MOF-Derived Co Nanoparticles Catalyst Assisted by F- and N-Doped Carbon Quantum Dots for Oxygen Reduction
Abstract
:1. Introduction
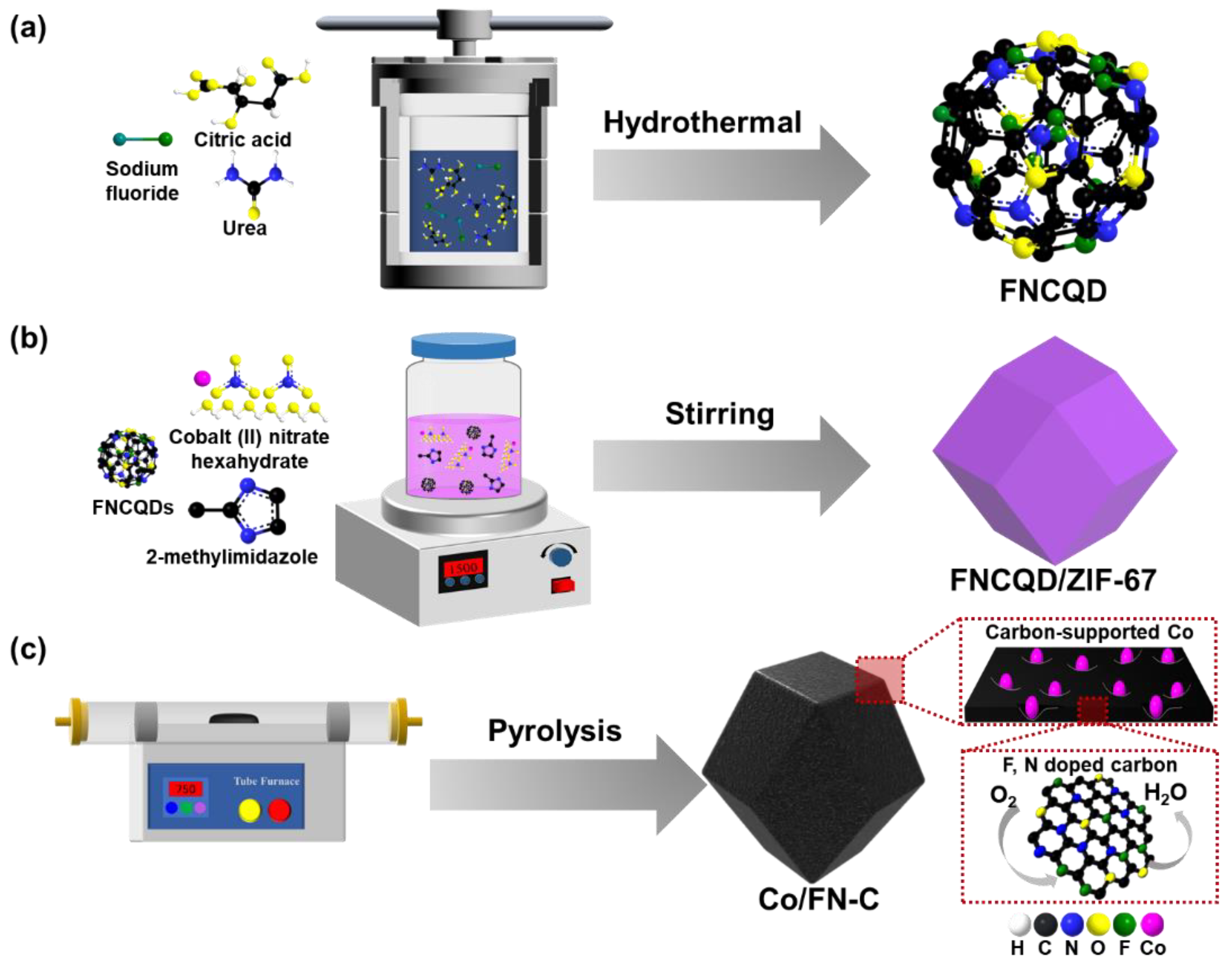
2. Materials and Methods
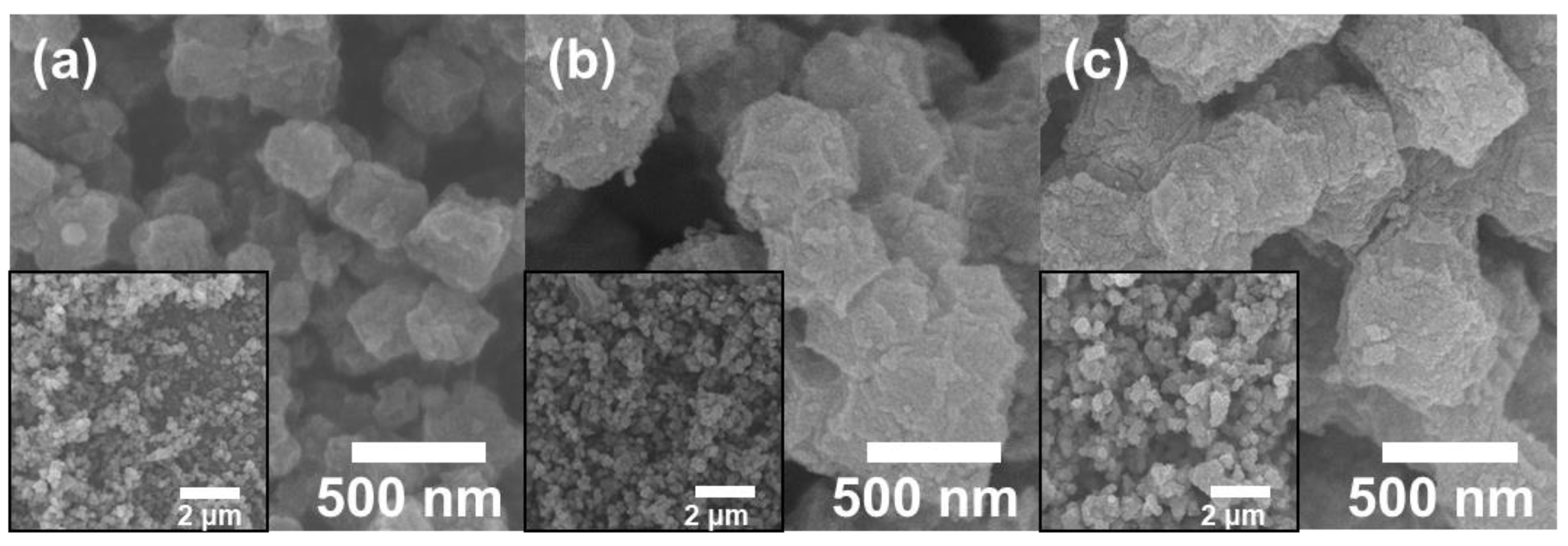
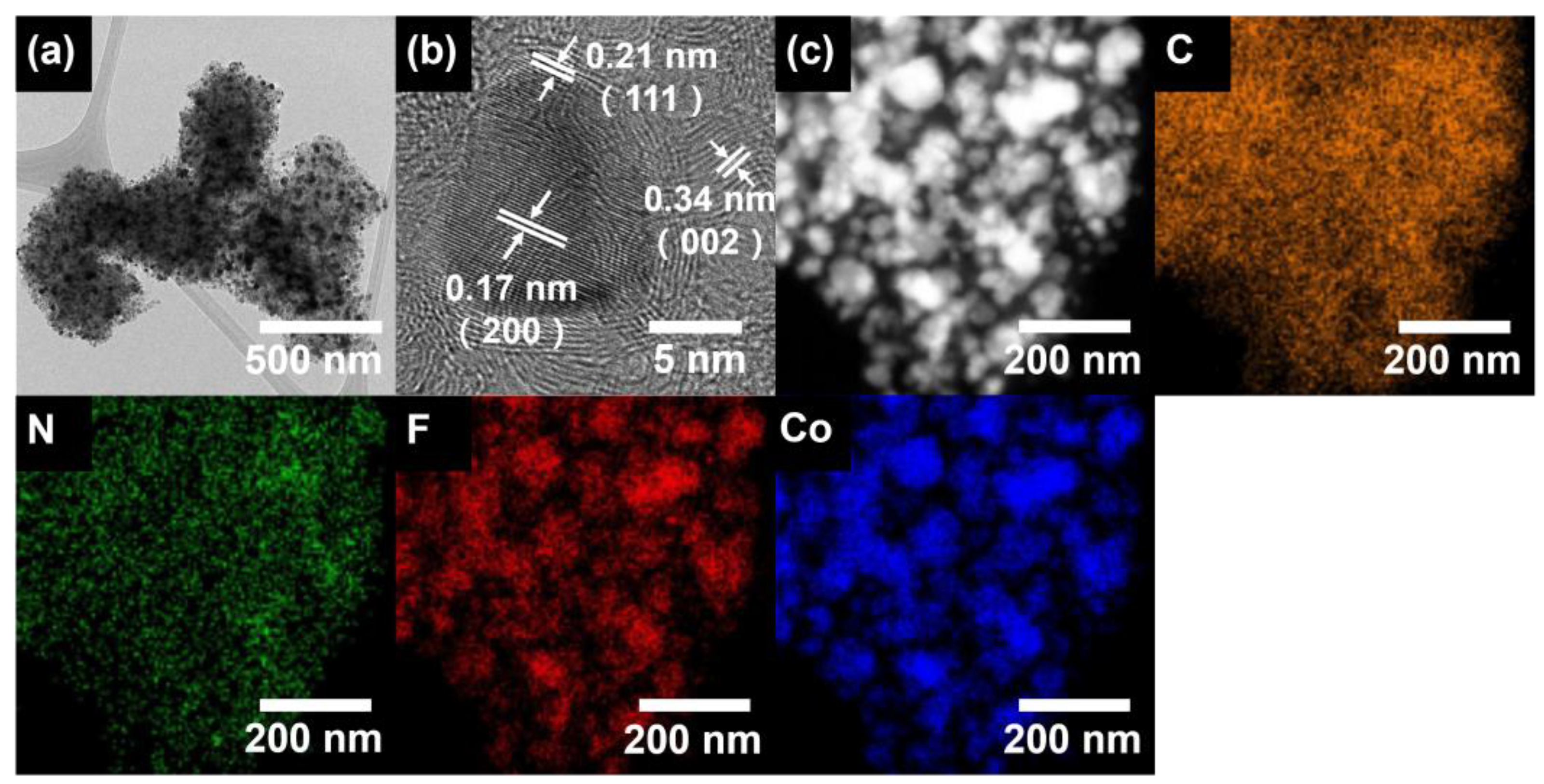
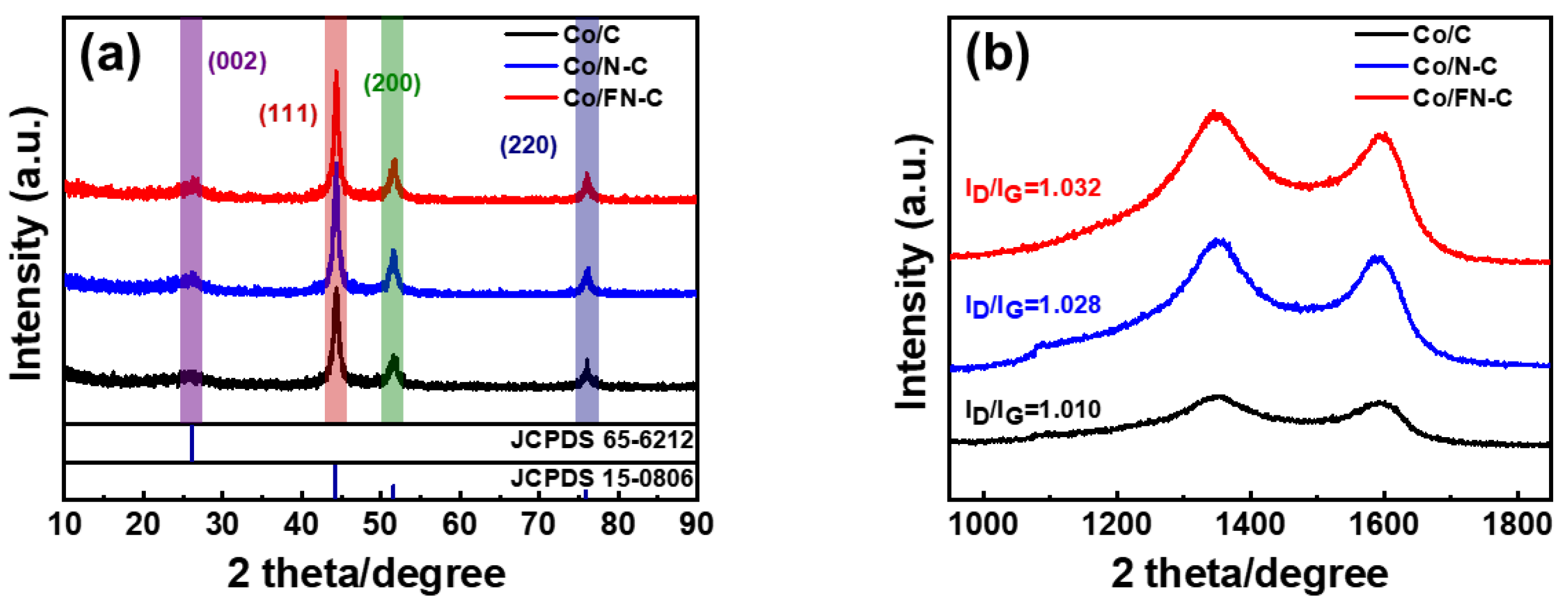
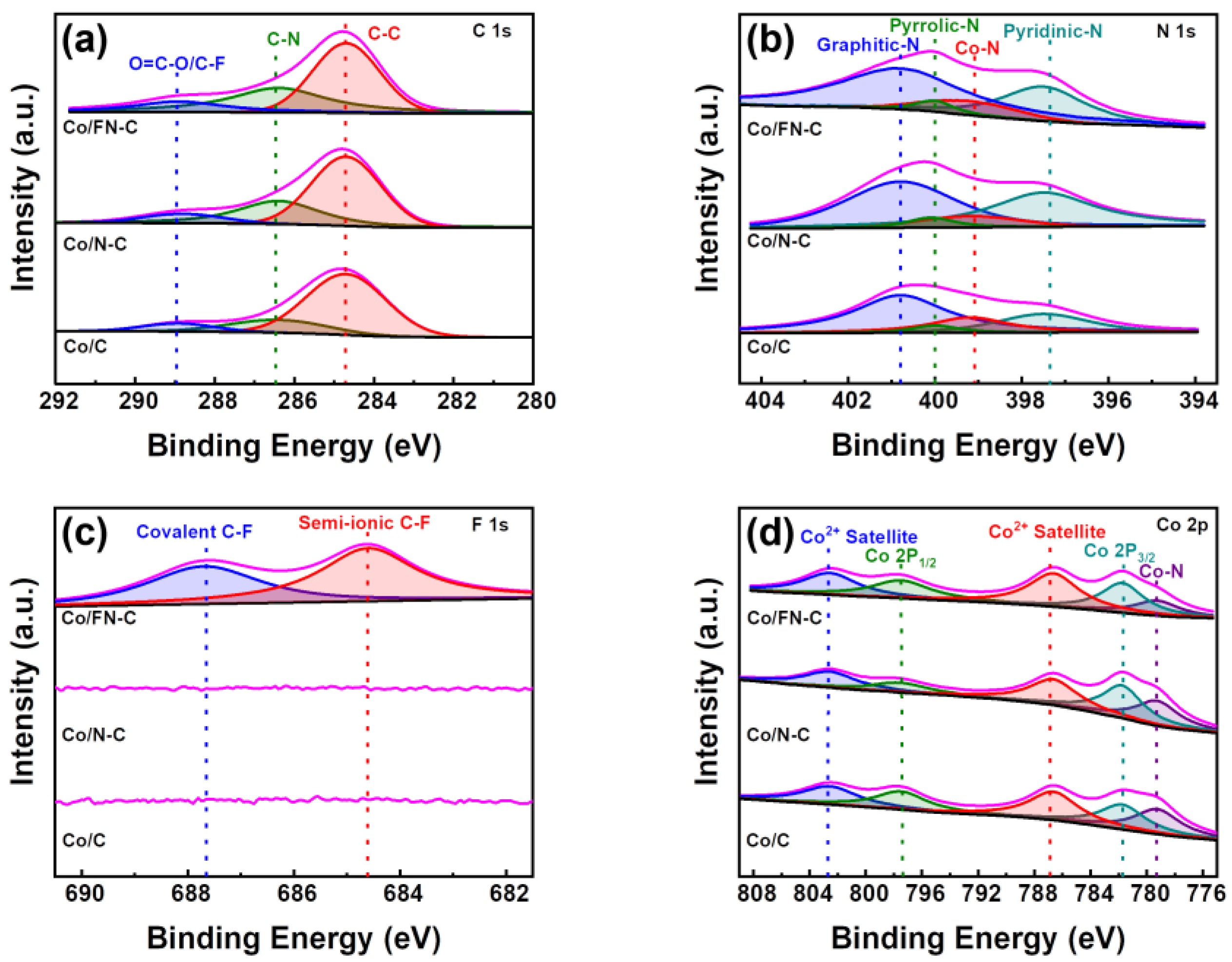
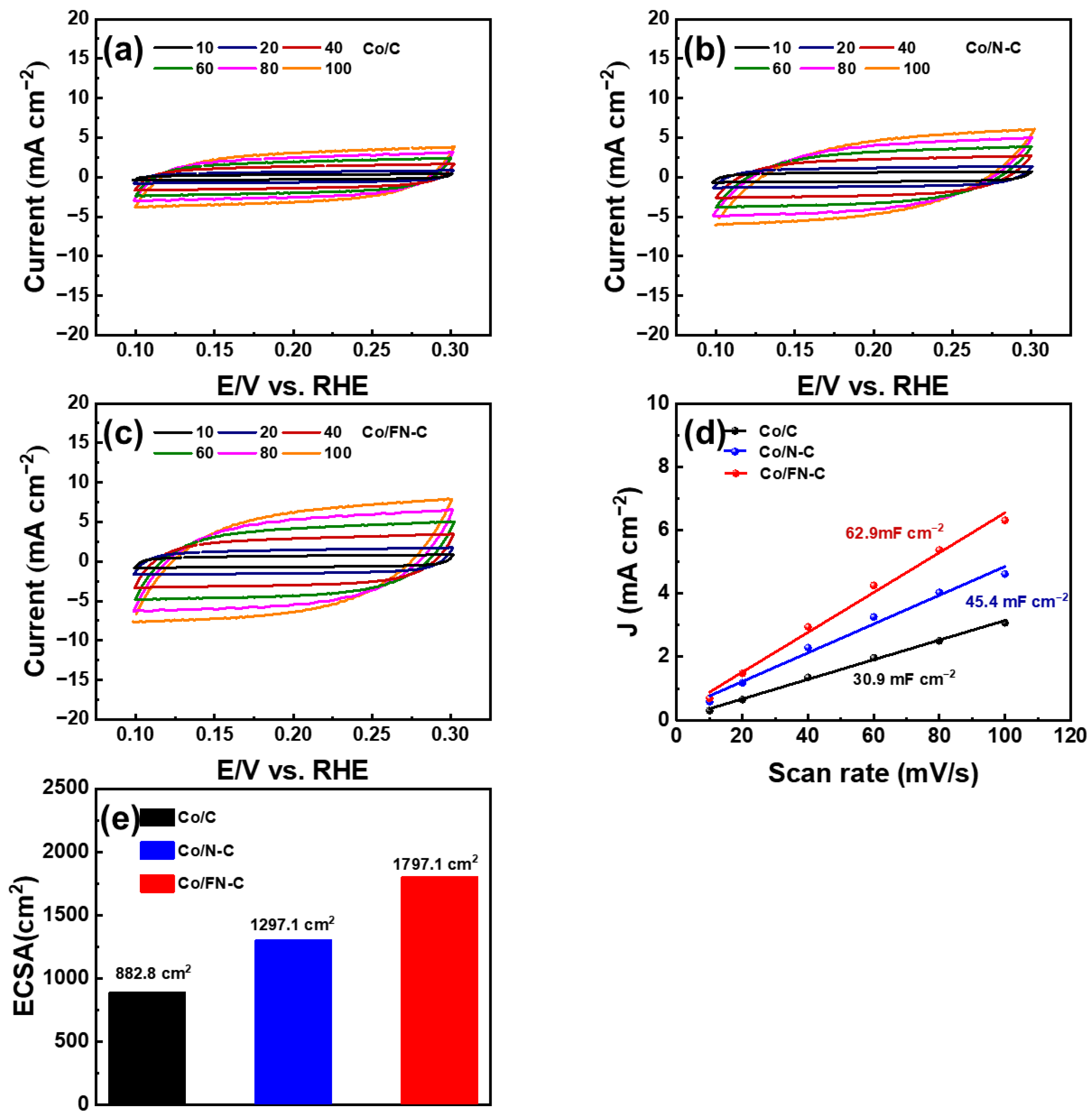
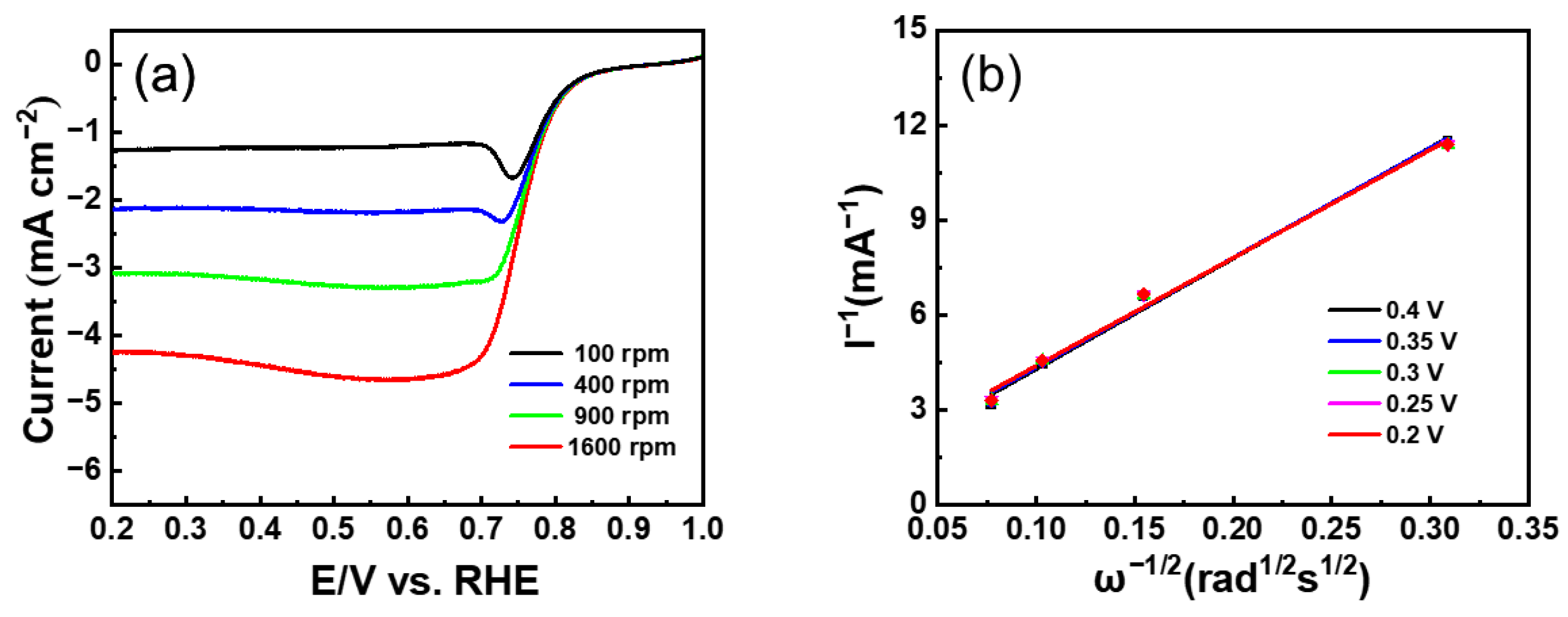
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Li, L.; Park, J.B. Aprotic and aqueous Li–O2 batteries. Chem. Rev. 2014, 114, 5611–5640. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, S. Structure and environmental impact of global energy consumption. Renew. Sustain. Energ. Rev. 2014, 38, 892–902. [Google Scholar] [CrossRef]
- Ibrahim, D. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energ. Rev. 2000, 4, 157–175. [Google Scholar]
- Peng, B.; Chen, J. Functional materials with high-efficiency energy storage and conversion for batteries and fuel cells. Coord. Chem. Rev. 2009, 253, 2805–2813. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S. Metal-air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2010, 1, 34–50. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, W.; Gu, Y.; Sun, Z. Metal-nitrogen-doped carbon materials as highly efficient catalysts: Progress and rational design. Adv. Sci. 2020, 7, 2001069. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Maia, G. Developing efficient catalysts for the OER and ORR using a combination of Co, Ni, and Pt oxides along with graphene nanoribbons and NiCo2O4. J. Mater. Chem. A 2020, 8, 17691–17705. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2018, 31, 1802234. [Google Scholar] [CrossRef]
- He, C.; Ma, Z.; Wu, Q. Promoting the ORR catalysis of Pt-Fe intermetallic catalysts by increasing atomic utilization and electronic regulation. Electrochim. Acta 2020, 330, 135119. [Google Scholar] [CrossRef]
