene fluoride (PVDF) *1 | Hydrophobic polypropylene (PP) *1 | Hydrophilized polyether sulfone (hPES) *1 |
| Mean pore size | 40.6 nm, with the largest being 300–400 nm (SEM) | 62.2 nm, with the largest being 300–400 nm (SEM) | 1153 nm | 40 nm (manufacturer) | 200 nm (manufacturer) |
| Thickness | 178 ± 13 μm | 178 ± 13 μm | 412.6 ± 106.2 μm *1 | 41.8 ± 2.2 μm | 85.5 ± 3.5 μm |
| Bulk porosity | 46% *2 | 47% | 24% *1 | 24% *1 or 40% (manufacturer) | 45% *1 |
| Shell surface mean pore size | Not applicable | Not applicable | 57 ± 35 nm *1 | 42 ± 17 nm *1 | 140 ± 87 nm *1 |
| Lumen surface mean pore size | Not applicable | Not applicable | 1346 ± 1086 *1 | 46 ± 27 nm *1 | 748 ± 896 nm *1 |
| Tortuosity | Not measured | Not measured | 3.1692 *1 | Not measured | Not measured |
| T (°C) | kobs (1/min) | ρm (g/L) | as (m2/g) | ksa (LMH) | Temperature-Normalized ksa (LMH) * |
|---|---|---|---|---|---|
| 22.5 °C | 0.0208 | 5 | 21.2 | 0.0118 | 0.0118 |
| 50 °C | 0.0424 | 5 | 21.2 | 0.0240 | 0.0108 |
| T (°C) | kobs (1/min) | ρm (g/L) | as (m2/g) | ksa (LMH) | Temperature-Normalized ksa (LMH) * |
|---|---|---|---|---|---|
| 23 °C | 0.0114 | 0.05 | 21.9 | 0.6247 | 0.6247 |
| 40 °C | 0.0247 | 0.05 | 21.9 | 1.3534 | 0.8147 |
| T (°C) | kobs (1/min) | ρm (g/L) | as (m2/g) | ksa (LMH) | Temperature-Normalized ksa (LMH) * |
|---|---|---|---|---|---|
| 23 °C | 0.0115 | 0.06 | 21.9 | 0.5251 | 0.5251 |
| 40 °C | 0.0993 | 0.06 | 21.9 | 4.5342 | 2.7293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mills, R.; Tvrdik, C.; Lin, A.; Bhattacharyya, D. Enhanced Degradation of Methyl Orange and Trichloroethylene with PNIPAm-PMMA-Fe/Pd-Functionalized Hollow Fiber Membranes. Nanomaterials 2023, 13, 2041. https://doi.org/10.3390/nano13142041
Mills R, Tvrdik C, Lin A, Bhattacharyya D. Enhanced Degradation of Methyl Orange and Trichloroethylene with PNIPAm-PMMA-Fe/Pd-Functionalized Hollow Fiber Membranes. Nanomaterials. 2023; 13(14):2041. https://doi.org/10.3390/nano13142041
Chicago/Turabian StyleMills, Rollie, Cameron Tvrdik, Andrew Lin, and Dibakar Bhattacharyya. 2023. "Enhanced Degradation of Methyl Orange and Trichloroethylene with PNIPAm-PMMA-Fe/Pd-Functionalized Hollow Fiber Membranes" Nanomaterials 13, no. 14: 2041. https://doi.org/10.3390/nano13142041
APA StyleMills, R., Tvrdik, C., Lin, A., & Bhattacharyya, D. (2023). Enhanced Degradation of Methyl Orange and Trichloroethylene with PNIPAm-PMMA-Fe/Pd-Functionalized Hollow Fiber Membranes. Nanomaterials, 13(14), 2041. https://doi.org/10.3390/nano13142041





