Microsecond All-Optical Modulation by Biofunctionalized Porous Silicon Microcavity
Abstract
1. Introduction
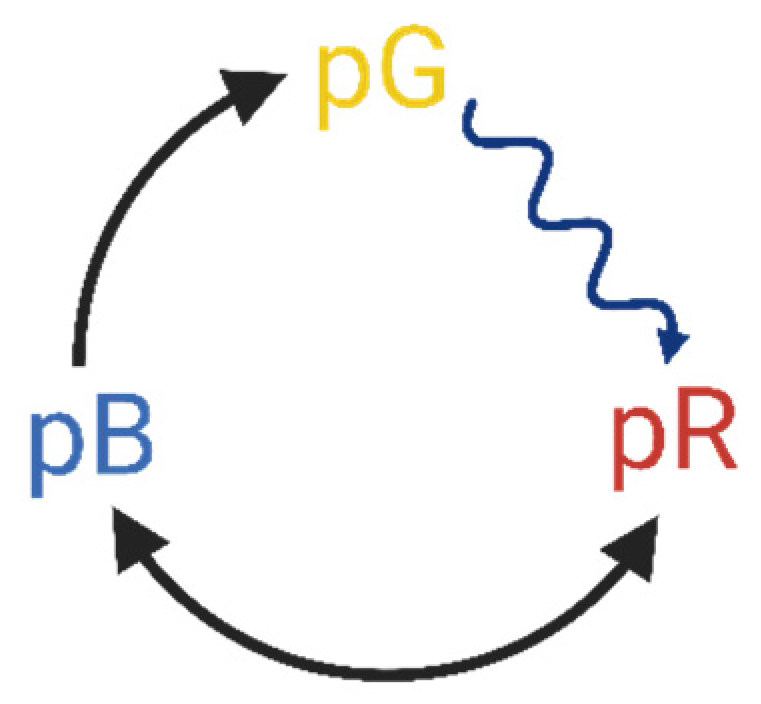
1.1. Optical Switching by Chromoproteins
1.2. Porous Silicon Structures
1.3. Aim of the Study
2. Materials and Methods
2.1. PYP Sample Preparation
2.2. Porous Silicon Microcavity (PSiMc) Fabrication
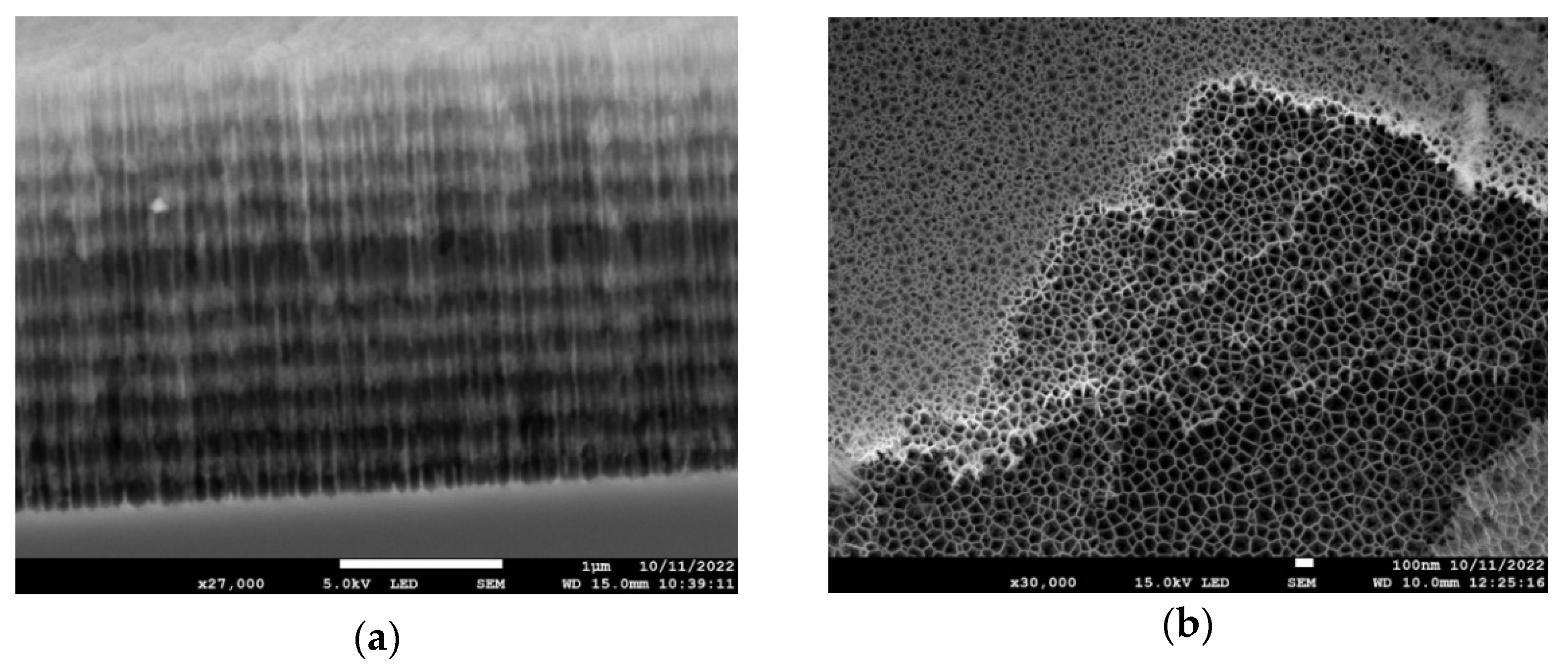
2.3. Scanning Electron Microscopy
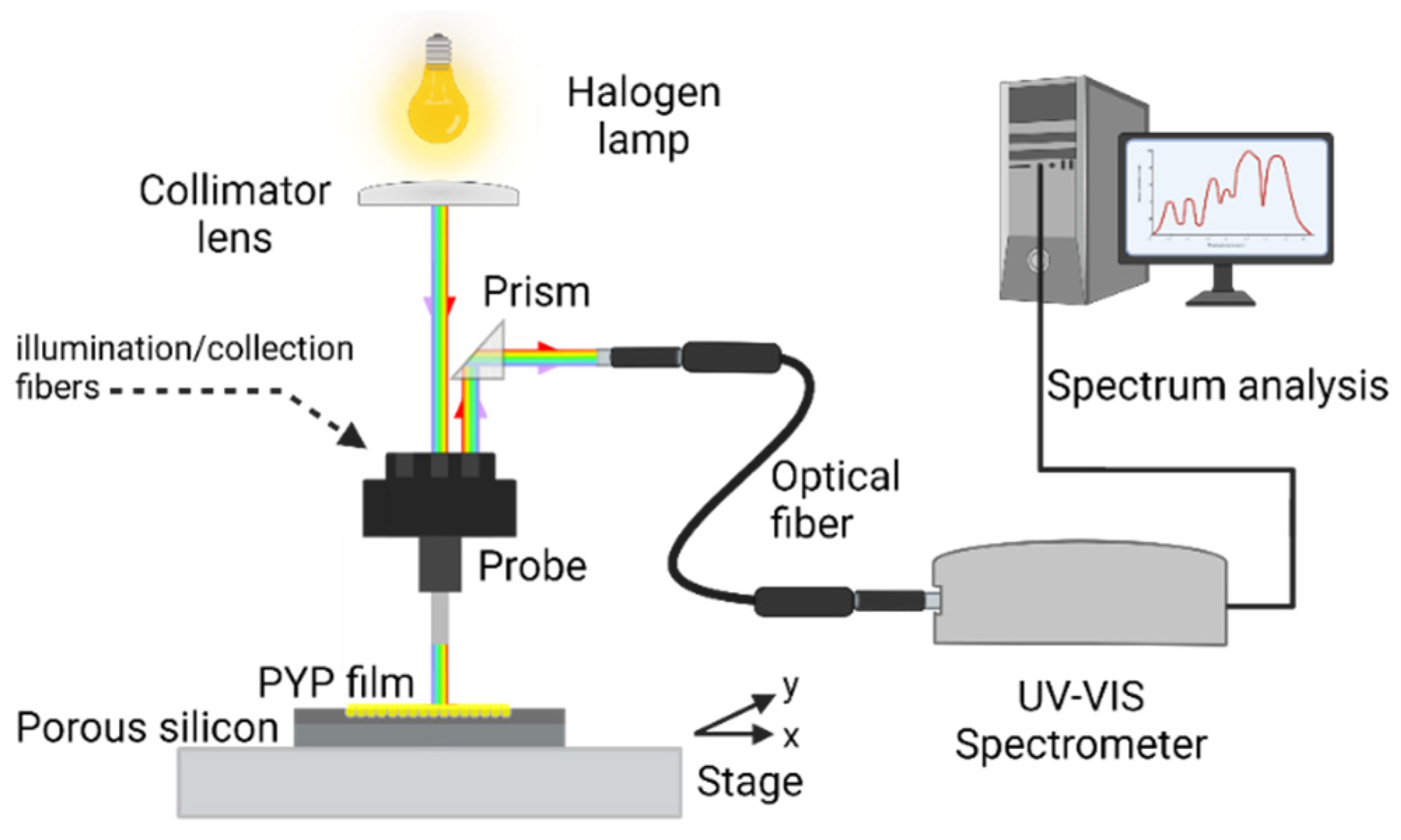
2.4. Experimental Setup
2.4.1. PYP Infiltrated PSiMc Reflectance Measurements
2.4.2. Photoinduced All-Optical Modulation
3. Results and Discussion
3.1. Structure of the PSi Microcavity Multilayer
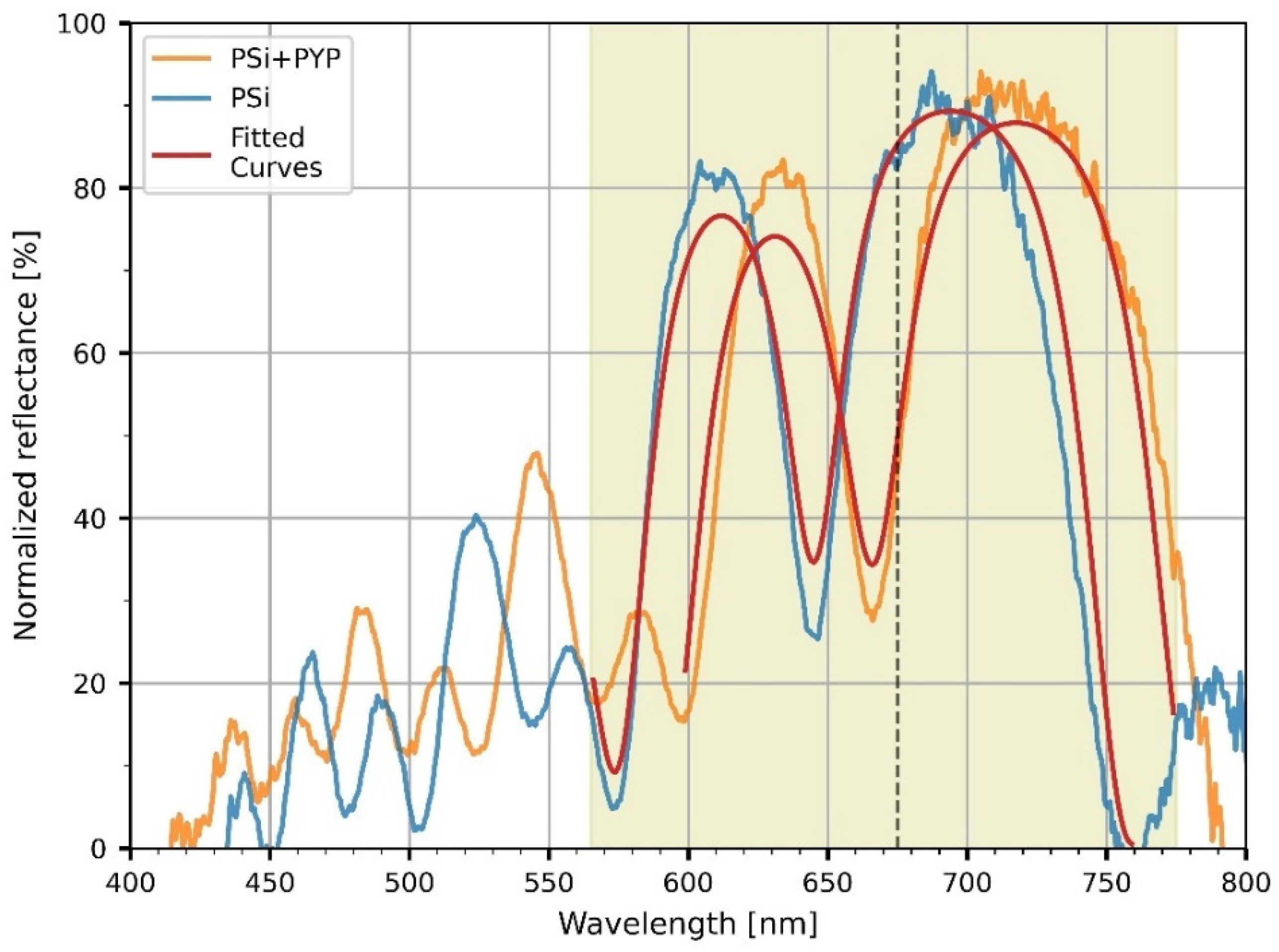
3.2. PYP-Functionalization of PSiMcs
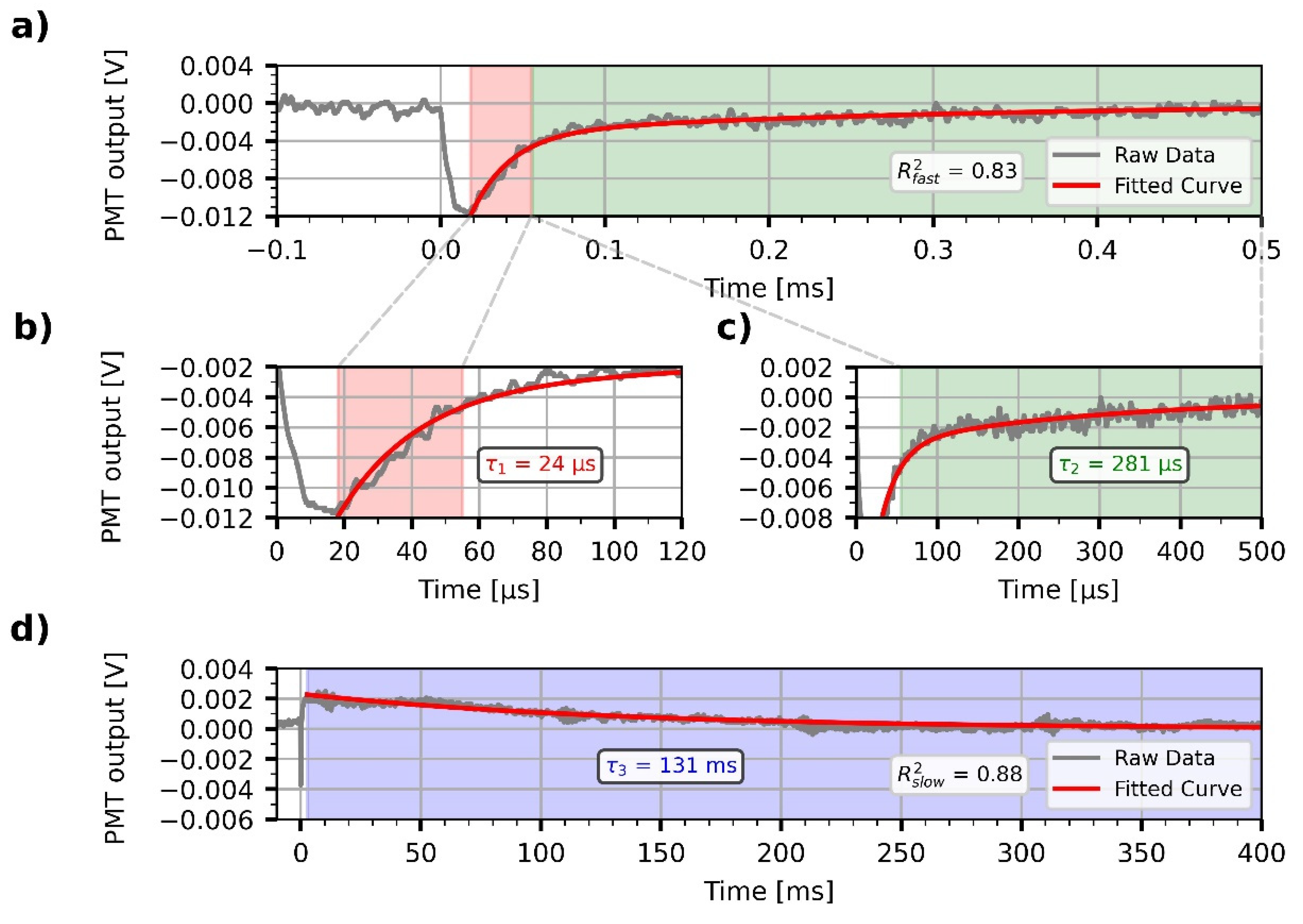
3.3. Demonstration of All-Optical Modulation on the Biofunctionalized PSiMc Samples
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ormos, P.; Fábián, L.; Oroszi, L.; Wolff, E.K.; Ramsden, J.J.; Dér, A. Protein-Based Integrated Optical Switching and Modulation. Appl. Phys. Lett. 2002, 80, 4060–4062. [Google Scholar] [CrossRef]
- Fábián, L.; Wolff, E.K.; Oroszi, L.; Ormos, P.; Dér, A. Fast Integrated Optical Switching by the Protein Bacteriorhodopsin. Appl. Phys. Lett. 2010, 97, 023305. [Google Scholar] [CrossRef]