- Wei, B.; Fu, Z.H.; Legut, D.; Germann, T.C.; Du, S.; Zhang, H.; Francisco, J.S.; Zhang, R.F. Rational design of highly stable and active MXene-based bifunctional ORR/OER double-atom catalysts. Adv. Mater. 2021, 33, 2102595. [Google Scholar] [CrossRef]
- Dou, S.; Wang, X.; Wang, S. Rational design of transition metal—Based materials for highly efficient electrocatalysis. Small Methods 2019, 3, 1800211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wilkinson, D.P.; Liu, Y.; Zhang, J.J. Progress in nanostructured (Fe or Co)/N/C non-noble metal electrocatalysts for fuel cell oxygen reduction reaction. Electrochim. Acta 2018, 262, 326–336. [Google Scholar] [CrossRef]
- Jin, H.; Yu, R.; Hu, C.; Ji, P.; Ma, Q.; Liu, B.; He, D.; Mu, S. Size-controlled engineering of cobalt metal catalysts through a coordination effect for oxygen electrocatalysis. Appl. Catal. B Environ. 2022, 317, 121766. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, C.; Dong, S. Cobalt and nitrogen—Cofunctionalized graphene as a durable non-precious metal catalyst with enhanced ORR activity. J. Mater. Chem. A 2013, 1, 3593–3599. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Wang, Z.; Bu, D.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. N and Mn dual-doped cactus-like cobalt oxide nanoarchitecture derived from cobalt carbonate hydroxide as efficient electrocatalysts for oxygen evolution reactions. J. Colloid Interface Sci. 2021, 597, 361–369. [Google Scholar] [CrossRef] [PubMed]
- David, S.C.; Nora, H.L. Single-atom catalysis for carbon dioxide dissociation using greigite-supported M1/Fe3S4(111) (M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) under electrostatic fields. J. Catal. Sci. 2023, 425, 89–104. [Google Scholar]
- Li, Y.; Li, W.; Fang, M. The preparation, performance and lithiation mechanism of cobalt-doped zinc oxide as a high performance anode material for LIB. Curr. Appl. Phys. 2017, 17, 1050–1057. [Google Scholar] [CrossRef]
