Introduce Ce3+ Ions to Realize Enhancement of C+L Band Luminescence of KMnF3: Yb, Er Nanoparticles
Abstract
:1. Introduction
2. Experiment Details
2.1. Preparation of KMnF3: Yb, Er, Ce NPs
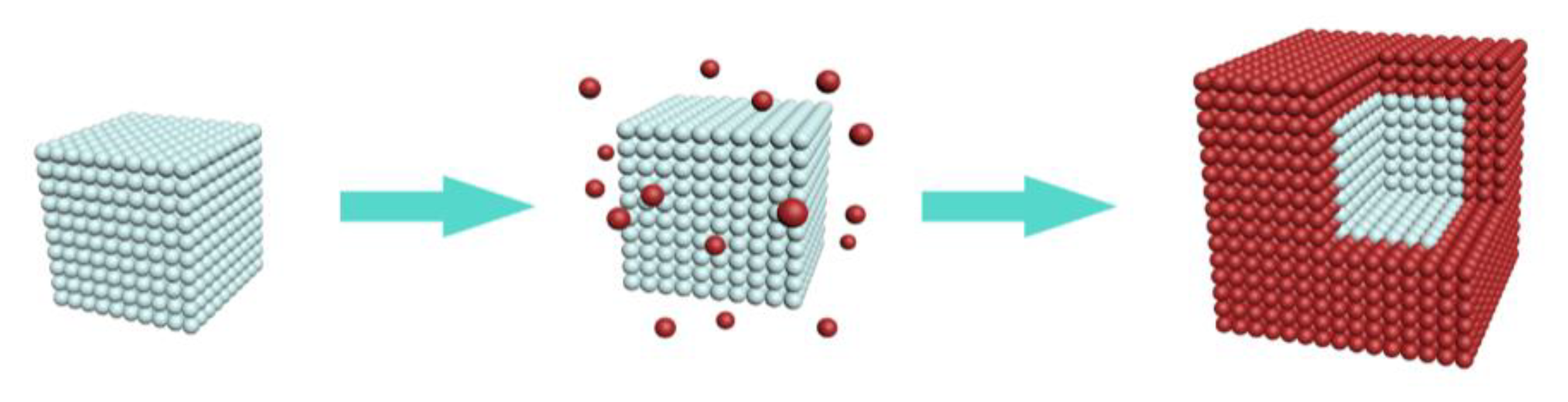
2.2. Preparation of KMnF3: Yb, Er, Ce@KMnF3: Yb Core-Shell NPs
2.3. Characterization
3. Results and Discussion
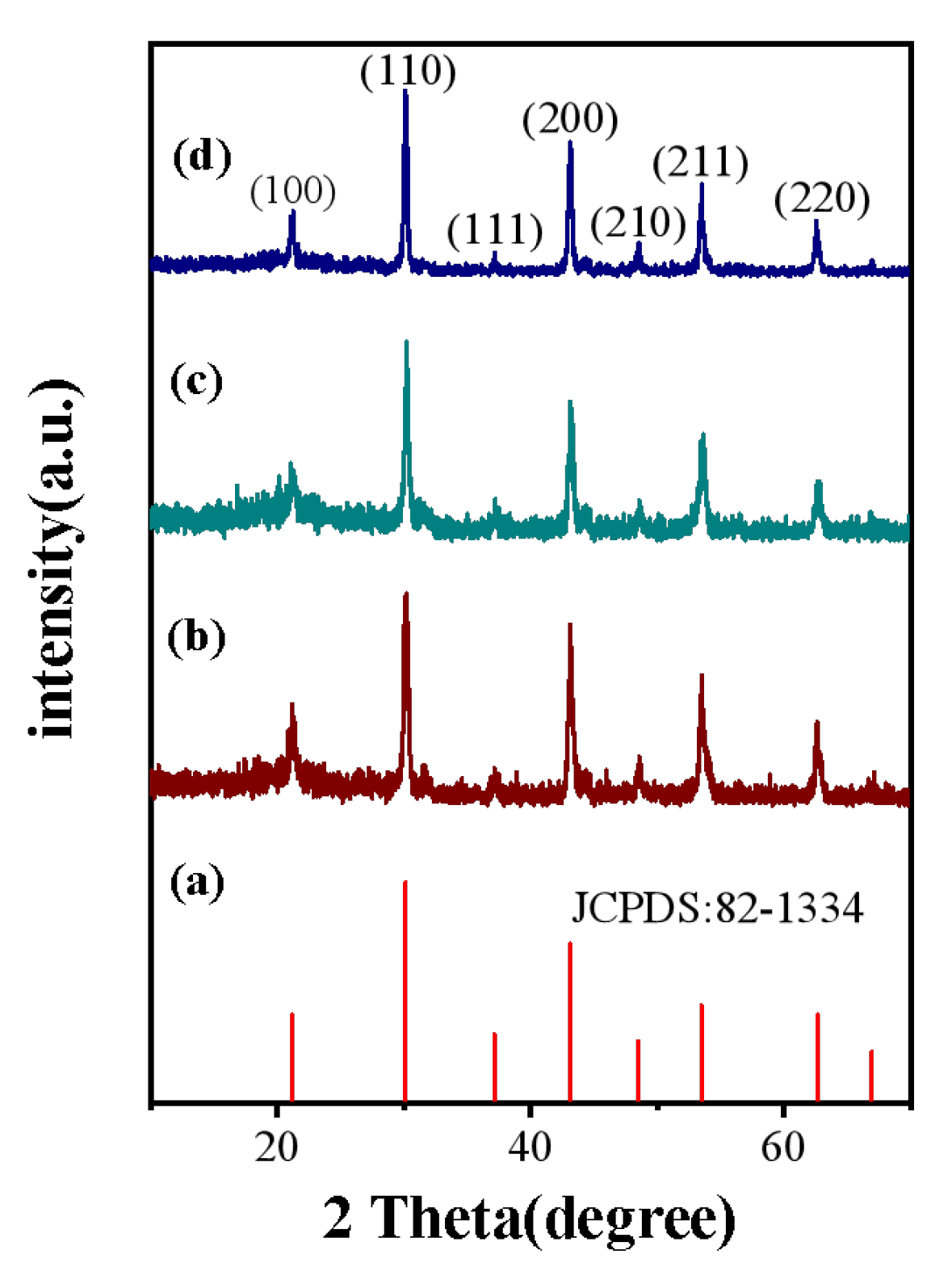
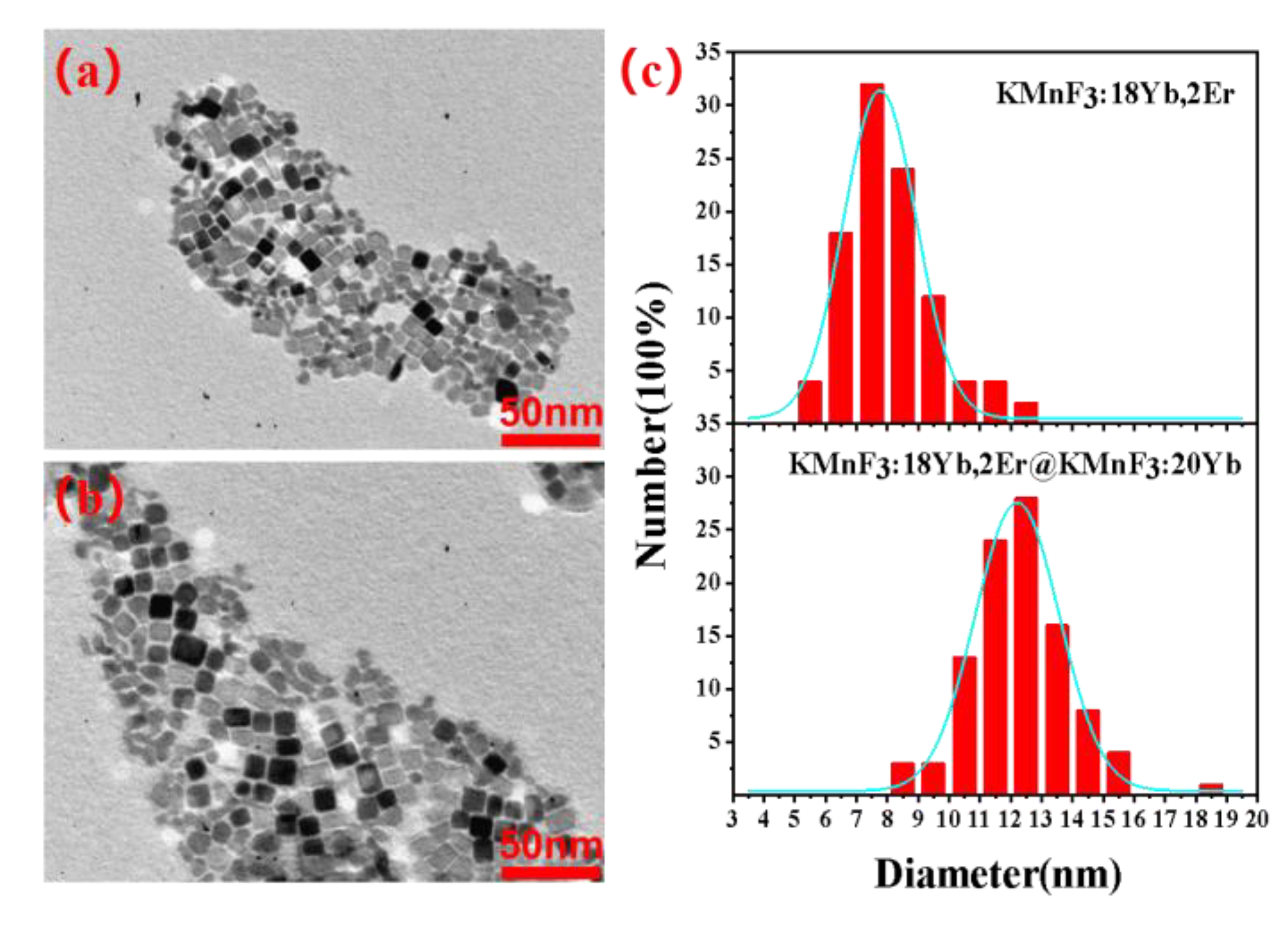
3.1. Crystal Structure and Morphology
3.2. Optical Properties
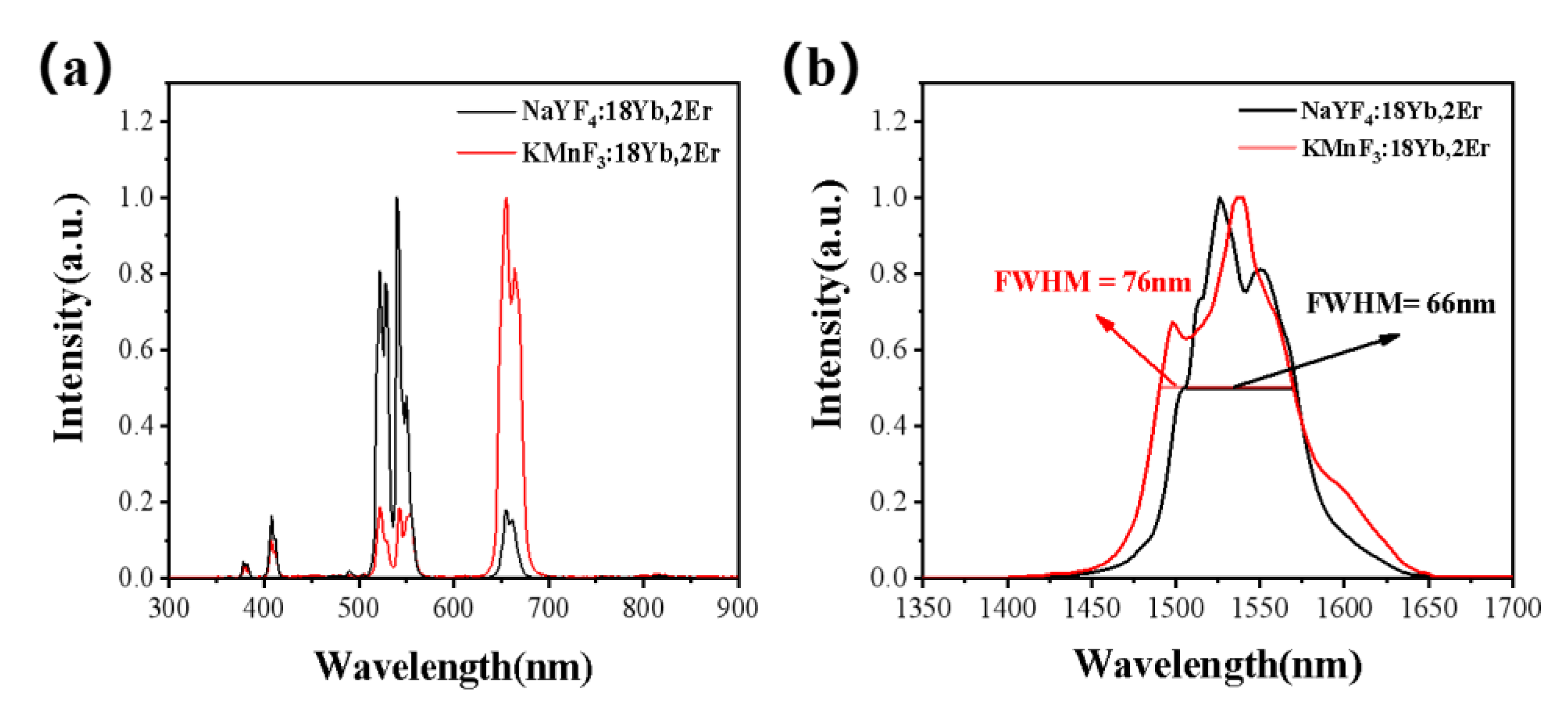
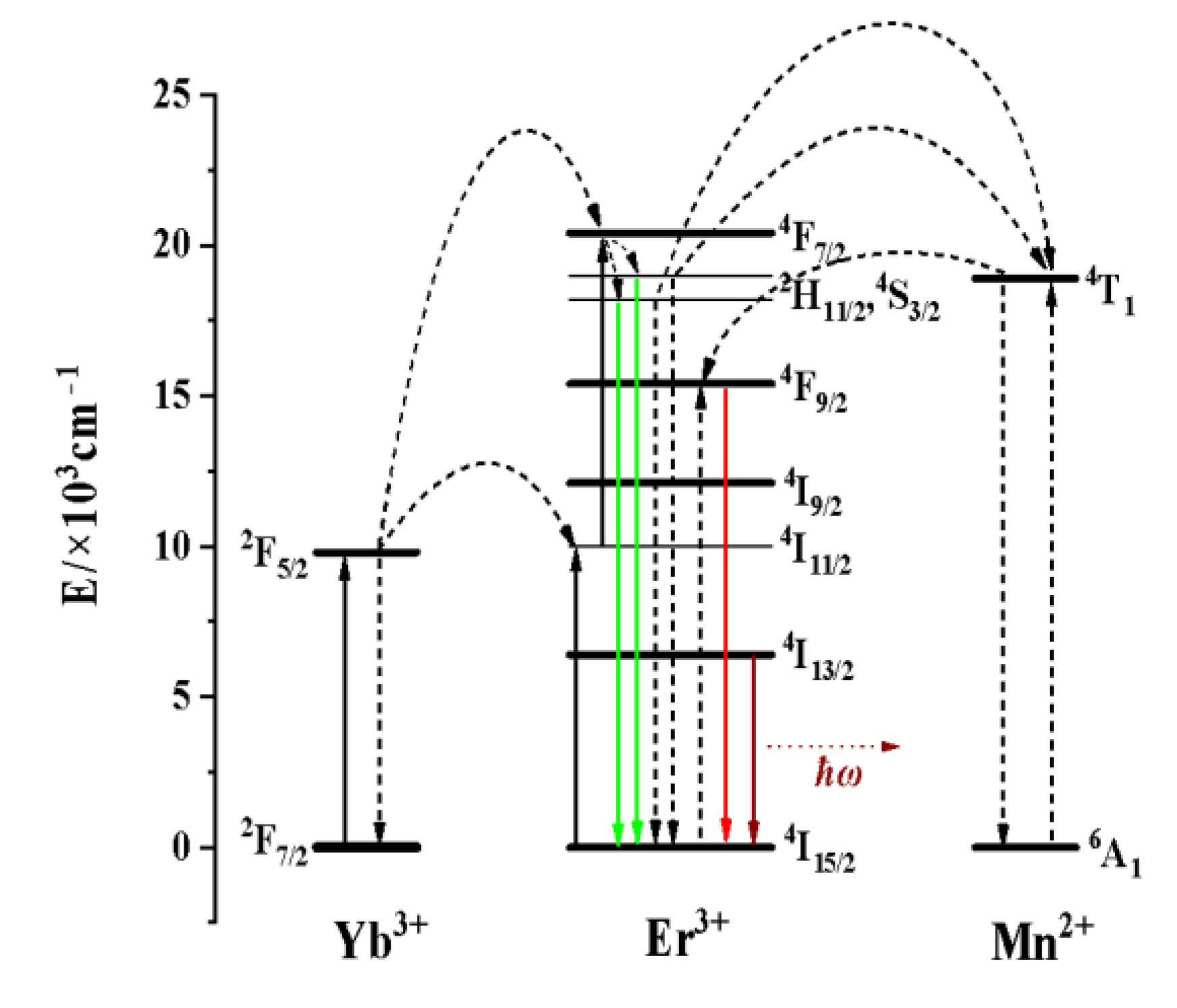
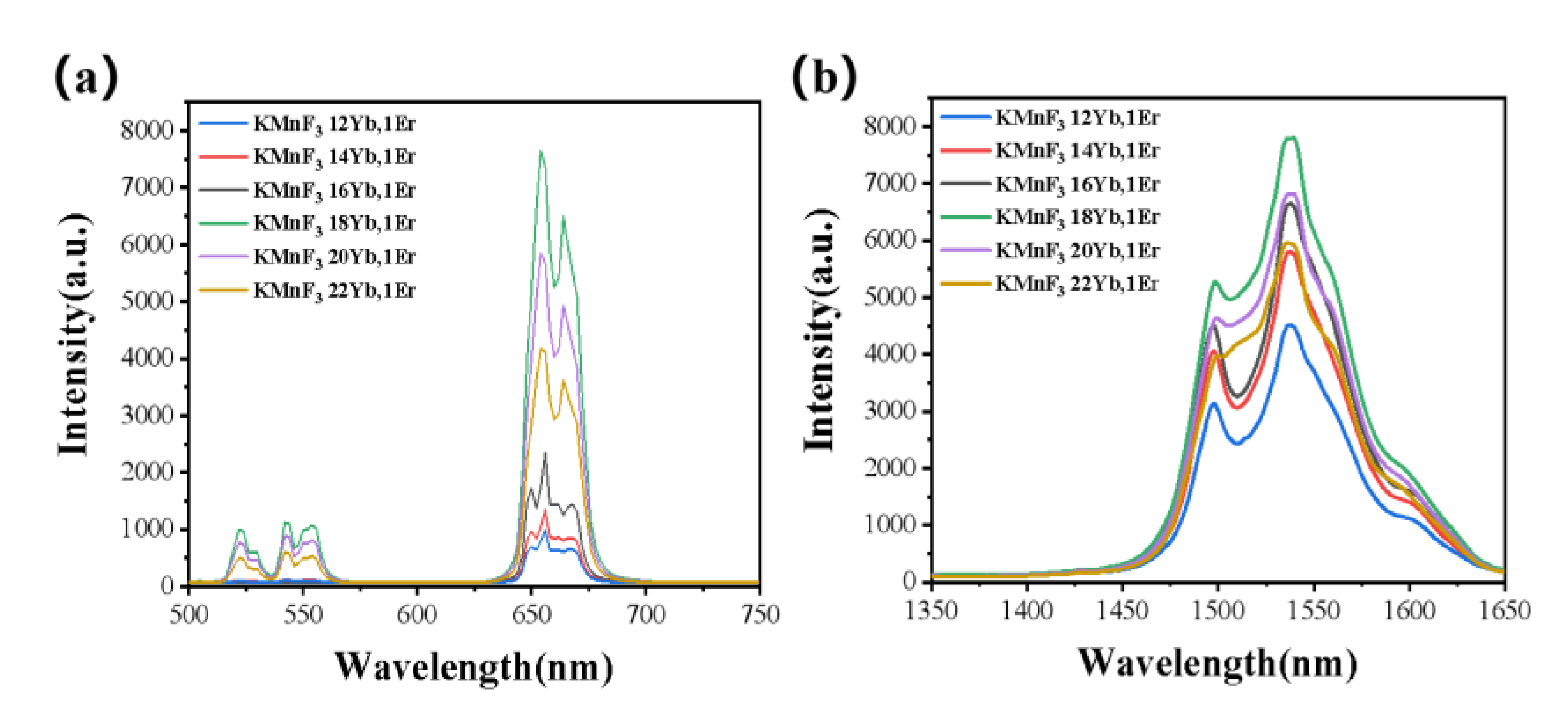
3.2.1. Effect of the Concentration of Yb3+ and Er3+ on the Luminescence Properties of KMnF3: Yb, Er NPs
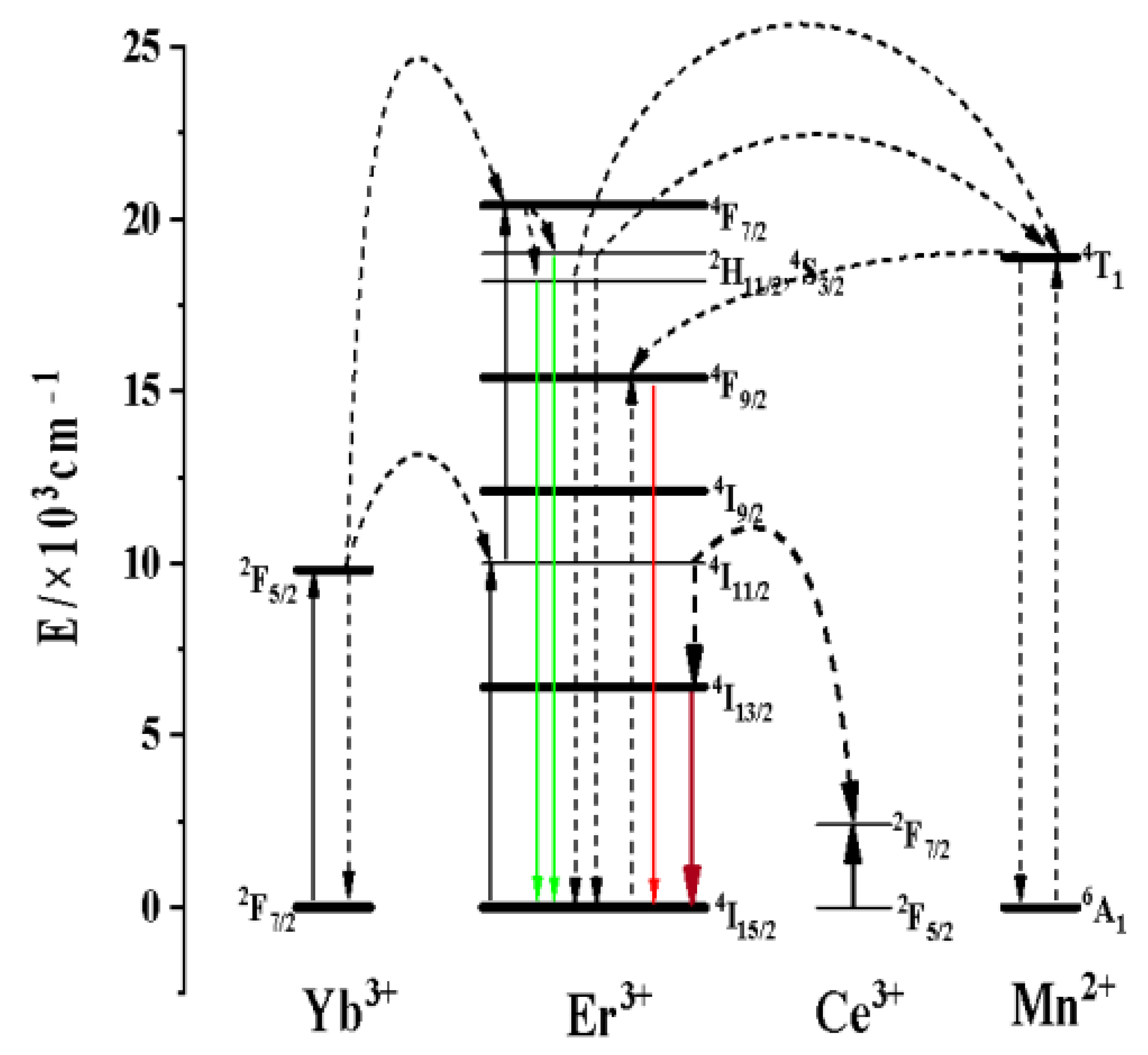
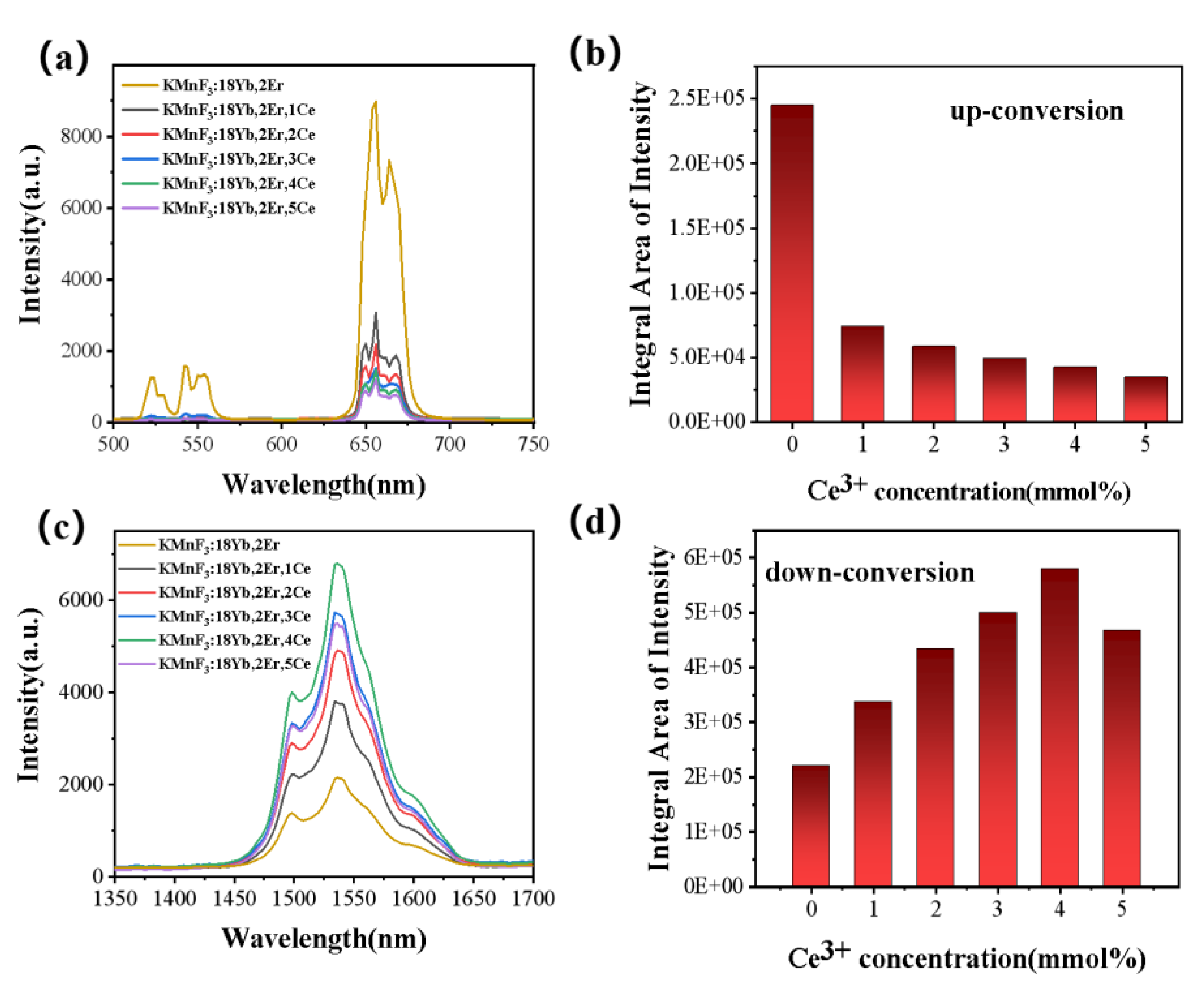
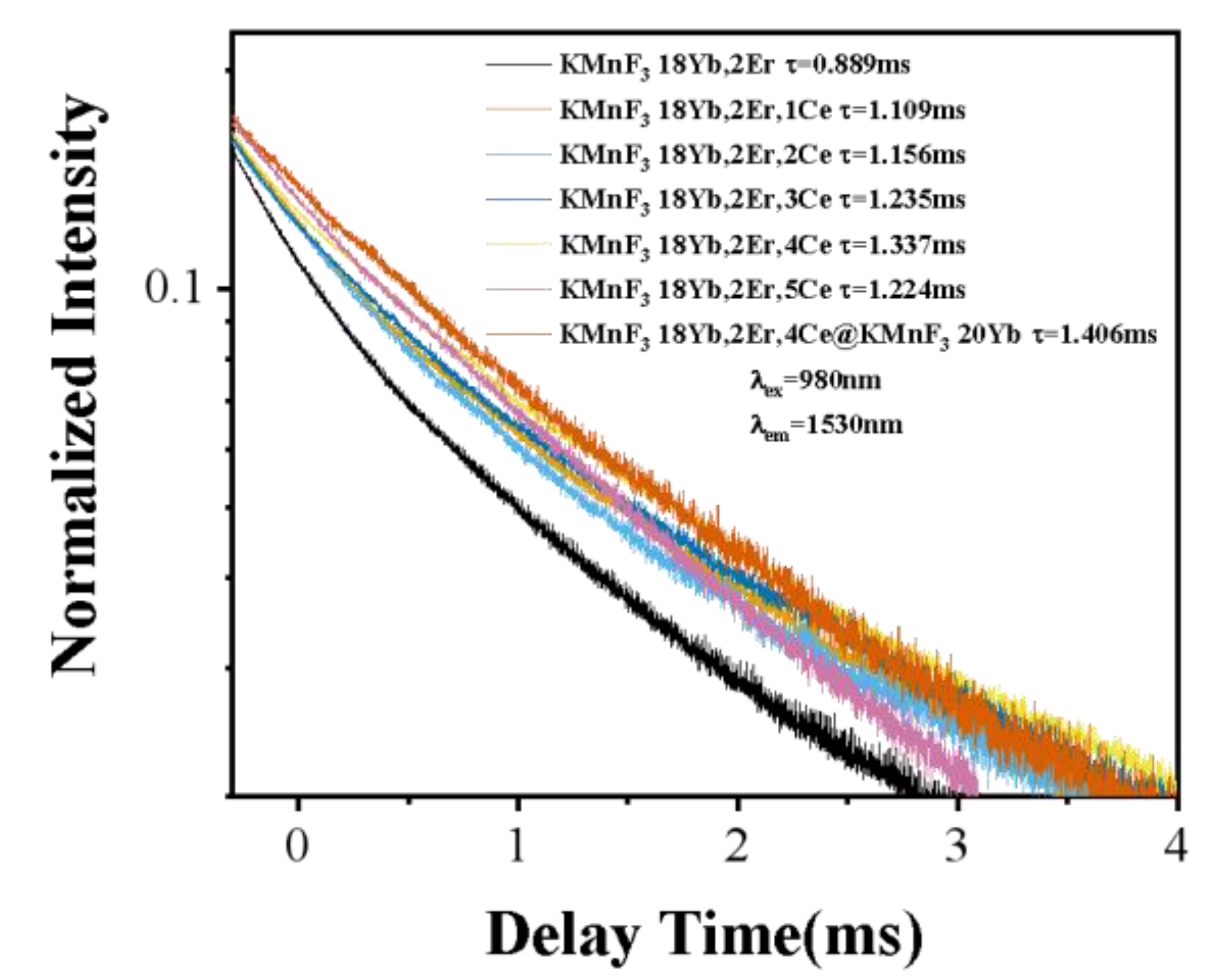
3.2.2. Effect of Ce3+ Concentration on the Luminescence Properties of KMnF3: Yb, Er NPs
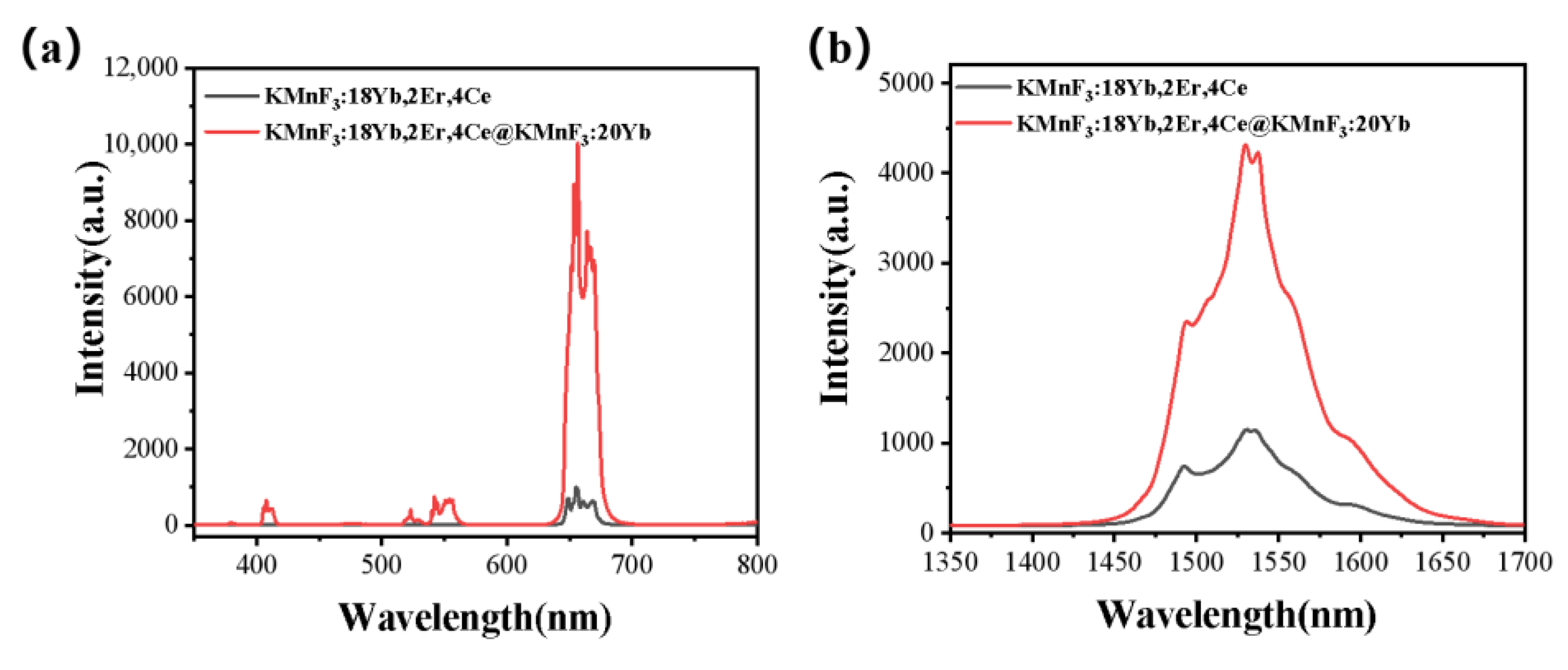
3.2.3. Up-Conversion Luminescence and Luminescence at 1.53 μm Characteristics of Core-Shell KMnF3: Yb, Er, Ce@KMnF3: Yb NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, F.; Zhao, D. Lab on upconversion nanoparticles: Optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 2015, 44, 1346–1378. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.B.; Demopoulos, G.P. Near-infrared sunlight harvesting in dye-sensitized solar cells via the insertion of an upconverter-TiO2 nanocomposite layer. Adv. Mater. 2010, 22, 4373–4377. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Cen, Y.; Huang, L.-J.; Yu, R.-Q.; Chu, X. Upconversion fluorescence resonance energy transfer biosensor for sensitive detection of human immunodeficiency virus antibodies in human serum. Chem. Commun. 2014, 50, 4759–4762. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Arandiyan, H.; Boyer, C.; Lim, M. Lanthanide-doped upconversion nanoparticles: Emerging intelligent light-activated drug delivery systems. Adv. Sci. 2016, 3, 1500437. [Google Scholar] [CrossRef] [Green Version]
- de Wild, J.; Rath, J.; Meijerink, A.; Van Sark, W.; Schropp, R. Enhanced near-infrared response of a-Si: H solar cells with β-NaYF4: Yb3+ (18%), Er3+ (2%) upconversion phosphors. Sol. Energy Mater. Sol. Cells 2010, 94, 2395–2398. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Zhang, D.; Zhao, D.; Wang, L.; Zheng, K. Near-infrared photocatalysis based on YF3: Yb3+, Tm3+/TiO2 core/shell nanoparticles. Chem. Commun. 2010, 46, 2304–2306. [Google Scholar] [CrossRef]
