Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature
Abstract
:1. Introduction
2. Computational Details
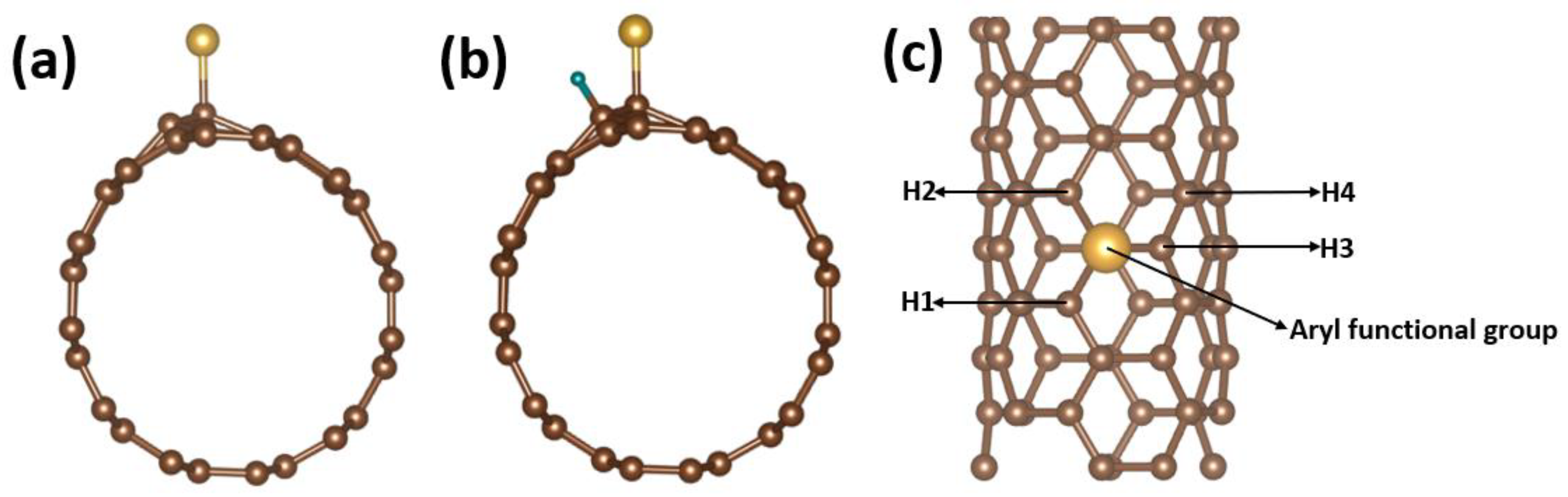
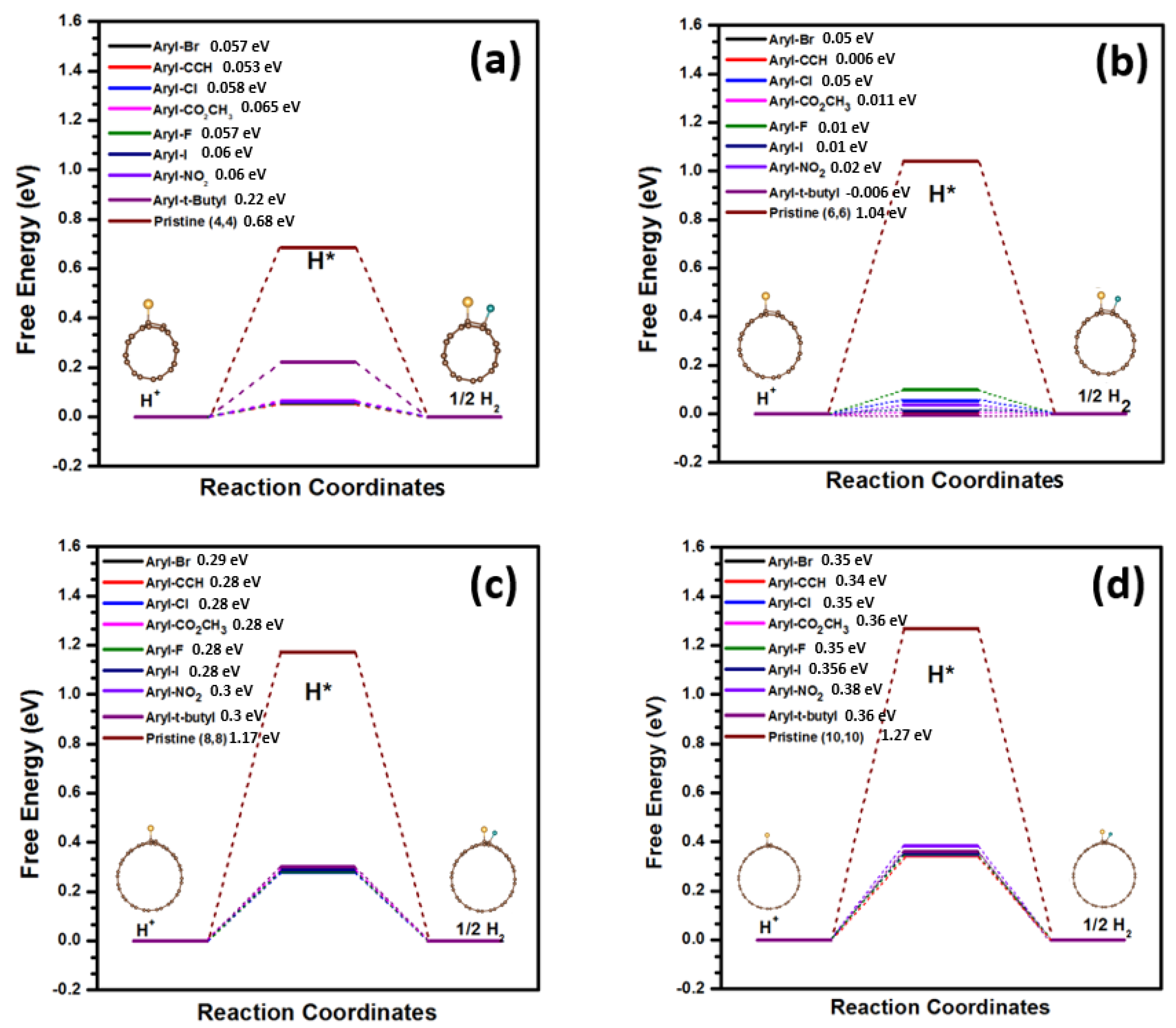
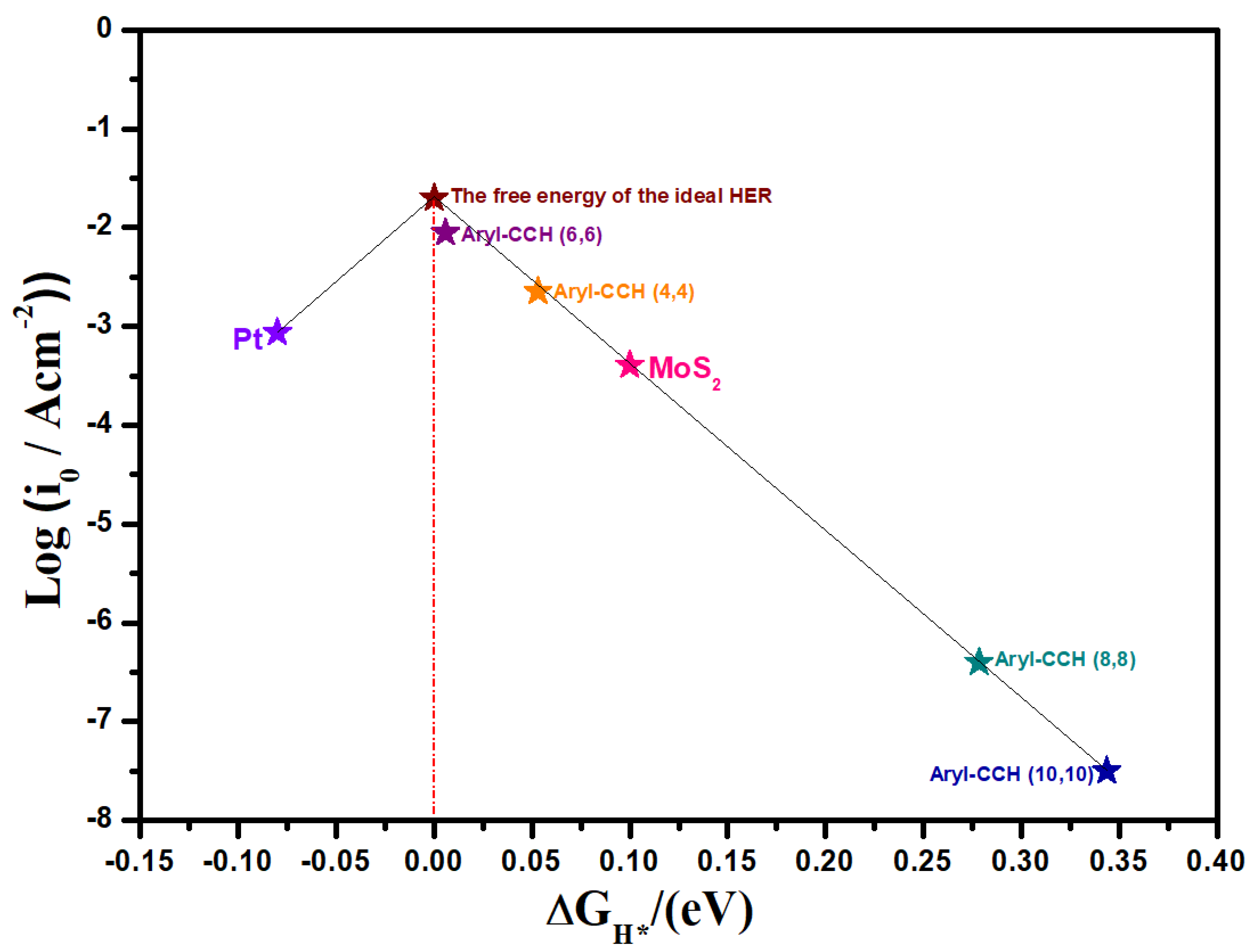
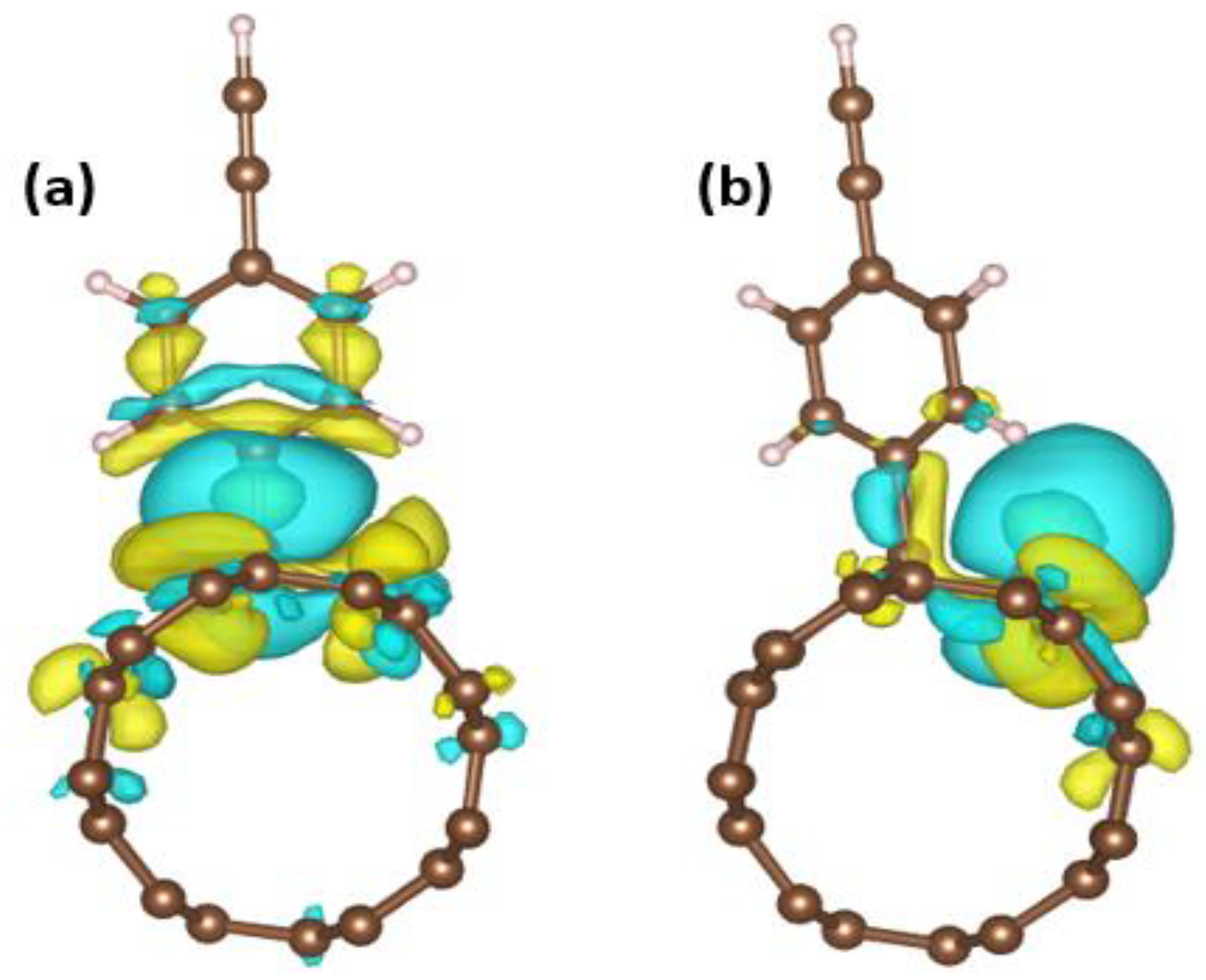
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, C.-H.; Huang, C.-W.; Wu, J.C. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Tekalgne, M.; Van Le, Q.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: Hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Murthya, A.; Madhavana, J.; Muruganb, K. Recent advances in hydrogen evolution reaction catalysts on carbon/carbon-based supports in acid media. J. Power Sources 2018, 398, 9–26. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions–a review. Int. J. Hydrogren Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Tahir, M.B.; Batool, A. Chapter 3—Recent development in sustainable technologies for clean hydrogen evolution: Current scenario and future perspectives. In Sustainable Materials and Green Processing for Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–130. [Google Scholar]
- Bockris, J.M.; Potter, E. The mechanism of the cathodic hydrogen evolution reaction. J. Electrochem. Soc. 1952, 99, 169. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogren Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Chen, R.; Yang, C.; Cai, W.; Wang, H.-Y.; Miao, J.; Zhang, L.; Chen, S.; Liu, B. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Lett. 2017, 2, 1070–1075. [Google Scholar] [CrossRef]
- Davodi, F.; Mühlhausen, E.; Tavakkoli, M.; Sainio, J.; Jiang, H.; Gökce, B.; Marzun, G.; Kallio, T. Catalyst Support Effect on the Activity and Durability of Magnetic Nanoparticles: Toward Design of Advanced Electrocatalyst for Full Water Splitting. ACS Appl. Mater. Intefaces 2018, 10, 31300–31311. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Zhang, M.; Tan, Y.; Jia, S.; Liu, A. Green electroless plating of cuprous oxide nanoparticles onto carbon nanotubes as efficient electrocatalysts for hydrogen evolution reaction. Appl. Surf. Sci. 2021, 548, 149218. [Google Scholar] [CrossRef]