- Yuan, N.; Jiang, Q.; Li, J.; Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Popov, B. Activity and stability of non–precious metal catalysts for oxygen reduction in acid and alkaline electrolytes. J. Power Sources Adv. 2010, 195, 6373–6378. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Meng, X.; Wu, X.; Li, S.; Chen, Y. Direct chemical synthesis of ultrathin holey iron doped cobalt oxide nanosheets on nickel foam for oxygen evolution reaction. Nano Energy 2018, 54, 238–250. [Google Scholar] [CrossRef]
- Huang, T.; Chen, Y.; Lee, J.M. A microribbon hybrid structure of CoOx–MoC encapsulated in N–doped carbon nanowire derived from MOF as efficient oxygen evolution electrocatalysts. Small 2017, 13, 1702753. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ji, Q.; Zhang, H. Ru-incorporated Co3O4 nanoparticles from self-sacrificial ZIF-67 template as efficient bifunctional electrocatalysts for rechargeable metal-air battery. J. Colloid Interface Sci. 2022, 606, 654–665. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Z.; Wang, H. Metal-organic framework-derived nanomaterials in environment related fields: Fundamentals, properties and applications. Coord. Chem. Rev. 2021, 429, 213618. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, X.; Ma, J.; Zheng, Q. Metal-organic framework-derived carbon–based composites for electromagnetic wave absorption: Dimension design and morphology regulation. J. Mater. Sci. Technol. 2023, 132, 223–251. [Google Scholar] [CrossRef]
- Song, K.; Feng, Y.; Zhang, W.; Zheng, W. MOFs fertilized transition–metallic single–atom electrocatalysts for highly-efficient oxygen reduction: Spreading the synthesis strategies and advanced identification. J. Energy Chem. 2022, 67, 391–422. [Google Scholar] [CrossRef]
- Wang, G.; Deng, J.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Turning on electrocatalytic oxygen reduction by creating robust Fe-Nx species in hollow carbon frameworks via in situ growth of Fe doped ZIFs on g-C3N4. Nanoscale 2020, 12, 5601–5611. [Google Scholar] [CrossRef]
