A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites
Abstract
:1. Introduction
2. Photocatalytic Wastewater Treatment
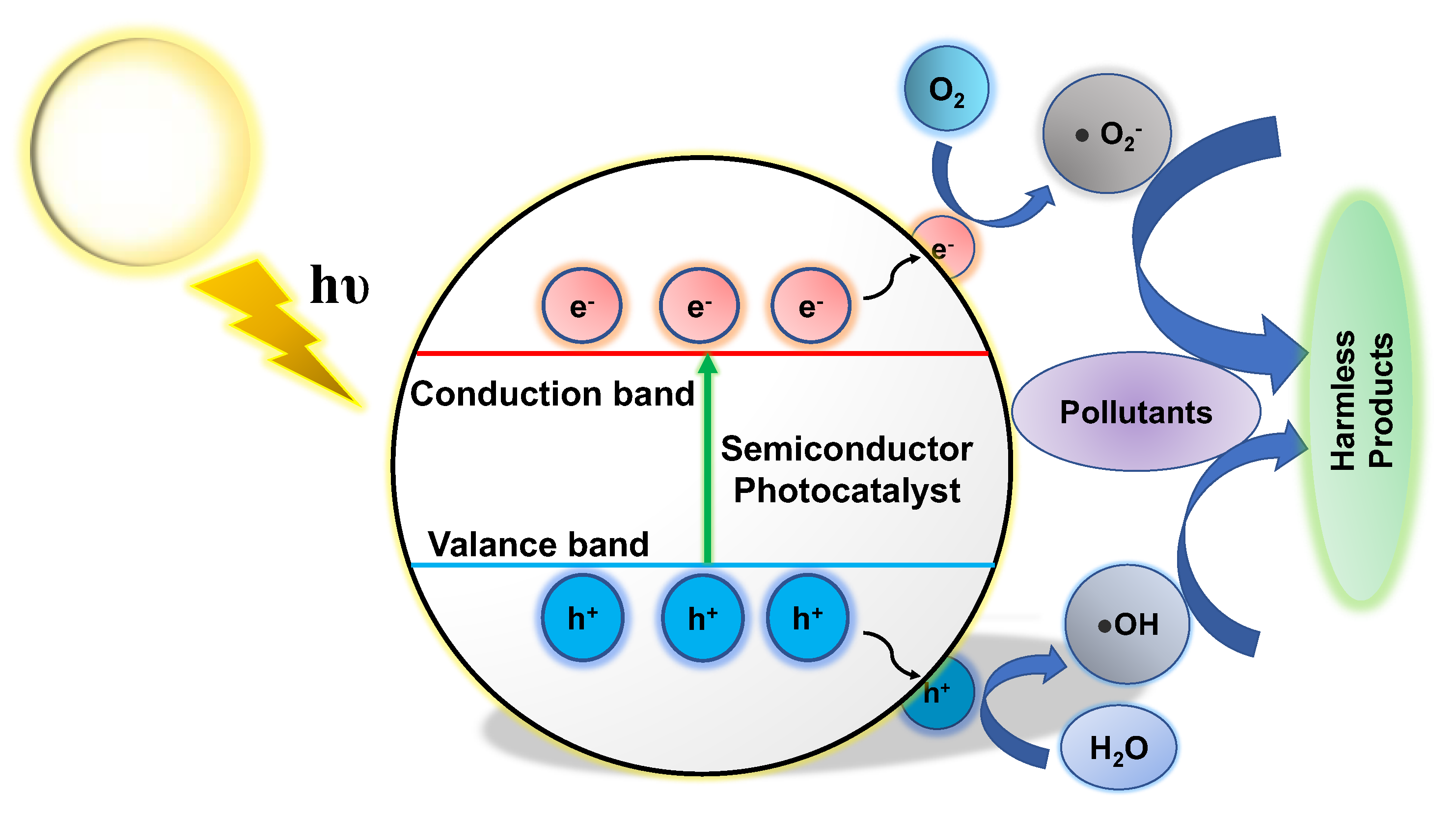
2.1. Basic Principles of Photocatalysis
2.2. Advantages of Photocatalytic Wastewater Treatment
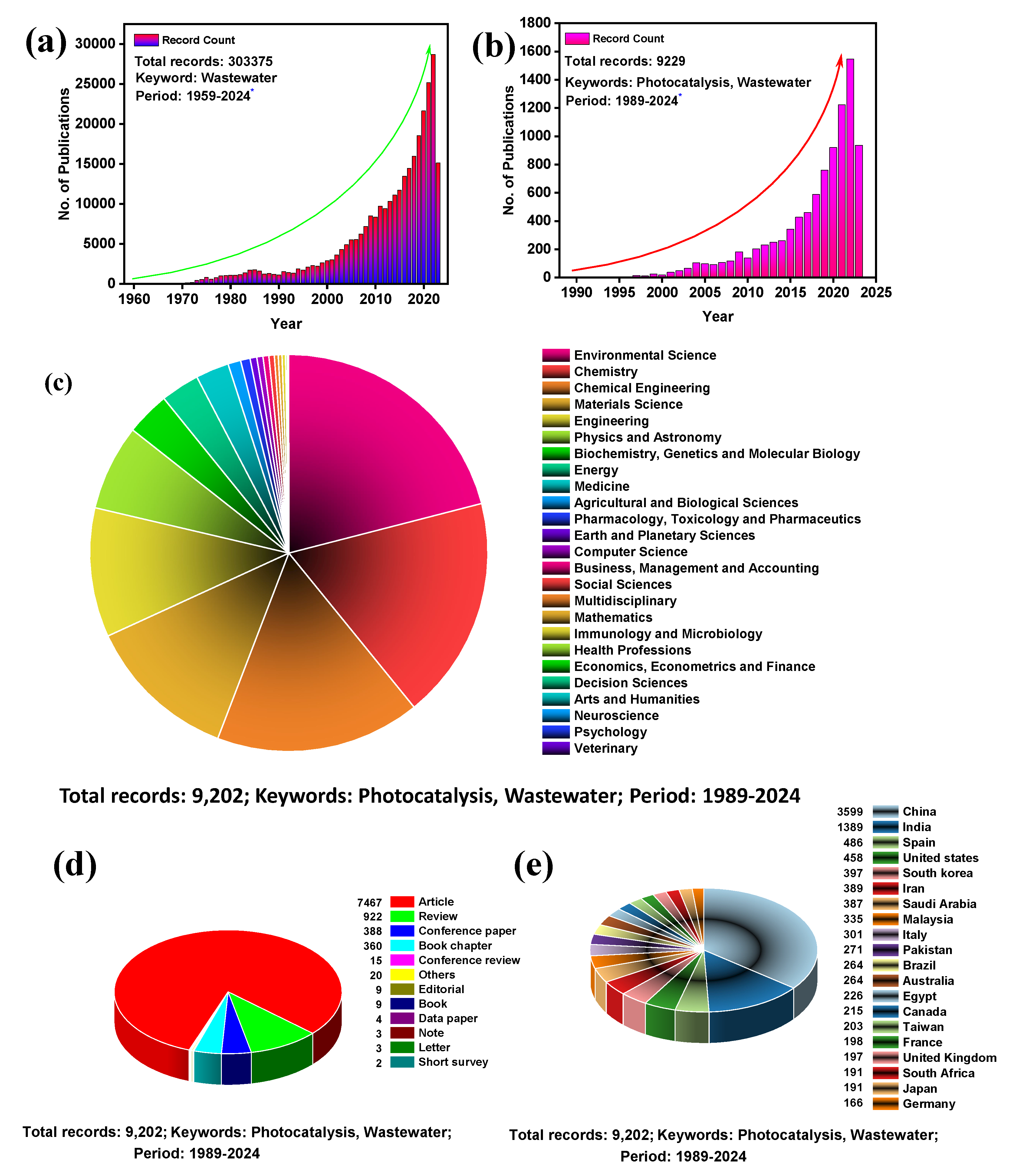
2.3. Global Research Trends
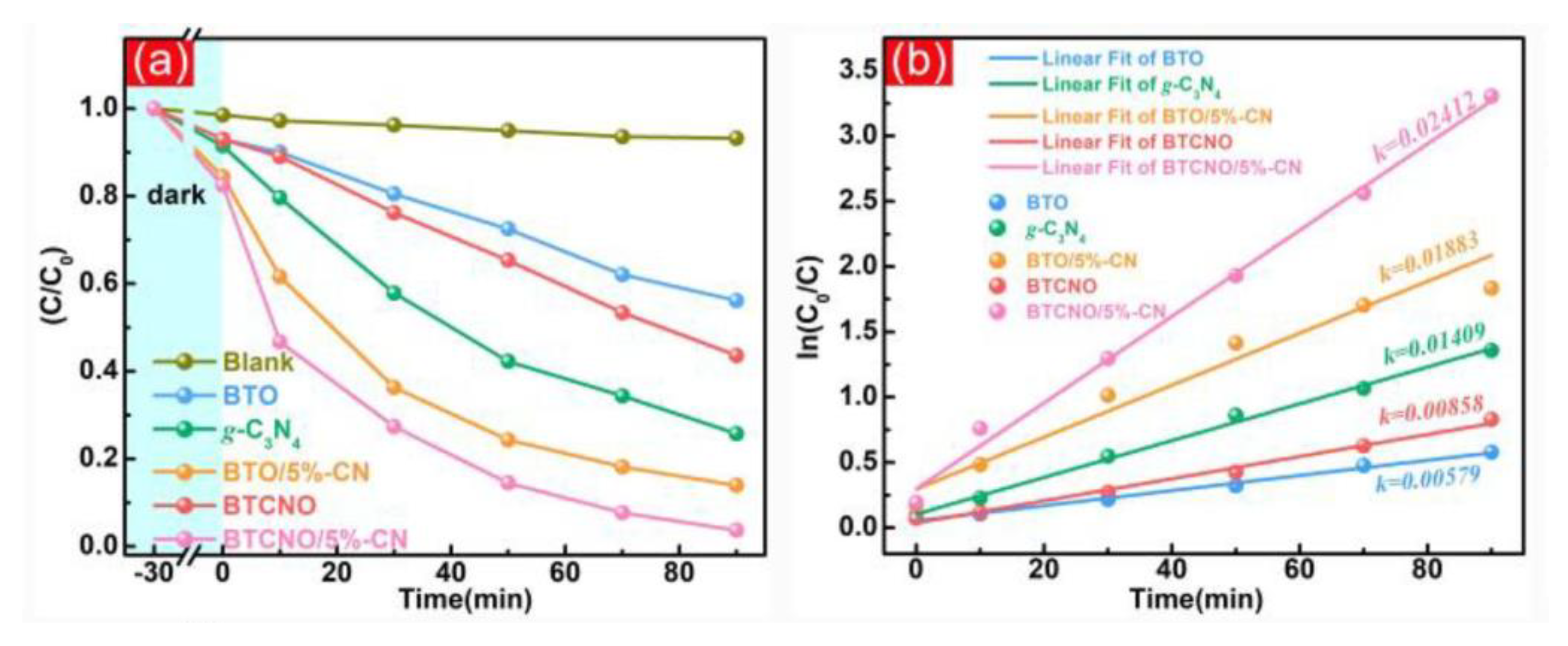
3. g-C3N4/TNP Nanocomposite as Photocatalysts
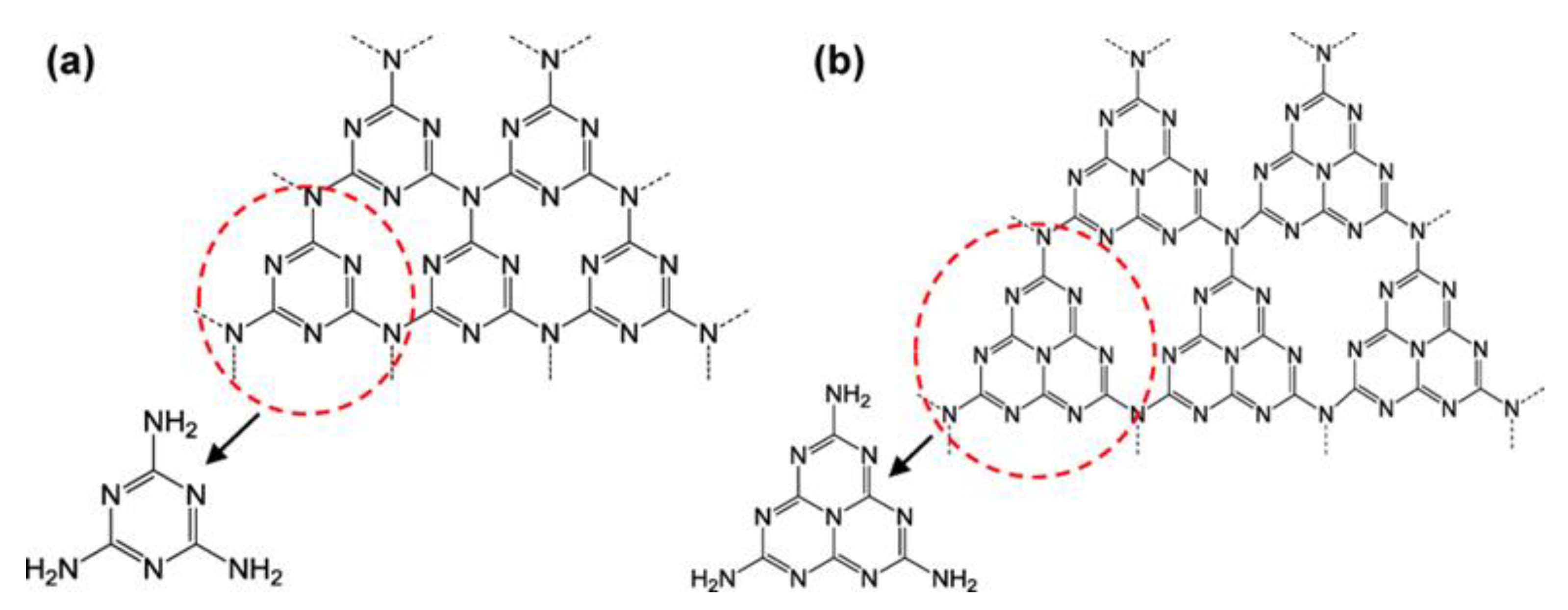
3.1. Overview of g-C3N4
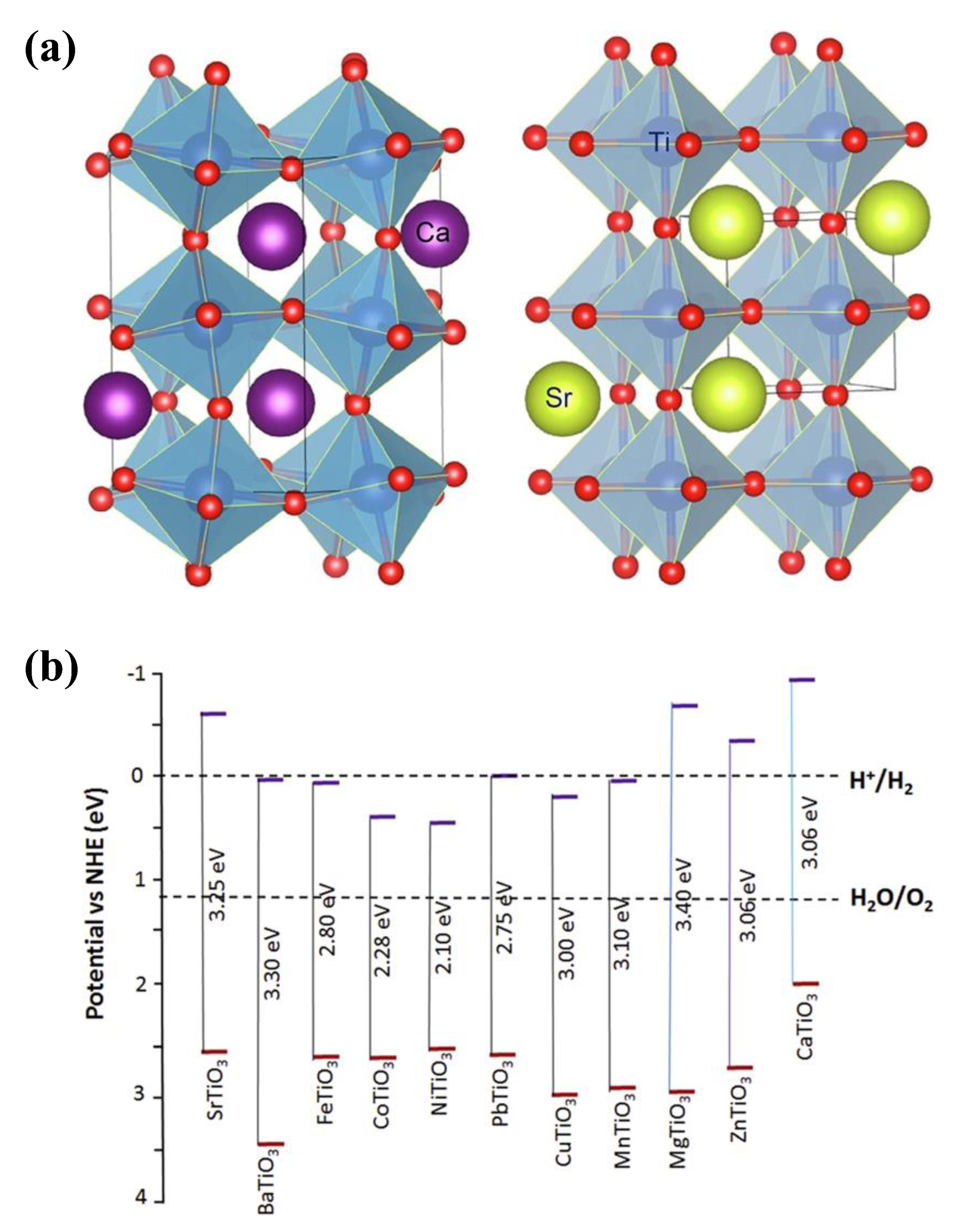
3.2. Overview of TNP Photocatalysts
| TNP Type | Synthesis Method | Morphology | Bandgap (eV) | Application | Ref. |
|---|---|---|---|---|---|
| ZnTiO3, CdTiO3, PbTiO3 | Solid state; solvo-combustion | Irregular | 3.7 4.0 2.75 | H2 production | [88] |
| BaTiO3, CaTiO3, SrTiO3 | Solid state | Elongated cylinders; spherical | 2.89 2.92 2.85 | Methyl orange (MO) degradation | [26] |
| Na/Fe co-doped BaTiO3 | Solid state | Spherical | 2.3 | RhB, malchite green (MG) degradation | [89] |
| SrTiO3 | Sol–gel | Tubular | 3.18 | MO degradation | [90] |
| Ag doped ZnTiO3 | Sol–gel | Hexagonal | 3.54–3.50 | MB degradation, antibacterial | [86] |
| ZnTiO3 | Sol–gel | Rod | 3.54–3.75 | Amoxicillin (AMX), TC, MO, MB degradation | [78] |
| ZnTiO3 | Sol–gel | Spherical | 3.2 | MO degradation | [91] |
| La2Ti2O7 | Sol–gel | Large particles | NA | Azophloxine degradation | [79] |
| Pt/CaTiO3 | Sol–gel | Cluster | 2.8 | Photoconversion of nitrobenzene (NTB) to aniline | [80] |
| SrTiO3 | Hydrothermal | Nanocubes | 3.19 | MB, Tartrazine (TZ) degradation | [81] |
| MTiO3 (M = Sr, Ba, Ca) | Hydrothermal | Spherical | 3.0–3.2 | H2 production, MB degradation | [76] |
| Bi4Ti3O12 | Hydrothermal | Spherical | 2.79 | MO degradation | [25] |
| Au@PbTiO3 | Hydrothermal | Nanoplates | 3.05 | RhB degradation | [92] |
| PbTiO3/CdS | Hydrothermal | Rectangular nanoplates | 2.85 (PbTiO3), 2.35 (CdS) | H2 production | [93] |
| PbTiO3 | Hydrothermal | Nanoplates | 3.08 | H2 production, RhB, MB, MO degradation | [27] |
| Ni@PbTiO3 | Hydrothermal | Nanoplates | 3.07 (PbTiO3), 3.25 (NiO) | RhB degradation | [87] |
| PbTiO3 | Hydrothermal | Nanoplates | NA | H2 production | [94] |
| Ag doped PbTiO3 | Hydrothermal | Irregular pores or foramen | 3.76–3.38 | MB degradation | [95] |
| NaTaO3, SrTiO3 | CVD | Orthorhombic, cauliflower | 3.12–4.01 | H2 production | [96] |
| CaTiO3-TiO2 | CVD | Spherical | 3.0 | H2 production | [97] |
| MgTi2O5 | CVD | Spherical | 3.4 | PEC water splitting | [98] |
| LaPO4/CdS | Self-assembly | Root nodule | NA | CO2 reduction | [82] |
| Zn/Cr-LDH-Pb2Nb3O10 | Self-assembly | Nanosheet | NA | O2 production | [99] |
| BaxSr1−xTiO3 | Molten salt | Cubic | 3.24 | RhB degradation | [100] |
3.3. Synthesis Routes for g-C3N4/TNP Nanocomposites
3.3.1. Hydrothermal Method
3.3.2. Solid-State/Heat Treatment Method
3.3.3. In Situ Method
3.3.4. Co-Precipitation Method
4. Photocatalytic Mechanism
4.1. Type II Heterojunction
4.2. Z-Scheme Heterojunction
4.3. S-Scheme Heterojunction
4.4. p–n Junction Heterojunction
5. Performance of g-C3N4/TNP Nanocomposites in Wastewater Treatment
6. Factors Affecting g-C3N4/TNP Photocatalytic Performance
6.1. Charge Carrier Separation, Transfer, and Reactive Species Generation
6.2. Catalyst Loading and Dosage
6.3. pH
6.4. Co-Catalysts and Dopants
7. Potential Impact and Significance
7.1. Environmental Impact
7.2. Water Resource Conservation
7.3. Public Health and Safety
7.4. Economic Opportunity
7.5. Technological Advancement
8. Challenges and Future Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Ray, S.K.; Cho, J.; Hur, J. A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manag. 2021, 290, 112679. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Ray, S.K.; Hur, J. Surface modifications, perspectives, and challenges of scheelite metal molybdate photocatalysts for removal of organic pollutants in wastewater. Ceram. Int. 2020, 46, 20608–20622. [Google Scholar] [CrossRef]