- Bashir, A.; Mehvish, A.; Khalil, M. Advanced Carbon Materials for Sustainable and Emerging Applications. In 21st Century Advanced Carbon Materials for Engineering Applications—A Comprehensive Handbook; Books on Demand: Copenhagen, Denmark, 2021. [Google Scholar]
- Melchionna, M.; Marchesan, S.; Pratoa, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef] [Green Version]
- Holmberg, N.; Laasonen, K. Ab initio electrochemistry: Exploring the hydrogen evolution reaction on carbon nanotubes. J. Phys. Chem. C 2015, 119, 16166–16178. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhang, D.; Guo, F.; Guo, H.; Liu, Y.; Yin, Y.; Hu, H.; Wang, X. Facile one-pot supercritical synthesis of MoS2/pristine graphene nanohybrid as a highly active advanced electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2020, 531, 147282. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, R.; Zhang, J.; Shi, Y.; Zhang, B. Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 2013, 49, 6656–6658. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Gong, M.; Chou, H.-L.; Pan, C.-J.; Chen, H.-A.; Wu, Y.; Lin, M.-C.; Guan, M.; Yang, J.; Chen, C.-W. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets–carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.; Zainal, Z.; Yusof, N. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Dai, Z.; Zhang, J.; Jin, Y.; Li, D.; Sun, C. Two-dimensional boron sheets as metal-free catalysts for hydrogen evolution reaction. J. Phys. Chem. C 2018, 122, 19051–19055. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Zhang, J.; Shi, Y.; Liu, D.; Zhang, B. Metallic WO2–carbon mesoporous nanowires as highly efficient electrocatalysts for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 6983–6986. [Google Scholar] [CrossRef]
- Shahraei, A.; Moradabadi, A.; Martinaiou, I.; Lauterbach, S.; Klemenz, S.; Dolique, S.; Kleebe, H.-J.; Kaghazchi, P.; Kramm, U.I. Elucidating the origin of hydrogen evolution reaction activity in mono-and bimetallic metal-and nitrogen-doped carbon catalysts (Me–N–C). ACS Appl. Mater. Interfaces 2017, 9, 25184–25193. [Google Scholar] [CrossRef]