- Fábián, L.; Heiner, Z.; Mero, M.; Kiss, M.; Wolff, E.K.; Ormos, P.; Osvay, K.; Dér, A. Protein-Based Ultrafast Photonic Switching. Opt. Express 2011, 19, 18861. [Google Scholar] [CrossRef]
- Topolancik, J.; Vollmer, F. All-Optical Switching in the near Infrared with Bacteriorhodopsin-Coated Microcavities. Appl. Phys. Lett. 2006, 89, 184103. [Google Scholar] [CrossRef]
- Roy, S.; Sethi, P.; Topolancik, J.; Vollmer, F. All-Optical Reversible Logic Gates with Optically Controlled Bacteriorhodopsin Protein-Coated Microresonators. Adv. Opt. Technol. 2012, 2012, 727206. [Google Scholar] [CrossRef]
- Roy, S.; Prasad, M.; Topolancik, J.; Vollmer, F. All-Optical Switching with Bacteriorhodopsin Protein Coated Microcavities and Its Application to Low Power Computing Circuits. J. Appl. Phys. 2010, 107, 053115. [Google Scholar] [CrossRef]
- Fábián, L.; Mathesz, A.; Dér, A. New Trends in Biophotonics. Acta Biol. Szeged. 2015, 59, 189–202. [Google Scholar]
- Topolancik, J.; Vollmer, F. Photoinduced Transformations in Bacteriorhodopsin Membrane Monitored with Optical Microcavities. Biophys. J. 2007, 92, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Dér, A.; Valkai, S.; Fábián, L.; Ormos, P.; Ramsden, J.J.; Wolff, E.K. Integrated Optical Switching Based on the Protein Bacteriorhodopsin. Photochem. Photobiol. 2007, 83, 393–396. [Google Scholar] [CrossRef]
- Stuart, J.A.; Marcy, D.L.; Wise, K.J.; Birge, R.R. Volumetric Optical Memory Based on Bacteriorhodopsin. Synth. Met. 2002, 127, 3–15. [Google Scholar] [CrossRef]