- Shen, J.-W.; Yang, C.-X.; Dong, L.-X.; Sun, H.-R.; Gao, K.; Yan, X.-P. Incorporation of computed tomography and magnetic resonance imaging function into NaYF4: Yb/Tm upconversion nanoparticles for in vivo trimodal bioimaging. Anal. Chem. 2013, 85, 12166–12172. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.; Xu, L.; Sun, C.; Yang, D.; Xu, J.; He, F.; Gai, S.; Yang, P. A novel core–shell structured upconversion nanorod as a multimodal bioimaging and photothermal ablation agent for cancer theranostics. J. Mater. Chem. B 2018, 6, 2597–2607. [Google Scholar] [CrossRef]
- Peng, J.; Sun, Y.; Zhao, L.; Wu, Y.; Feng, W.; Gao, Y.; Li, F. Polyphosphoric acid capping radioactive/upconverting NaLuF4: Yb, Tm, 153Sm nanoparticles for blood pool imaging in vivo. Biomaterials 2013, 34, 9535–9544. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef]
- Adusumalli, V.N.; Sarkar, S.; Mahalingam, V. Strong Single-Band Blue Emission from Colloidal Ce3+/Tm3+-Doped NaYF4 Nanocrystals for Light-Emitting Applications. Chemphyschem 2015, 16, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.; Siwale, R.; Singh, A.; Mody, H. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Iqbal, M.A.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of silver nanoparticles for biomedical applications: A mini review. Inorg. Chem. Commun. 2022, 2022, 109980. [Google Scholar] [CrossRef]
- Malik, M.; Iqbal, M.A.; Malik, M.; Raza, M.A.; Shahid, W.; Choi, J.R.; Pham, P.V. Biosynthesis and Characterizations of Silver Nanoparticles from Annona squamosa Leaf and Fruit Extracts for Size-Dependent Biomedical Applications. Nanomaterials 2022, 12, 616. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Wang, F.; Zhao, D.; Qu, C.; Wang, X.; Yi, Y.; Cassan, E.; Zhang, D. High-gain polymer optical waveguide amplifiers based on core-shell NaYF4/NaLuF4: Yb(3+), Er(3+) NPs-PMMA covalent-linking nanocomposites. Sci. Rep. 2016, 6, 36729. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.-L.; Chow, C.-F.; Tsang, K.-C.; Lei, E.N.Y.; Roy, V.A.L.; Lam, M.H.W.; Lee, C.S.; Pun, E.Y.B.; Li, J. Long aliphatic chain coated rare-earth nanocrystal as polymer-based optical waveguide amplifiers. J. Mater. Chem. 2010, 20, 7526–7529. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, D.; Li, T.; Zhang, D.; Song, L.; Zhen, Z. Erbium-ytterbium codoped waveguide amplifier fabricated with solutionprocessable complex. Appl. Phys. Lett. 2009, 94, 041119. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, P.; Wang, D.; Qin, Z.; Wang, F.; Zhang, D.; Zhao, D.; Qin, G.; Qin, W. KMnF3: Yb3+,Er3+Core-Active-Shell Nanoparticles with Broadband Down-Shifting Luminescence at 1.5 mum for Polymer-Based Waveguide Amplifiers. Nanomaterials 2019, 9, 463. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Qiu, J.; Xu, X.; Zhou, D. Effect of Ce3+ Concentration on the Luminescence Properties of Ce3+/Er3+Nb3+ Tri-Doped NaYF4 Nanocrystals. J. Nanosci. Nanotechnol. 2016, 16, 3749–3753. [Google Scholar] [CrossRef]
- Huang, B.; Zhou, Y.; Yang, F.; Wu, L.; Qi, Y.; Li, J. The 1.53μm spectroscopic properties of Er3+/Ce3+/Yb3+ tri-doped tellurite glasses containing silver nanoparticles. Opt. Mater. 2016, 51, 9–17. [Google Scholar] [CrossRef]
- Ren, W.; Tian, G.; Jian, S.; Gu, Z.; Zhou, L.; Yan, L.; Jin, S.; Yin, W.; Zhao, Y. TWEEN coated NaYF4:Yb,Er/NaYF4 core/shell upconversion nanoparticles for bioimaging and drug delivery. RSC Adv. 2012, 2, 7037–7041. [Google Scholar] [CrossRef]