- Xiao, P.; Sk, M.A.; Thia, L.; Ge, X.; Lim, R.J.; Wang, J.-Y.; Lim, K.H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629. [Google Scholar] [CrossRef] [Green Version]
- Rahman, U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.; Khan, A. MXenes as Emerging Materials: Synthesis, Properties, and Applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Xiao, P.; Buijnsters, J.G.; Zhao, Y.; Yu, H.; Xu, X.; Zhu, Y.; Tang, D.; Zhu, J.; Zhao, Z. Fullerene-like WS2 supported Pd catalyst for hydrogen evolution reaction. J. Catal. 2019, 380, 215–223. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A new family of promising catalysts for the hydrogen evolution reaction. Acs Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Fang, W.; Wang, J.; Hu, Y.; Cui, X.; Zhu, R.; Zhang, Y.; Yue, C.; Dang, J.; Cui, W.; Zhao, H. Metal-organic framework derived Fe-Co-CN/reduced graphene oxide for efficient HER and OER. Electrochim. Acta 2021, 365, 137384. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Lee, D.-E.; Tonda, S. Cobalt-and iron-coordinated graphitic carbon nitride on reduced graphene oxide: A nonprecious bimetallic M–Nx–C analogue electrocatalyst for efficient oxygen reduction reaction in acidic media. Appl. Surf. Sci. 2020, 531, 147367. [Google Scholar] [CrossRef]
- Yu, P.; Wang, F.; Shifa, T.A.; Zhan, X.; Lou, X.; Xia, F.; He, J. Earth abundant materials beyond transition metal dichalcogenides: A focus on electrocatalyzing hydrogen evolution reaction. Nano Energy 2019, 58, 244–276. [Google Scholar] [CrossRef]
- Zahra, R.; Pervaiz, E.; Yang, M.; Rabi, O.; Saleem, Z.; Ali, M.; Farrukh, S. A review on nickel cobalt sulphide and their hybrids: Earth abundant, pH stable electro-catalyst for hydrogen evolution reaction. Int. J. Hydrogren Energy 2020, 45, 24518–24543. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting–A review. J. Energy Chem. 2019, 34, 111–160. [Google Scholar] [CrossRef]