- Lv, M.; Yan, L.; Liu, C.; Su, C.; Zhou, Q.; Zhang, X.; Lan, Y.; Zheng, Y.; Lai, L.; Liu, X.; et al. Non-covalent functionalized graphene oxide (GO) adsorbent with an organic gelator for co-adsorption of dye, endocrine-disruptor, pharmaceutical and metal ion. J. Chem. Eng. 2018, 349, 791–799. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [Green Version]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Hadei, M.; Mesdaghinia, A.; Nabizadeh, R.; Mahvi, A.H.; Rabbani, S.; Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. Environ. Sci. Pollut. Res. 2021, 28, 13055–13071. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Lhomme, L.; Brosillon, S.; Wolbert, D. Photocatalytic degradation of pesticides in pure water and a commercial agricultural solution on TiO2 coated media. Chemosphere 2008, 70, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A.; Abdel-Salam, O.E.; Mostafa, A.A.; Mohamed, R.M.; Hanafy, M. Rapid synthesis of titania–silica nanoparticles photocatalyst by a modified sol–gel method for cyanide degradation and heavy metals removal. J. Alloys Compd. 2013, 551, 1–7. [Google Scholar] [CrossRef]
- Bi, R.; Yin, D.; Lei, B.; Chen, F.; Zhang, R.; Li, W. Mercaptocarboxylic acid intercalated MgAl layered double hydroxide adsorbents for removal of heavy metal ions and recycling of spent adsorbents for photocatalytic degradation of organic dyes. Sep. Purif. Technol. 2022, 289, 120741. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. Synergistic effect of organic-inorganic hybrid nanocomposite ion exchanger on photocatalytic degradation of Rhodamine-B dye and heavy metal ion removal from industrial effluents. J. Environ. Chem. Eng. 2018, 6, 7091–7101. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile organic compounds (VOCs) removal by photocatalysts: A review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef]
- Li, J.; Chen, R.; Cui, W.; Dong, X.A.; Wang, H.; Kim, K.-H.; Chu, Y.; Sheng, J.; Sun, Y.; Dong, F. Synergistic Photocatalytic Decomposition of a Volatile Organic Compound Mixture: High Efficiency, Reaction Mechanism, and Long-Term Stability. ACS Catal. 2020, 10, 7230–7239. [Google Scholar] [CrossRef]
- He, F.; Weon, S.; Jeon, W.; Chung, M.W.; Choi, W. Self-wetting triphase photocatalysis for effective and selective removal of hydrophilic volatile organic compounds in air. Nat. Commun. 2021, 12, 6259. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, H.; Luo, Y.; Yu, S.; Liu, A.; Zou, W.; Gao, F.; Dong, L. Facile two-step treatment of carbon nitride for preparation of highly efficient visible-light photocatalyst. Appl. Catal. B Environ. 2018, 227, 541–547. [Google Scholar] [CrossRef]
- Das, S.; Deka, T.; Ningthoukhangjam, P.; Chowdhury, A.; Nair, R.G. A critical review on prospects and challenges of metal-oxide embedded g-C3N4-based direct Z-scheme photocatalysts for water splitting and environmental remediation. Surf. Sci. Adv. 2022, 11, 100273. [Google Scholar] [CrossRef]
- Jiang, F.; Yan, T.; Chen, H.; Sun, A.; Xu, C.; Wang, X. A g-C3N4–CdS composite catalyst with high visible-light-driven catalytic activity and photostability for methylene blue degradation. Appl. Surf. Sci. 2014, 295, 164–172. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhang, C.; Liu, X.; Xu, S. Long-term photochemical stability of heteroaromatic dye-functionalised g-C3N4 via covalent linkage for efficient photocatalytic hydrogen evolution. Dyes Pigment. 2023, 212, 111128. [Google Scholar] [CrossRef]
- Hayat, A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Jawad Ali Shah, S.; Amin, M.A.; Ali, T.; Bashir, T.; Palamanit, A.; et al. Graphitic carbon nitride (g–C3N4)–based semiconductor as a beneficial candidate in photocatalysis diversity. Int. J. Hydrogen Energy 2022, 47, 5142–5191. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 2021, 143, 111417. [Google Scholar] [CrossRef]
- Lin, X.; Lv, P.; Guan, Q.; Li, H.; Zhai, H.; Liu, C. Bismuth titanate microspheres: Directed synthesis and their visible light photocatalytic activity. Appl. Surf. Sci. 2012, 258, 7146–7153. [Google Scholar] [CrossRef]
- Karakozov, B.K.; Kozlovskiy, A.L.; Janseitov, D.M.; Zdorovets, M.V. Solid-phase synthesis and study of the structural, optical, and photocatalytic properties of the ATiO3, A=Ca, Sr, Ba ceramic. J. Mater. Sci. Mater. Electron. 2021, 32, 24436–24445. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, M.; Liu, H.; Li, W.; Li, H.; Bian, Z. Charge separation and interfacial selectivity induced by synergistic effect of ferroelectricity and piezoelectricity on PbTiO3 monocrystalline nanoplates. Nano Energy 2020, 73, 104768. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Bai, J.; Xiang, J.; Chen, C.; Guo, C. Piezoelectric-effect-enhanced photocatalytic performance in Cr/Nb modified Bi4Ti3O12/g-C3N4 Z-scheme system. J. Chem. Eng. 2023, 456, 141095. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Jamil, T.S.; El-Seesy, I.E.; Souaya, E.R.; Nasr, R.A. Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2. J. Chem. Eng. 2011, 168, 446–454. [Google Scholar] [CrossRef]