- Hristova, S.G.; Varo, G.; Der, A. Long Time Stability of Purple Membranes from Halobacterium Halobium. Acta Biochim. Biophys. Acad. Sci. Hung. 1984, 19, 215–219. [Google Scholar] [PubMed]
- Hampp, N.; Oesterhelt, D. Bacteriorhodopsin and Its Potential in Technical Applications. In Nanobiotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 146–167. [Google Scholar]
- Krekic, S.; Nagy, D.; Taneva, S.G.; Fábián, L.; Zimányi, L.; Dér, A. Spectrokinetic Characterization of Photoactive Yellow Protein Films for Integrated Optical Applications. Eur. Biophys. J. 2019, 48, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Krekic, S.; Zakar, T.; Gombos, Z.; Valkai, S.; Mero, M.; Zimányi, L.; Heiner, Z.; Dér, A. Nonlinear Optical Investigation of Microbial Chromoproteins. Front. Plant Sci. 2020, 11, 547818. [Google Scholar] [CrossRef]
- Petrovszki, D.; Krekic, S.; Valkai, S.; Heiner, Z.; Dér, A. All-Optical Switching Demonstrated with Photoactive Yellow Protein Films. Biosensors 2021, 11, 432. [Google Scholar] [CrossRef]
- Krekic, S.; Mero, M.; Dér, A.; Heiner, Z. Ultrafast All-Optical Switching Using Doped Chromoprotein Films. J. Phys. Chem. C 2023, 127, 1499–1506. [Google Scholar] [CrossRef]