- McKone, J.R.; Marinescu, S.C.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci. 2014, 5, 865–878. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-L.; Hao, X.-F.; Jiang, Z.; Sun, X.-P.; Xu, D.; Wang, J.; Zhong, H.-X.; Meng, F.-L.; Zhang, X.-B. C and N hybrid coordination derived Co–C–N complex as a highly efficient electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 15070–15073. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, M.S.; Atarod, M.; Sajjadi, M.; Isaabadi, Z. An Introduction to Green Nanotechnology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sulaiman, M.R.; Gupta, R.K. Advanced carbon-based nanostructured materials for fuel cells. In Nanotechnology in Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–227. [Google Scholar]
- Yang, L.; Lv, Y.; Cao, D. Co, N-codoped nanotube/graphene 1D/2D heterostructure for efficient oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2018, 6, 3926–3932. [Google Scholar] [CrossRef]
- Katsnelson, M. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Li, T.; Luo, G.; Liu, K.; Li, X.; Sun, D.; Xu, L.; Li, Y.; Tang, Y. Encapsulation of Ni3Fe nanoparticles in N-doped carbon nanotube–grafted carbon nanofibers as high-efficiency hydrogen evolution electrocatalysts. Adv. Funct. Mater. 2018, 28, 1805828. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co nanoparticles encapsulated in carbon-nanotubes-grafted graphene sheets as advanced electrocatalysts for the hydrogen evolution reaction. Adv. Mater. 2018, 30, 1802011. [Google Scholar] [CrossRef]
- Jin, D.; Johnson, L.R.; Raman, A.S.; Ming, X.; Gao, Y.; Du, F.; Wei, Y.; Chen, G.; Vojvodic, A.; Gogotsi, Y. Computational screening of 2D ordered double transition-metal carbides (MXenes) as electrocatalysts for hydrogen evolution reaction. J. Phys. Chem. C 2020, 124, 10584–10592. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Zheng, W.; Feng, W.; Cao, W.; Hu, W.; Hu, P. Vertical 2D MoO2/MoSe2 Core–Shell Nanosheet Arrays as High-Performance Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 8537–8544. [Google Scholar] [CrossRef]