- Alam, U.; Pandey, K.; Verma, N. Photocatalytic oxidation of glyphosate and reduction of Cr(VI) in water over ACF-supported CoNiWO4-gCN composite under batch and flow conditions. Chemosphere 2022, 297, 134119. [Google Scholar] [CrossRef]
- Samad, A.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic oxidation and simultaneous removal of arsenite with CuO/ZnO photocatalyst. J. Photochem. Photobiol. A Chem. 2016, 325, 97–103. [Google Scholar] [CrossRef]
- Sohrabian, M.; Mahdikhah, V.; Alimohammadi, E.; Sheibani, S. Improved photocatalytic performance of SrTiO3 through a Z-scheme polymeric-perovskite heterojunction with g-C3N4 and plasmonic resonance of Ag mediator. Appl. Surf. Sci. 2023, 618, 156682. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, S.; Sun, M.; Yang, L.; Luo, S.; Crittenden, J.C. A Critical Review on Energy Conversion and Environmental Remediation of Photocatalysts with Remodeling Crystal Lattice, Surface, and Interface. ACS Nano 2019, 13, 9811–9840. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Kumara, A.; Jayanetti, M.; Usgodaarachchi, L.; Liyanaarachchi, H.; Lansakara, B. Fabrication of dual Z-scheme g-C3N4/Fe2TiO5/Fe2O3 ternary nanocomposite using natural ilmenite for efficient photocatalysis and photosterilization under visible light. Surf. Sci. Adv. 2022, 12, 100337. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mat. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Z.-T.; Yang, P.-C.; Ding, Y.-X.; Lin, K.-Y.A.; Nguyen, B.-S. Self-assembly L-cysteine based 2D g-C3N4 nanoflakes for light-dependent degradation of rhodamine B and tetracycline through photocatalysis. J. Taiwan Inst. Chem. Eng. 2021, 123, 219–227. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Molaei, M.J. Graphitic carbon nitride (g-C3N4) synthesis and heterostructures, principles, mechanisms, and recent advances: A critical review. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L.-A. A critical review of g-C3N4-based photocatalytic membrane for water purification. J. Chem. Eng. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, S.; Zhang, A.; Li, Y.; Fu, Y.; Zhou, Q. Intramolecular built-in electric field enhanced polymerized nitrogen-carbon homojunction π*-electron delocalization enrichment promotes photocatalytic uranium (VI) reduction. Appl. Catal. B Environ. 2023, 338, 123023. [Google Scholar] [CrossRef]
- Tan, M.; Yu, C.; Li, J.; Li, Y.; Tao, C.; Liu, C.; Meng, H.; Su, Y.; Qiao, L.; Bai, Y. Engineering of g-C3N4-based photocatalysts to enhance hydrogen evolution. Adv. Colloid Interface Sci. 2021, 295, 102488. [Google Scholar] [CrossRef]
- Lei, H.; He, Q.; Wu, M.; Xu, Y.; Sun, P.; Dong, X. Piezoelectric polarization promoted spatial separation of photoexcited electrons and holes in two-dimensional g-C3N4 nanosheets for efficient elimination of chlorophenols. J. Hazard. Mater. 2022, 421, 126696. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, H.; Wang, Z.; Yu, Z.; Wang, L.; Mueller, J.F.; Guo, J. Efficient photocatalytic destruction of recalcitrant micropollutants using graphitic carbon nitride under simulated sunlight irradiation. Environ. Sci. Ecotechnol. 2021, 5, 100079. [Google Scholar] [CrossRef]
- Zuluaga, S.; Liu, L.-H.; Shafiq, N.; Rupich, S.M.; Veyan, J.-F.; Chabal, Y.J.; Thonhauser, T. Structural band-gap tuning in g-C3N4. Phys. Chem. Chem. Phys. 2015, 17, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wang, Y.; Zuo, C.; Wang, J.; Sheng, X.; Huang, Y.; Zhang, Y.; Zhou, Y. Bimetallic PtNi alloy modified 2D g-C3N4 nanosheets as an efficient cocatalyst for enhancing photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.K.A.; Dao, D.Q.; Shin, E.W. Ethanol Solvothermal Treatment on Graphitic Carbon Nitride Materials for Enhancing Photocatalytic Hydrogen Evolution Performance. Nanomaterials 2022, 12, 179. [Google Scholar] [CrossRef]
- Muhmood, T.; Uddin, A. Fabrication of spherical-graphitic carbon nitride via hydrothermal method for enhanced photo-degradation ability towards antibiotic. Chem. Phys. Lett. 2020, 753, 137604. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Lin, L.; Valério, A.; He, D. Synthesis of carbon nitride nanosheets with tunable size by hydrothermal method for tetracycline degradation. Mater. Lett. 2020, 264, 127005. [Google Scholar] [CrossRef]