- Mahboobeh, S.; Mojtaba, M. Synthesis and characterization of GO/ZIF-67 nanocomposite: Investigation of catalytic activity for the determination of epinine in the presence of dobutamine. Micromachines 2022, 13, 88. [Google Scholar]
- Panchariya, D.; Rai, R.; Kumar, E.; Singh, S. Core–shell zeolitic imidazolate frameworks for enhanced hydrogen storage. ACS Omega 2018, 3, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.N.; Wang, S.B.; Wang, X.C. Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst. Appl. Catal. B Environ. 2017, 209, 476–482. [Google Scholar] [CrossRef]
- Gong, H.M.; Zhang, X.J.; Wang, G.R.; Liu, Y.; Li, Y.B.; Jin, Z.L. Dodecahedron ZIF-67 anchoring ZnCdS particles for photocatalytic hydrogen evolution. Mol. Catal. 2020, 485, 110832. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-Atom Catalysts: Emerging Multifunctional Materials in Heterogeneous Catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Wu, H.; Wu, M.; Wang, B.; Yong, X.; Liu, Y.; Li, B.; Liu, B.; Lu, S. Interface electron collaborative migration of Co–Co3O4/carbon dots: Boosting the hydrolytic dehydrogenation of ammonia borane. J. Energy Chem. 2020, 48, 43–53. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, C.; Dong, C.; Sun, W.; Ji, D.; Ding, Y. Carbon quantum dots assisted strategy to synthesize Co@ Nc for boosting photocatalytic hydrogen evolution performance of Cds. Chem. Eng. J. 2020, 389, 124432. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Tang, L.; Wei, Q.; Wei, X.; Liang, J.; Wang, L. Rationally constructing a nano MOF-derived Ni and CQD embedded N-doped carbon nanosphere for the hydrogenation of petroleum resin at low temperature. ACS Appl. Mater. Interfaces 2021, 13, 10855–10869. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Hu, F. Green synthesis of carbon dots from pork and application as nanosensors for uric acid detection, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 360–367. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.H.; Xie, L.L.; Zhu, L.M.; Cao, X.Y. ZIF-67-derived N-doped Co/C nanocubes as high–performance anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 16619–16628. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised polyaniline and polypyrrole: Towards advanced nitrogen-containing carbon materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Yang, L.J.; Jiang, S.J.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.Z.; Wu, Q.; Ma, J.; Ma, Y.W.; Hu, Z. Boron-doped carbon nanotubes as metal–free electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Mesostructured carbon—Based nanocages: An advanced platform for energy chemistry. Adv. Mater. 2020, 32, 1904177. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Chen, S.; Wang, X.; Ma, Y.; Wu, Q.; Jiang, Y.; Qian, W.; Hu, Z. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J. Am. Chem. Soc. 2013, 135, 1201–1204. [Google Scholar] [CrossRef]
- Behan, J.A.; Mates-Torres, E.; Stamatin, S.N.; Dominguez, C.; Iannaci, A.; Fleischer, K.; Hoque, M.K.; Perova, T.S.; Garcia-Melchor, M.; Colavita, P.E. Untangling cooperative effects of pyridinic and graphitic nitrogen sites at metal–free N doped carbon electrocatalysts for the oxygen reduction reaction. Small 2019, 15, 1902081. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, B.; Zhang, J.; Xu, H.; Xu, K.; Chen, G. Photoluminescence and photodetecting properties of the hydrothermally synthesized nitrogen-doped carbon quantum dots. J. Phys. Chem. C 2019, 123, 25570–25578. [Google Scholar] [CrossRef]
- Ma, Y.; Sung, K.W.; Ahn, H.J. N- and F-co-doped carbon quantum dots coated on a Ni foam substrate as current collector for highly stable Li-air batteries. Int. J. Energy Res. 2023, 2023, 5310171. [Google Scholar] [CrossRef]
- Jo, H.G.; Kim, K.H.; Ahn, H.J. Nitrogen-doped carbon quantum dots decorated on platinum catalysts for improved oxygen reduction reaction. Appl. Surf. Sci. 2021, 554, 149594. [Google Scholar] [CrossRef]