- Liang, X.; Wu, C.-M.L. Metal-free two-dimensional phosphorus carbide as an efficient electrocatalyst for hydrogen evolution reaction comparable to platinum. Nano Energy 2020, 71, 104603. [Google Scholar] [CrossRef]
- Yao, Y.; Jin, Z.; Chen, Y.; Gao, Z.; Yan, J.; Liu, H.; Wang, J.; Li, Y.; Liu, S.F. Graphdiyne-WS2 2D-Nanohybrid electrocatalysts for high-performance hydrogen evolution reaction. Carbon 2018, 129, 228–235. [Google Scholar] [CrossRef]
- Zhang, S.; Chowdari, B.; Wen, Z.; Jin, J.; Yang, J. Constructing highly oriented configuration by few-layer MoS2: Toward high-performance lithium-ion batteries and hydrogen evolution reactions. ACS Nano 2015, 9, 12464–12472. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Nanomaterials for lithium-ion batteries and hydrogen energy. Pure Appl. Chem. 2017, 89, 1185–1194. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Sun, Y.; Zhang, P.; Gao, X.; Zhang, W.; Guo, J. MoS2-graphene hybrid nanosheets constructed 3D architectures with improved electrochemical performance for lithium-ion batteries and hydrogen evolution. Electrochim. Acta 2016, 189, 224–230. [Google Scholar] [CrossRef]
- Lasia, A. Hydrogen evolution reaction. In Handbook of Fuel Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; p. 815. [Google Scholar]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrogren Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Kamran, M.; Fazal, M. Chapter 1—Fundamentals of renewable energy systems. In Renewable Energy Conversion Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–19. [Google Scholar]
- Dincer, I.; Rosen, M. Chapter 4—Exergy, environment, and sustainable development. In Environment and Sustainable Development; Springer: Warsaw, Poland, 2021; pp. 61–89. [Google Scholar]
- Zorn, N.; Berger, F.; Zaumseil, J. Charge Transport in and Electroluminescence from sp3-Functionalized Carbon Nanotube Networks. ACS Nano 2021, 15, 10451–10463. [Google Scholar] [CrossRef]