- Gu, Z.; Cui, Z.; Wang, Z.; Qin, K.S.; Asakura, Y.; Hasegawa, T.; Tsukuda, S.; Hongo, K.; Maezono, R.; Yin, S. Carbon vacancies and hydroxyls in graphitic carbon nitride: Promoted photocatalytic NO removal activity and mechanism. Appl. Catal. B Environ. 2020, 279, 119376. [Google Scholar] [CrossRef]
- Hong, Z.; Shen, B.; Chen, Y.; Lin, B.; Gao, B. Enhancement of photocatalytic H2 evolution over nitrogen-deficient graphitic carbon nitride. J. Mater. Chem. 2013, 1, 11754–11761. [Google Scholar] [CrossRef]
- Li, K.; Bao, L.; Cao, S.; Xue, Y.; Yan, S.; Gao, H. Template-Assisted Surface Hydrophilicity of Graphitic Carbon Nitride for Enhanced Photocatalytic H2 Evolution. ACS Appl. Energy Mater. 2021, 4, 12965–12973. [Google Scholar] [CrossRef]
- Yang, F.; Liu, D.; Li, Y.; Cheng, L.; Ye, J. Salt-template-assisted construction of honeycomb-like structured g-C3N4 with tunable band structure for enhanced photocatalytic H2 production. Appl. Catal. B Environ. 2019, 240, 64–71. [Google Scholar] [CrossRef]
- Maeda, K.; An, D.; Kuriki, R.; Lu, D.; Ishitani, O. Graphitic carbon nitride prepared from urea as a photocatalyst for visible-light carbon dioxide reduction with the aid of a mononuclear ruthenium(II) complex. Beilstein J. Org. Chem. 2018, 14, 1806–1812. [Google Scholar] [CrossRef]
- Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Kalarikkal, N.; Lennartz, B. Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation. Nanomaterials 2023, 13, 762. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Oxygen functional groups in graphitic carbon nitride for enhanced photocatalysis. J. Colloid Interface Sci. 2016, 468, 176–182. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J. Direct microwave synthesis of graphitic C3N4 with improved visible-light photocatalytic activity. Ceram. Int. 2016, 42, 4063–4071. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Ho, W.; Han, S.; Wang, P.; Lee, S.; Zhang, Z. Modulation of sulfur vacancies at ZnIn2S4-δ/g-C3N4 heterojunction interface for successive C-H secession in photocatalytic gaseous formaldehyde complete oxidation. Appl. Catal. B Environ. 2023, 338, 123048. [Google Scholar] [CrossRef]
- Chakraborty, R.; Vilya, K.; Pradhan, M.; Nayak, A.K. Recent advancement of biomass-derived porous carbon based materials for energy and environmental remediation applications. J. Mater. Chem. 2022, 10, 6965–7005. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, K.; Wang, X.; Guo, Q.; Wu, Y.; Du, Y.; Zhang, L.; Xia, J.; Tang, H.; Zhang, X.; et al. 0D/2D Schottky junction synergies with 2D/2D S-scheme heterojunction strategy to achieve uniform separation of carriers in 0D/2D/2D quasi CNQDs/TCN/ZnIn2S4 towards photocatalytic remediating petroleum hydrocarbons polluted marine. Appl. Catal. B Environ. 2023, 325, 122387. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Er, C.-C.; Kong, X.Y.; Ng, B.-J.; Chew, Y.-H.; Tan, L.-L.; Mohamed, A.R.; Chai, S.-P. Two-dimensional interface engineering of g-C3N4/g-C3N4 nanohybrid: Synergy between isotype and p-n heterojunctions for highly efficient photocatalytic CO2 reduction. J. Chem. Eng. 2023, 466, 143287. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, Y.; Xia, L.; Yu, X.-F.; Zhang, K.; Du, Y.; Zhang, L.; Tang, H.; Cheng, J.; Shang, J.; et al. Stitching electron localized heptazine units with “carbon patches” to regulate exciton dissociation behavior of carbon nitride for photocatalytic elimination of petroleum hydrocarbons. J. Chem. Eng. 2023, 452, 139092. [Google Scholar] [CrossRef]
- Xia, L.; Sun, Z.; Wu, Y.; Yu, X.-F.; Cheng, J.; Zhang, K.; Sarina, S.; Zhu, H.-Y.; Weerathunga, H.; Zhang, L.; et al. Leveraging doping and defect engineering to modulate exciton dissociation in graphitic carbon nitride for photocatalytic elimination of marine oil spill. J. Chem. Eng. 2022, 439, 135668. [Google Scholar] [CrossRef]
- Xu, Z.; Kong, L.; Wang, H.; Ma, Q.; Shen, X.; Wang, J.; Premlatha, S. Soft-template assisted preparation of hierarchically porous graphitic carbon nitride layers for high-performance supercapacitors. J. Appl. Polym. Sci. 2022, 139, e52947. [Google Scholar] [CrossRef]