- Boyer, J.C.; Gagnon, J.; Cuccia, L.A.; Capobianco, J.A. Synthesis, Characterization, and Spectroscopy of NaGdF4: Ce3+, Tb3+/NaYF4 Core/Shell Nanoparticles. Chem. Mater. 2007, 19, 3358–3360. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, L.; Gu, Z.; Tian, G.; Yan, L.; Ren, W.; Yin, W.; Liu, X.; Zhang, X.; Hu, Z.; et al. A new near infrared photosensitizing nanoplatform containing blue-emitting up-conversion nanoparticles and hypocrellin A for photodynamic therapy of cancer cells. Nanoscale 2013, 5, 11910–11918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Tao, L.; Tsang, Y.H.; Jin, W. Core–shell nanoarchitecture: A strategy to significantly enhance white-light upconversion of lanthanide-doped nanoparticles. J. Mater. Chem. C 2013, 1, 4313–4318. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Li, G.; Shang, M.; Kang, X.; Lin, J. Colloidal synthesis and remarkable enhancement of the upconversion luminescence of BaGdF5:Yb3+/Er3+ nanoparticles by active-shell modification. J. Mater. Chem. 2011, 21, 5923–5927. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Y.; Sun, T.; Tang, Y.; Li, J.; Cui, H.; Qin, W.; Wang, F.; Qin, G.; Zhao, D. Polymer-based S-band waveguide amplifier using NaYF4:Yb,Tm-PMMA nanocomposite as gain medium. Opt. Lett. 2022, 47, 154–157. [Google Scholar] [CrossRef]