- Li, M.; Riaz, A.; Wederhake, M.; Fink, K.; Saha, A.; Dehm, S.; He, X.; Schöppler, F.; Kappes, M.; Htoon, H.; et al. Electroluminescence from Single-Walled Carbon Nanotubes with Quantum Defects. ACS Nano 2022, 16, 11742–11754. [Google Scholar] [CrossRef]
- Zaumseil, J. Luminescent Defects in Single-Walled Carbon Nanotubes for Applications. Adv. Opt. Mater. 2022, 10, 2101576. [Google Scholar] [CrossRef]
- Oskin, P.; Demkina, I.; Dmitrieva, E.; Alferov, S. Functionalization of Carbon Nanotubes Surface by Aryl Groups: A Review. Nanomaterials 2023, 13, 1630. [Google Scholar] [CrossRef]
- Vautrin-Ul, C. Modification of sp2 carbon Allotropes with Diazonium Salts-Focus on Carbon Nanotubes Functionalization. Aryl Diazonium Salts Relat. Compd. Surf. Chem. Appl. 2022, 31, 137–156. [Google Scholar]
- Zhou, F.; Zhou, Y.; Liu, G.-G.; Wang, C.-T.; Wang, J. Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Met. 2021, 40, 3375–3405. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Gross, E.; Kohn, W. Time-Dependent Density-Functional Theory. Adv. Quantum Chem. 1990, 21, 255–291. [Google Scholar]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Atkins, P.; Atkins, P.W.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef] [Green Version]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Wang, H.; Huang, B.; Dai, Y. Multifunctional electrocatalyst PtM with low Pt loading and high activity T towards hydrogen and oxygen electrode reactions: A computational study. Appl. Catal. B Environ. 2019, 255, 117743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Kour, G.; Sun, Z.; Du, A.; Mao, X. Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature. Nanomaterials 2023, 13, 2122. https://doi.org/10.3390/nano13142122
Ahmed M, Kour G, Sun Z, Du A, Mao X. Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature. Nanomaterials. 2023; 13(14):2122. https://doi.org/10.3390/nano13142122
Chicago/Turabian StyleAhmed, Muhammad, Gurpreet Kour, Ziqi Sun, Aijun Du, and Xin Mao. 2023. "Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature" Nanomaterials 13, no. 14: 2122. https://doi.org/10.3390/nano13142122