- Gasidit, P.; Nagahiro, S.; Takahiro, I. Simple one-step synthesis of fluorine-doped carbon nanoparticles as potential alternative metal-free electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9972–9981. [Google Scholar]
- Li, X.; Tang, Y.; Zhu, J.; Zhi, C.; Li, H. Boosting the cycling stability of aqueous flexible Zn batteries via F doping in nickel—Cobalt carbonate hydroxide cathode. Small 2020, 16, 2001935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Chen, Y.; Dou, S.; Chen, X.; Dong, B.; Guo, B.; Liu, D.; Liu, C.; Chai, Y. Hydrogen evolution under large-current—Density based on fluorine-doped cobalt-iron phosphides. Chem. Eng. J. 2020, 399, 125831. [Google Scholar] [CrossRef]
- Liu, M.X.; Gan, L.H.; Xiong, W.; Zhao, F.Q.; Fan, X.Z.; Zhu, D.Z.; Xu, Z.J.; Hao, Z.X.; Chen, L.W. Nickel-doped activated mesoporous carbon microspheres with partially graphitic structure for supercapacitors. Energy Fuels 2013, 27, 1168–1173. [Google Scholar] [CrossRef]
- Lee, J.S.; Rajan, H.; Christy, M. Optimization of active sites by sulfurization of the core–shell ZIF 67@ZIF 8 for rapid oxygen reduction kinetics in acidic media. Int. J. Hydrogen Energy 2021, 46, 10739–10748. [Google Scholar] [CrossRef]
- Sirisomboonchai, S.; Li, S.; Yoshida, A.; Guana, G. Terephthalic acid induced binder-free NiCoP-carbon nanocomposite for highly efficient electrocatalysis of hydrogen evolution eeaction. Catal. Sci. Technol. 2019, 9, 4651–4658. [Google Scholar] [CrossRef]
- Lin, S.; Luo, J.; Xiao, C. Facile one-step synthesis of porous hybrid material fabricated by 2D nanosheets of molybdenum disulfide and reduced graphene oxide for efficient electrocatalytic hydrogen evolution. Coord. Chem. Rev. 2020, 27, 123–131. [Google Scholar] [CrossRef]
- Liu, B.; Cao, B.; Cheng, Y.; Jing, P. Ultrafine CoP/Co2P nanorods encapsulated in Janus/Twins-type honeycomb 3D nitrogen-doped carbon nanosheets for efficient hydrogen evolution. iScience 2020, 23, 101264. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Yilmaz, G.; Tan, C.F.; Hong, M. Functional defective metal-organic coordinated network of mesostructured nanoframes for enhanced electrocatalysis. Adv. Funct. Mater. 2018, 28, 1704177. [Google Scholar]