- Ge, M.; Li, X.; Liu, Z.; Zhang, M. Enhanced photocatalytic degradation performance of cyano groups-modified carbon nitride nanosheets synthesized by magadiite template-assisted thermal treatment. Mater. Sci. Semicond. Process. 2022, 141, 106420. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Q.; Wang, J. The ultra-rapid synthesis of 2D graphitic carbon nitride nanosheets via direct microwave heating for field emission. Chem. Commun. 2016, 52, 3396–3399. [Google Scholar] [CrossRef]
- Meng, L.; Ushakova, E.V.; Zhou, Z.; Liu, E.; Li, D.; Zhou, D.; Tan, Z.; Qu, S.; Rogach, A.L. Microwave-assisted in situ large scale synthesis of a carbon dots@g-C3N4 composite phosphor for white light-emitting devices. Mater. Chem. Front. 2020, 4, 517–523. [Google Scholar] [CrossRef]
- Dai, H.; Gao, X.; Liu, E.; Yang, Y.; Hou, W.; Kang, L.; Fan, J.; Hu, X. Synthesis and characterization of graphitic carbon nitride sub-microspheres using microwave method under mild condition. Diam. Relat. Mater. 2013, 38, 109–117. [Google Scholar] [CrossRef]
- Ma, H.; Shi, Z.; Li, S.; Liu, N. Large-scale production of graphitic carbon nitride with outstanding nitrogen photofixation ability via a convenient microwave treatment. Appl. Surf. Sci. 2016, 379, 309–315. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.-Q.; Huang, H.; Feng, J.-J.; Wang, A.-J. Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens. Actuators B Chem. 2016, 226, 506–511. [Google Scholar] [CrossRef]
- Paramanik, L.; Subudhi, S.; Parida, K.M. Visible light active titanate perovskites: An overview on its synthesis, characterization and photocatalytic applications. Mater. Res. Bull. 2022, 155, 111965. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A Review on Visible Light Active Perovskite-Based Photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Alammar, T.; Hamm, I.; Wark, M.; Mudring, A.-V. Low-temperature route to metal titanate perovskite nanoparticles for photocatalytic applications. Appl. Catal. B Environ. 2015, 178, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Cui, W.; Yang, L.; Zhang, G.; Li, X.; Shen, Y.; Dong, F.; Sun, Y. The structural differences of perovskite ATiO3 (A=Ca, Sr) dictate the photocatalytic VOCs mineralization efficiency. J. Chem. Eng. 2021, 425, 130613. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Wang, C.-P.; Chen, P.-C.; Lin, K.-Y.A.; Ghosh, S.; Huang, C.-W.; Nguyen, V.-H. Perovskite Zinc Titanate Photocatalysts Synthesized by the Sol–Gel Method and Their Application in the Photocatalytic Degradation of Emerging Contaminants. Catalysts 2021, 11, 854. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Ma, Z.; Li, H.; Wang, H. Photocatalytic degradation of azophloxine on porous La2Ti2O7 prepared by sol-gel method. Solid State Sci. 2019, 87, 58–63. [Google Scholar] [CrossRef]
- Shawky, A.; Alhaddad, M.; Al-Namshah, K.S.; Mohamed, R.M.; Awwad, N.S. Synthesis of Pt-decorated CaTiO3 nanocrystals for efficient photoconversion of nitrobenzene to aniline under visible light. J. Mol. Liq. 2020, 304, 112704. [Google Scholar] [CrossRef]
- Kiran, K.S.; Shashanka, R.; Lokesh, S.V. Enhanced Photocatalytic Activity of Hydrothermally Synthesized Perovskite Strontium Titanate Nanocubes. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Pan, B.; Qin, J.; Wang, X.; Su, W. Efficient self-assembly synthesis of LaPO4/CdS hierarchical heterostructure with enhanced visible-light photocatalytic CO2 reduction. Appl. Surf. Sci. 2020, 504, 144379. [Google Scholar] [CrossRef]
- Solís, R.R.; Bedia, J.; Rodríguez, J.J.; Belver, C. A review on alkaline earth metal titanates for applications in photocatalytic water purification. J. Chem. Eng. 2021, 409, 128110. [Google Scholar] [CrossRef]
- Bin Adnan, M.A.; Arifin, K.; Minggu, L.J.; Kassim, M.B. Titanate-based perovskites for photochemical and photoelectrochemical water splitting applications: A review. Int. J. Hydrogen Energy 2018, 43, 23209–23220. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, P.; Bansal, N.K.; Paul, M.; Dixit, H.; Porwal, S.; Mishra, S.; Singh, T. Recent progress in perovskite transition metal oxide-based photocatalyst and photoelectrode materials for solar-driven water splitting. J. Environ. Chem. Eng. 2022, 10, 108429. [Google Scholar] [CrossRef]