- Meyer, T.E.; Yakali, E.; Cusanovich, M.A.; Tollin, G. Properties of a Water-Soluble, Yellow Protein Isolated from a Halophilic Phototrophic Bacterium That Has Photochemical Activity Analogous to Sensory Rhodopsin. Biochemistry 1987, 26, 418–423. [Google Scholar] [CrossRef]
- Kamikubo, H.; Shimizu, N.; Harigai, M.; Yamazaki, Y.; Imamoto, Y.; Kataoka, M. Characterization of the solution structure of the M intermediate of photoactive yellow protein using high-angle solution X-Ray scattering. Biophys. J. 2007, 92, 3633–3642. [Google Scholar] [CrossRef]
- Golovan, L.A. Nonlinear Optical Properties of Porous Silicon In Handbook of Porous Silicon; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 1–32. [Google Scholar] [CrossRef]
- Palestino, G.; Agarwal, V.; Aulombard, R.; Pérez, E.; Gergely, C. Biosensing and Protein Fluorescence Enhancement by Functionalized Porous Silicon Devices. Langmuir 2008, 24, 13765–13771. [Google Scholar] [CrossRef]
- Palestino, G.; Martin, M.; Agarwal, V.; Legros, R.; Cloitre, T.; Zimányi, L.; Gergely, C. Detection and Light Enhancement of Glucose Oxidase Adsorbed on Porous Silicon Microcavities. Proc. Phys. Status Solidi (C) Curr. Top. Solid State Phys. 2009, 6, 1624–1628. [Google Scholar] [CrossRef]
- Martin, M.; Palestino, G.; Cloitre, T.; Agarwal, V.; Zimányi, L.; Gergely, C. Three-Dimensional Spatial Resolution of the Nonlinear Photoemission from Biofunctionalized Porous Silicon Microcavity. Appl. Phys. Lett. 2009, 94, 223313. [Google Scholar] [CrossRef]