- Thirukumaran, P.; Raji, A.; Shakila, A.; Madhappan, P. N-Doped mesoporous carbon prepared from a polybenzoxazine precursor for high performance supercapacitors. Polymers 2021, 13, 2048. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zaman, W.; Sun, W.; Cao, L.; Tariq, M.; Yang, J. Cultivating crystal lattice distortion in IrO2 via coupling with MnO2 to boost the oxygen evolution reaction with high intrinsic activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef]
- Pu, Z.; Cheng, R.; Zhao, J.; Hu, Z. Anion-modulated platinum for high-performance multifunctional electrocatalysis toward HER, HOR, and ORR. iScience 2020, 23, 101793. [Google Scholar] [CrossRef]
- Ong, A.L.; Inglis, K.K.; Whelligan, D.K.; Murphy, S.; Varcoe, J.R. Effect of cationic molecules on the oxygen reduction reaction on fuel cell grade Pt/C (20 wt%) catalyst in potassium hydroxide (aq, 1 mol dm-3). Phys. Chem. Chem. Phys. 2015, 17, 12135–12145. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Baldizzone, C.; Grote, J.P.; Schuppert, A.K.; Jaouen, F.; Mayrhofer, K. Stability of Fe6N6C catalysts in acidic medium studied by operando spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 1275361275. [Google Scholar] [CrossRef]
- Ding, W.; Li, L.; Xiong, K.; Wang, Y.; Li, W.; Nie, Y.; Chen, S.G.; Qi, X.Q.; Wei, Z.D. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 54146542. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Min, W.; Han, C.; Sung, H. N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694–3699. [Google Scholar]
- Lin, Q.P.; Bu, X.H.; Kong, A.G.; Mao, C.Y.; Bu, F.; Feng, P.Y. Heterometal-embedded organic conjugate frameworks from alternating monomeric iron and cobalt metalloporphyrins and their application in design of porous carbon catalysts. Adv. Mater. 2015, 27, 343163436. [Google Scholar] [CrossRef] [PubMed]