- Zhai, X. Enhancement of 1.53 μm emission band in NaYF4:Er3+,Yb3+,Ce3+ nanocrystals for polymer-based optical waveguide amplifiers. Opt. Mater. Express 2013, 3, 270–277. [Google Scholar] [CrossRef]
- Ding, M.Y.; Hou, J.J.; Yuan, Y.J.; Bai, W.F.; Lu, C.H.; Xi, J.H.; Ji, Z.G.; Chen, D.Q. Nd3+/Yb3+ cascade-sensitized single-band red upconversion emission in active-core/active-shell nanocrystals. Nanotechnology 2018, 29, 345704. [Google Scholar] [CrossRef]
- Sani, E.; Toncelli, A.; Tonelli, M. Effect of Cerium codoping on Er:BaY2F8 crystals. Opt. Express 2005, 13, 8980–8992. [Google Scholar] [CrossRef]
- Shen, S.; Richards, B.; Jha, A. Enhancement in pump inversion efficiency at 980 nm in Er3+, Er3+/Eu3+ and Er3+/Ce3+ doped tellurite glass fibers. Opt. Express 2006, 14, 5050–5054. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yoshimura, T.; Fukue, K.; Higashihata, M.; Nakata, Y.; Okada, T. Large improvement in quantum fluorescence yield of Er3+-doped fluorozirconate and fluoroindate glasses by Ce3+ codoping. J. Appl. Phys. 2000, 88, 2187–2190. [Google Scholar] [CrossRef]