- Abirami, R.; Kalaiselvi, C.R.; Kungumadevi, L.; Senthil, T.S.; Kang, M. Synthesis and characterization of ZnTiO3 and Ag doped ZnTiO3 perovskite nanoparticles and their enhanced photocatalytic and antibacterial activity. J. Solid State Chem. 2020, 281, 121019. [Google Scholar] [CrossRef]
- Xie, Z.; Shi, J.; Tang, X.; Wang, Y.; Yuan, G.; Liu, J.-M. Piezotronic effect boosted photocatalytic performance of NiO@PbTiO3 p-n heterojunction. Ceram. Int. 2022, 48, 16707–16714. [Google Scholar] [CrossRef]
- Carrasco-Jaim, O.A.; Mora-Hernandez, J.M.; Torres-Martínez, L.M.; Moctezuma, E. A comparative study on the photocatalytic hydrogen production of ATiO3 (A = Zn, Cd and Pb) perovskites and their photoelectrochemical properties. J. Photochem. Photobiol. A Chem. 2019, 371, 98–108. [Google Scholar] [CrossRef]
- Yakout, S.M. Influence of Na and Na/Fe doping on the dielectric constant, ferromagnetic and sunlight photocatalytic properties of BaTiO3 perovskite. J. Solid State Chem. 2020, 290, 121517. [Google Scholar] [CrossRef]
- Kiran, K.S.; Ashwath Narayana, B.; Lokesh, S. Enhanced Photocatalytic Activity of Perovskite SrTiO3 nanorods. Solid State Technol. 2020, 63, 1913–1920. [Google Scholar]
- Salavati-Niasari, M.; Soofivand, F.; Sobhani-Nasab, A.; Shakouri-Arani, M.; Yeganeh Faal, A.; Bagheri, S. Synthesis, characterization, and morphological control of ZnTiO3 nanoparticles through sol-gel processes and its photocatalyst application. Adv. Powder Technol. 2016, 27, 2066–2075. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.; Zhou, Y.; Li, H.; He, W.; Fa, W. Polarization-induced selective growth of Au islands on single-domain ferroelectric PbTiO3 nanoplates with enhanced photocatalytic activity. Appl. Surf. Sci. 2019, 466, 274–281. [Google Scholar] [CrossRef]
- Huang, X.; Lei, R.; Yuan, J.; Gao, F.; Jiang, C.; Feng, W.; Zhuang, J.; Liu, P. Insight into the piezo-photo coupling effect of PbTiO3/CdS composites for piezo-photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 282, 119586. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, S.; Xie, H.; Zhu, J.; Shi, Q.; Ta, N.; Chen, R.; Gao, Y.; An, H.; Nie, W.; et al. Internal-Field-Enhanced Charge Separation in a Single-Domain Ferroelectric PbTiO3 Photocatalyst. Adv. Mater. 2020, 32, 1906513. [Google Scholar] [CrossRef]
- Abirami, R.; Senthil, T.S.; Kalpana, S.; Kungumadevi, L.; Kang, M. Hydrothermal synthesis of pure PbTiO3 and silver doped PbTiO3 perovskite nanoparticles for enhanced photocatalytic activity. Mater. Lett. 2020, 279, 128507. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Chen, J.; Torres-Martínez, L.M.; Ito, A.; Moctezuma, E.; Goto, T. Laser assisted chemical vapor deposition of nanostructured NaTaO3 and SrTiO3 thin films for efficient photocatalytic hydrogen evolution. Fuel 2017, 197, 174–185. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Naeem, R.; McKee, V.; Rehman, A.; Hakeem, A.S.; Mazhar, M. Fabrication of photoactive CaTiO3–TiO2 composite thin film electrodes via facile single step aerosol assisted chemical vapor deposition route. J. Mater. Sci. Mater. Electron. 2019, 30, 1411–1424. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Naeem, R.; McKee, V.; Hakeem, A.S.; Mazhar, M. MgTi2O5 thin films from single molecular precursor for photoelectrochemical water splitting. Sol. Energy Mater. Sol. Cells 2017, 161, 328–337. [Google Scholar] [CrossRef]
- Zheng, B.; Mao, L.; Shi, J.; Chen, Q.; Hu, Y.; Zhang, G.; Yao, J.; Lu, Y. Facile layer-by-layer self-assembly of 2D perovskite niobate and layered double hydroxide nanosheets for enhanced photocatalytic oxygen generation. Int. J. Hydrogen Energy 2021, 46, 34276–34286. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Liu, J.; Zhang, H.; Li, F.; Tseng, C.-W.; Yang, B.; Smith, G.; Zhai, J.; Zhang, Z.; et al. Domain Wall Free Polar Structure Enhanced Photodegradation Activity in Nanoscale Ferroelectric BaxSr1-xTiO3. Adv. Energy Mater. 2020, 10, 2001802. [Google Scholar] [CrossRef]
- Nkwachukwu, O.V.; Arotiba, O.A. Perovskite Oxide–Based Materials for Photocatalytic and Photoelectrocatalytic Treatment of Water. Front. Chem. 2021, 9, 634630. [Google Scholar] [CrossRef]
- Yan, L.; Zhong, S.; Xiao, C.; Chen, Y. Optimization of hydrothermal pretreatment to prepare g-C3N4 with enhanced catalyst yield and photocatalytic activity for aqueous contaminant removal. Mater. Today Sustain. 2023, 22, 100374. [Google Scholar] [CrossRef]