- Hajdu, K.; Gergely, C.; Martin, M.; Zimányi, L.; Agarwal, V.; Palestino, G.; Hernádi, K.; Németh, Z.; Nagy, L. Light-Harvesting Bio-Nanomaterial Using Porous Silicon and Photosynthetic Reaction Center. Nanoscale Res. Lett. 2012, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, K.; Gergely, C.; Martin, M.; Cloitre, T.; Zimányi, L.; Tenger, K.; Khoroshyy, P.; Palestino, G.; Agarwal, V.; Hernádi, K.; et al. Porous Silicon/Photosynthetic Reaction Center Hybrid Nanostructure. Langmuir 2012, 28, 11866–11873. [Google Scholar] [CrossRef] [PubMed]
- Márquez, J.; Cházaro-Ruiz, L.F.; Zimányi, L.; Palestino, G. Immobilization Strategies and Electrochemical Evaluation of Porous Silicon Based Cytochrome c Electrode. Electrochim. Acta 2014, 140, 550–556. [Google Scholar] [CrossRef]
- Agarwal, V.; del Río, J.A. Tailoring the Photonic Band Gap of a Porous Silicon Dielectric Mirror. Appl. Phys. Lett. 2003, 82, 1512–1514. [Google Scholar] [CrossRef]
- Agarwal, V.; del Río, J.A.; Malpuech, G.; Zamfirescu, M.; Kavokin, A.; Coquillat, D.; Scalbert, D.; Vladimirova, M.; Gil, B. Photon Bloch Oscillations in Porous Silicon Optical Superlattices. Phys. Rev. Lett. 2004, 92, 97401. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, D.A.G. Berechnung Verschiedener Physikalischer Konstanten von Heterogenen Substanzen. I. Dielektrizitätskonstanten Und Leitfähigkeiten Der Mischkörper Aus Isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Salzen, K.; Zhang, F. Explicit solutions to the mixing rules with three-component inclusions. J. Quant. Spectrosc. Radiat. Transf. 