- Johnson, N.J.; He, S.; Diao, S.; Chan, E.M.; Dai, H.; Almutairi, A. Direct Evidence for Coupled Surface and Concentration Quenching Dynamics in Lanthanide-Doped Nanocrystals. J. Am. Chem. Soc. 2017, 139, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Lang, Y.; Yuan, Z.; Zhao, D.; Qin, G.; Qin, W. Synthesis of ultra-small BaLuF5:Yb3+,Er3+@BaLuF5:Yb3+ active-core–active-shell nanoparticles with enhanced up-conversion and down-conversion luminescence by a layer-by-layer strategy. J. Mater. Chem. C 2015, 3, 2045–2053. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Li, D.; Yang, Y.; Fu, Y.; Dong, Y.; Yin, J.; Qin, W.; Jia, Z.; Zhao, D. Introduce Ce3+ Ions to Realize Enhancement of C+L Band Luminescence of KMnF3: Yb, Er Nanoparticles. Nanomaterials 2023, 13, 2153. https://doi.org/10.3390/nano13152153
Cui H, Li D, Yang Y, Fu Y, Dong Y, Yin J, Qin W, Jia Z, Zhao D. Introduce Ce3+ Ions to Realize Enhancement of C+L Band Luminescence of KMnF3: Yb, Er Nanoparticles. Nanomaterials. 2023; 13(15):2153. https://doi.org/10.3390/nano13152153
Chicago/Turabian StyleCui, Hao, Daguang Li, Yu Yang, Yuewu Fu, Yanhui Dong, Jing Yin, Weiping Qin, Zhixu Jia, and Dan Zhao. 2023. "Introduce Ce3+ Ions to Realize Enhancement of C+L Band Luminescence of KMnF3: Yb, Er Nanoparticles" Nanomaterials 13, no. 15: 2153. https://doi.org/10.3390/nano13152153