- Yang, B.; Wu, C.; Wang, J.; Bian, J.; Wang, L.; Liu, M.; Du, Y.; Yang, Y. When C3N4 meets BaTiO3: Ferroelectric polarization plays a critical role in building a better photocatalyst. Ceram. Int. 2020, 46, 4248–4255. [Google Scholar] [CrossRef]
- Chen, X.; Tan, P.; Zhou, B.; Dong, H.; Pan, J.; Xiong, X. A green and facile strategy for preparation of novel and stable Cr-doped SrTiO3/g-C3N4 hybrid nanocomposites with enhanced visible light photocatalytic activity. J. Alloys Compd. 2015, 647, 456–462. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Huang, J.; Feng, Z.; Luo, J. Novel g-C3N4/h′ZnTiO3-a′TiO2 direct Z-scheme heterojunction with significantly enhanced visible-light photocatalytic activity. J. Alloys Compd. 2019, 774, 768–778. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Kumari, A.; Guo, C.; Naushad, M.; Vo, D.-V.N.; Iqbal, J.; Stadler, F.J. Construction of dual Z-scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 heterojunction for visible and solar powered coupled photocatalytic antibiotic degradation and hydrogen production: Boosting via I−/I3− and Bi3+/Bi5+ redox mediators. Appl. Catal. B Environ. 2021, 284, 119808. [Google Scholar] [CrossRef]
- Song, T.; Yu, X.; Tian, N.; Huang, H.-W. Preparation, structure and application of g-C3N4/BiOX composite photocatalyst. Int. J. Hydrogen Energy 2021, 46, 1857–1878. [Google Scholar] [CrossRef]
- Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. Photocatalytic disinfection efficiency of 2D structure graphitic carbon nitride-based nanocomposites: A review. J. Mater. Sci. 2019, 54, 12206–12235. [Google Scholar] [CrossRef]
- Shi, H.; Fu, J.; Jiang, W.; Wang, Y.; Liu, B.; Liu, J.; Ji, H.; Wang, W.; Chen, Z. Construction of g-C3N4/Bi4Ti3O12 hollow nanofibers with highly efficient visible-light-driven photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126063. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Nguyen Thi, V.N.; Tran, H.H.; Tran Thi, T.P.; Truong, T.T.; Vo, V. A facile synthesis of g-C3N4/BaTiO3 photocatalyst with enhanced activity for degradation of methylene blue under visible light. Bull. Mater. Sci 2021, 44, 28. [Google Scholar] [CrossRef]
- Ashouri, F.; Khoobi, M.; Ganjali, M.R.; Karimi, M.S. Construction, characterization, and photocatalytic study of La2Ti2O7/C3N4+xHy and La2Ti2O7/GO nanocomposites as efficient catalysts toward photodegradation of harmful organic dyes. J. Photochem. Photobiol. A Chem. 2023, 435, 114279. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Yang, X.; Wu, Z.; Li, Y. Construction of novel 2D/1D g-C3N4/CaTiO3 heterojunction with face-to-face contact for boosting photodegradation of triphenylmethane dyes under simulated sunlight. J. Taiwan Inst. Chem. Eng. 2020, 107, 98–109. [Google Scholar] [CrossRef]
- Nan, Y.; Yang, D.; Tong, Z.; Sun, Y.; Jiang, Z. Fabrication of nanoplate-like g-C3N4/Bi12TiO20 heterojunction with enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2017, 93, 91–101. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Zhang, W.; Jin, B.; Feng, Q.; Huang, J.; Jiao, Z. Advances in Z-scheme semiconductor photocatalysts for the photoelectrochemical applications: A review. Carbon Energy 2022, 4, 294–331. [Google Scholar] [CrossRef]
- Xu, C.-X.; Kong, Y.-L.; Zhang, W.-J.; Yang, M.-D.; Wang, K.; Chang, L.; Chen, W.; Huang, G.-B.; Zhang, J. S-scheme 2D/2D FeTiO3/g-C3N4 hybrid architectures as visible-light-driven photo-Fenton catalysts for tetracycline hydrochloride degradation. Sep. Purif. Technol. 2022, 303, 122266. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Gao, Z.; Zhu, X.; Liu, Y.; Wei, Z.; Zhao, W.; Sun, C. A simple and effective method for fabricating novel p–n heterojunction photocatalyst g-C3N4/Bi4Ti3O12 and its photocatalytic performances. Appl. Catal. B Environ. 2016, 192, 57–71. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, H.; Zhao, X. Enhanced photocatalytic performance of g-C3N4/Bi4Ti3O12 heterojunction nanocomposites. Mater. Sci. Eng. B 2018, 229, 160–172. [Google Scholar] [CrossRef]
- Kadkhodayan, H.; Alizadeh, T. Highly efficient photocatalytic degradation of hazardous organic and inorganic contaminants by heterogeneous nZVI-doped Al2ZnTiO6 double perovskite/g-C3N4 photocatalyst under visible light irradiation. J. Alloys Compd. 2023, 960, 170772. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.; Bai, Y.; Chen, J.; Zhang, C. Simple synthesis of CaTiO3/g-C3N4 heterojunction for efficient photodegradation of methylene blue and levofloxacin. Opt. Mater. 2023, 135, 113239. [Google Scholar] [CrossRef]