2018, 207, 78–82. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E.; Bhatia, A.B.; Clemmow, P.C.; Gabor, D.; Stokes, A.R.; Taylor, A.M.; Wayman, P.A.; Wilcock, W.L. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, 7th ed.; Cambridge University Press: Singapore, 1999; ISBN 9780521642224. [Google Scholar]
- Fábián, L.; Krekic, S.; Tóth-Boconádi, R.; Taneva, S.G.; Bálint, A.M.; Nánai, L.; Dér, A. Integrated optical investigation of two light-sensitive proteins. In Proceedings of the High Energy Gamma-Ray Astronomy: 6th International Meeting on High Energy Gamma-Ray Astronomy, Heidelberg, Germany, 4 January 2017; p. 040001. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Z.; Lv, X.; Liu, Y. Design, analysis and optimization of porous silicon microcavity based on silicon-on-insulator at optical communication wavelengths. Optik 2014, 125, 557–560. [Google Scholar] [CrossRef]
- Reece, P.J.; Lerondel, G.; Mulders, J.; Zheng, W.H.; Gal, M. Fabrication and tuning of high quality porous silicon microcavities. Phys. Stat. Sol. (A) 2003, 197, 321–325. [Google Scholar] [CrossRef]
- Cencha, L.G.; Antonio Hernández, C.; Forzani, L.; Urteaga, R.; Koropecki, R.R. Optical performance of hybrid porous silicon–porous alumina multilayers. J. Appl. Phys. 2018, 123, 183101. [Google Scholar] [CrossRef]
- Ivanov, I.; Skryshevsky, V.; Belouci, A. Engineering Porous Silicon-Based Microcavity for Chemical Sensing. ACS Omega 2023, 8, 21265–21276. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovszki, D.; Valkai, S.; Kelemen, L.; Nagy, L.; Agarwal, V.; Krekic, S.; Zimányi, L.; Dér, A. Microsecond All-Optical Modulation by Biofunctionalized Porous Silicon Microcavity. Nanomaterials 2023, 13, 2070. https://doi.org/10.3390/nano13142070
Petrovszki D, Valkai S, Kelemen L, Nagy L, Agarwal V, Krekic S, Zimányi L, Dér A. Microsecond All-Optical Modulation by Biofunctionalized Porous Silicon Microcavity. Nanomaterials. 2023; 13(14):2070. https://doi.org/10.3390/nano13142070
Chicago/Turabian StylePetrovszki, Dániel, Sándor Valkai, Lóránd Kelemen, László Nagy, Vivechana Agarwal, Szilvia Krekic, László Zimányi, and András Dér. 2023. "Microsecond All-Optical Modulation by Biofunctionalized Porous Silicon Microcavity" Nanomaterials 13, no. 14: 2070. https://doi.org/10.3390/nano13142070
APA StylePetrovszki, D., Valkai, S., Kelemen, L., Nagy, L., Agarwal, V., Krekic, S., Zimányi, L., & Dér, A. (2023). Microsecond All-Optical Modulation by Biofunctionalized Porous Silicon Microcavity. Nanomaterials, 13(14), 2070. https://doi.org/10.3390/nano13142070