- Karim, K. High efficiency platinum nanoparticles based on carbon quantum dot and its application for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 11605–11613. [Google Scholar]
- Muthusankar, G.; Sangili, A.; Chen, S.M.; Karkuzhali, R.; Sethupathi, M.; Gopu, G.; Karthicka, S.; Devi, R.; Sengottuvelan, N. In situ assembly of sulfur–doped carbon quantum dots surrounded iron (III) oxide nanocomposite; a novel electrocatalyst for highly sensitive detection of antipsychotic drug olanzapine. J. Mol. Liq. 2018, 26, 471–480. [Google Scholar] [CrossRef]
- Martins, N.; Ângelo, J.; Girão, A.; Trindade, T.; Andrade, L.; Mendes, A. N–doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Int. J. Hydrogen Energy 2016, 193, 67–74. [Google Scholar] [CrossRef]

| Material | Eonset (V) | E1/2 (V) |
|---|---|---|
| Co-NSC 200 | 0.81 | 0.74 |
| Fe-N-C | 0.85 | 0.74 |
| CAPANI-Fe-NaC | Not mentioned | 0.73 |
| PNGr | 0.87 | 0.64 |
| FeCo/C680 | 0.9 | 0.76 |
| Co/FN-C | 0.917 | 0.753 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Sung, K.-W.; Ahn, H.-J. MOF-Derived Co Nanoparticles Catalyst Assisted by F- and N-Doped Carbon Quantum Dots for Oxygen Reduction. Nanomaterials 2023, 13, 2093. https://doi.org/10.3390/nano13142093
Ma Y, Sung K-W, Ahn H-J. MOF-Derived Co Nanoparticles Catalyst Assisted by F- and N-Doped Carbon Quantum Dots for Oxygen Reduction. Nanomaterials. 2023; 13(14):2093. https://doi.org/10.3390/nano13142093
Chicago/Turabian StyleMa, Yuqi, Ki-Wook Sung, and Hyo-Jin Ahn. 2023. "MOF-Derived Co Nanoparticles Catalyst Assisted by F- and N-Doped Carbon Quantum Dots for Oxygen Reduction" Nanomaterials 13, no. 14: 2093. https://doi.org/10.3390/nano13142093
APA StyleMa, Y., Sung, K.-W., & Ahn, H.-J. (2023). MOF-Derived Co Nanoparticles Catalyst Assisted by F- and N-Doped Carbon Quantum Dots for Oxygen Reduction. Nanomaterials, 13(14), 2093. https://doi.org/10.3390/nano13142093