- Konstas, P.-S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts 2018, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Luo, D.; Duan, M.; Feng, L.; Zhang, S.; Liu, M. Based on a dual Z-scheme heterojunction and magnetically separable CoFe2O4/g-C3N4/Bi4Ti3O12 flower-like composite for efficient visible-light photocatalytic degradation of organic pollutants. J. Alloys Compd. 2022, 911, 164907. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, P.; Xu, J.; Duan, M.; Zhang, S.; Wu, X. Highly Efficient Bi4Ti3O12/g-C3N4/BiOBr Dual Z-Scheme Heterojunction Photocatalysts with Enhanced Visible Light-Responsive Activity for the Degradation of Antibiotics. Langmuir 2022, 38, 9532–9545. [Google Scholar] [CrossRef] [PubMed]
- Rakibuddin, M.; Kim, H.; Ehtisham Khan, M. Graphite-like carbon nitride (C3N4) modified N-doped LaTiO3 nanocomposite for higher visible light photocatalytic and photo-electrochemical performance. Appl. Surf. Sci. 2018, 452, 400–412. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Li, R.; Xian, T. Design of ternary CaTiO3/g-C3N4/AgBr Z-scheme heterostructured photocatalysts and their application for dye photodegradation. Solid State Sci. 2020, 100, 106102. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Gu, S.; Li, H.; Liu, X.; Ren, C. Visible-light-driven heterojunction photocatalysts based on g-C3N4 decorated La2Ti2O7: Effective transportation of photogenerated carriers in this heterostructure. Catal. Commun. 2017, 96, 50–53. [Google Scholar] [CrossRef]
- Ferreira, M.A.; da Silva, G.T.S.T.; Lopes, O.F.; Mastelaro, V.R.; Ribeiro, C.; Pires, M.J.M.; Malagutti, A.R.; Avansi, W.; Mourão, H.A.J.L. Fabrication of SrTiO3/g-C3N4 heterostructures for visible light-induced photocatalysis. Mater. Sci. Semicond. Process. 2020, 108, 104887. [Google Scholar] [CrossRef]
- Tan, C.-E.; Lee, J.-T.; Su, E.-C.; Wey, M.-Y. Facile approach for Z-scheme type Pt/g-C3N4/SrTiO3 heterojunction semiconductor synthesis via low-temperature process for simultaneous dyes degradation and hydrogen production. Int. J. Hydrogen Energy 2020, 45, 13330–13339. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, W.-K. Sustainable treatment of harmful dyeing industry pollutants using SrZnTiO3/g-C3N4 heterostructure with a light source-dependent charge transfer mechanism. Appl. Catal. B Environ. 2019, 242, 171–177. [Google Scholar] [CrossRef]
- Xu, T.; Niu, P.; Wang, S.; Li, L. High visible light photocatalytic activities obtained by integrating g-C3N4 with ferroelectric PbTiO3. J. Mater. Sci. Technol. 2021, 74, 128–135. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Younis, S.A.; Ali, H.R.; Nada, A.A. Solar-driven photocatalytic transformation of toluene to benzoic acid over perovskite-type NiTiO3 decorated with reduced GO and g-C3N4 nanosheets. J. Environ. Chem. Eng. 2023, 11, 109477. [Google Scholar] [CrossRef]
- Chen, L.; Shen, D.; Li, B.; Xiao, Z.; Sun, W.; Liu, X.; Ma, J.; Li, C.; Wang, W. The efficient electrocatalytic and photocatalytic reduction of nitric oxide into ammonia over 0D/3D g-C3N4 quantum dots/3DOMM-TiO2-x heterojunction. Ceram. Int. 2023, 49, 23129–23139. [Google Scholar] [CrossRef]
- Zhu, M.; Guan, D.; Hu, Z.; Lin, H.-J.; Chen, C.-T.; Sheu, H.-S.; Wang, S.; Zhou, J.; Zhou, W.; Shao, Z. Synergistic effects in ordered Co oxides for boosting catalytic activity in advanced oxidation processes. Appl. Catal. B Environ. 2021, 297, 120463. [Google Scholar] [CrossRef]
- Eghbali, P.; Hassani, A.; Sündü, B.; Metin, Ö. Strontium titanate nanocubes assembled on mesoporous graphitic carbon nitride (SrTiO3/mpg-C3N4): Preparation, characterization and catalytic performance. J. Mol. Liq. 2019, 290, 111208. [Google Scholar] [CrossRef]
- Zeng, X.; Shu, S.; Meng, Y.; Wang, H.; Wang, Y. Enhanced photocatalytic degradation of sulfamethazine by g-C3N4/Cu, N-TiO2 composites under simulated sunlight irradiation. J. Chem. Eng. 2023, 456, 141105. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Ma, H.; Zhang, C.; Dong, X.; Zhang, X. Enhanced peroxymonosulfate activation on dual active sites of N vacancy modified g-C3N4 under visible-light assistance and its selective removal of organic pollutants. Sci. Total Environ. 2021, 756, 144139. [Google Scholar] [CrossRef]

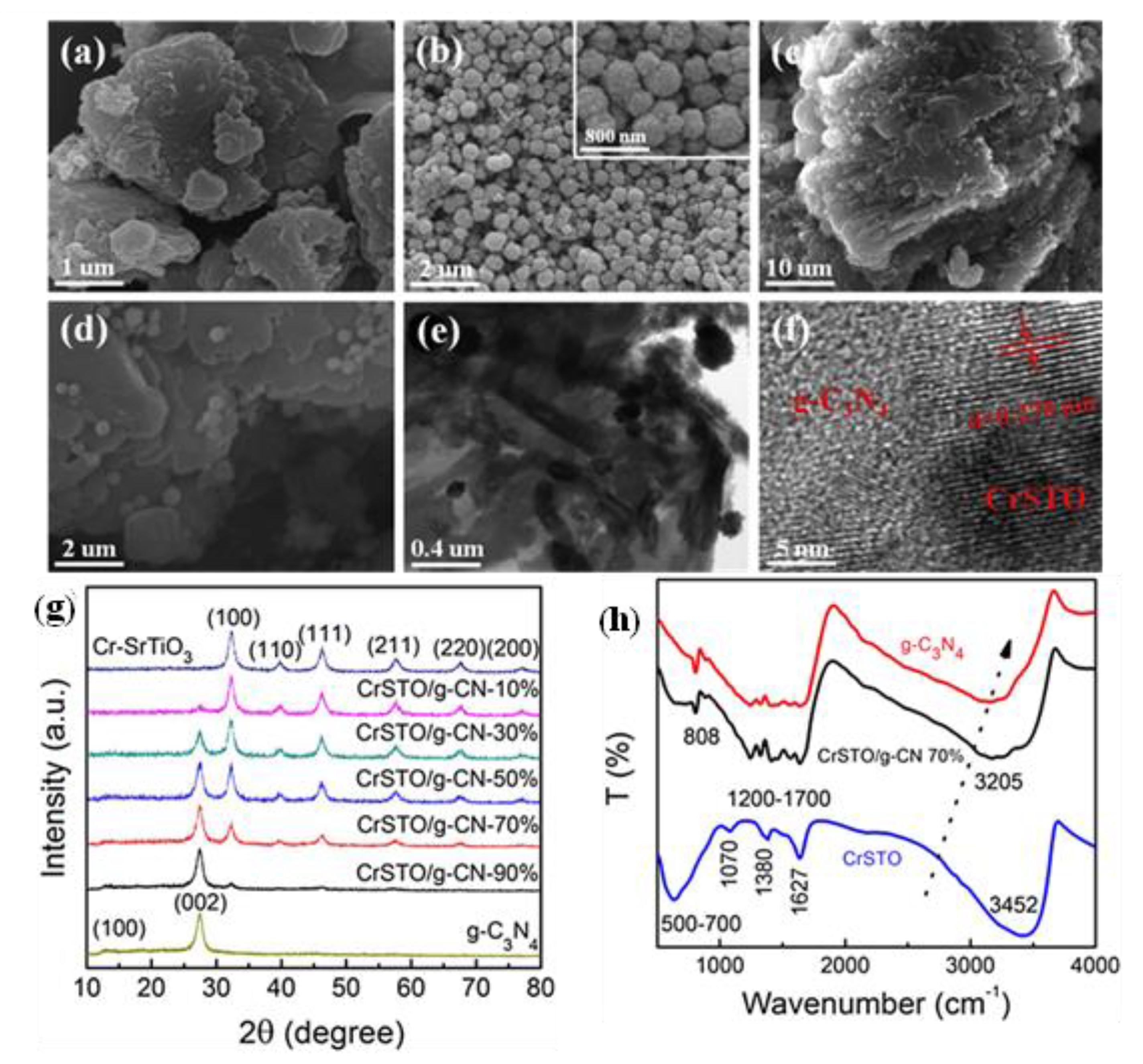

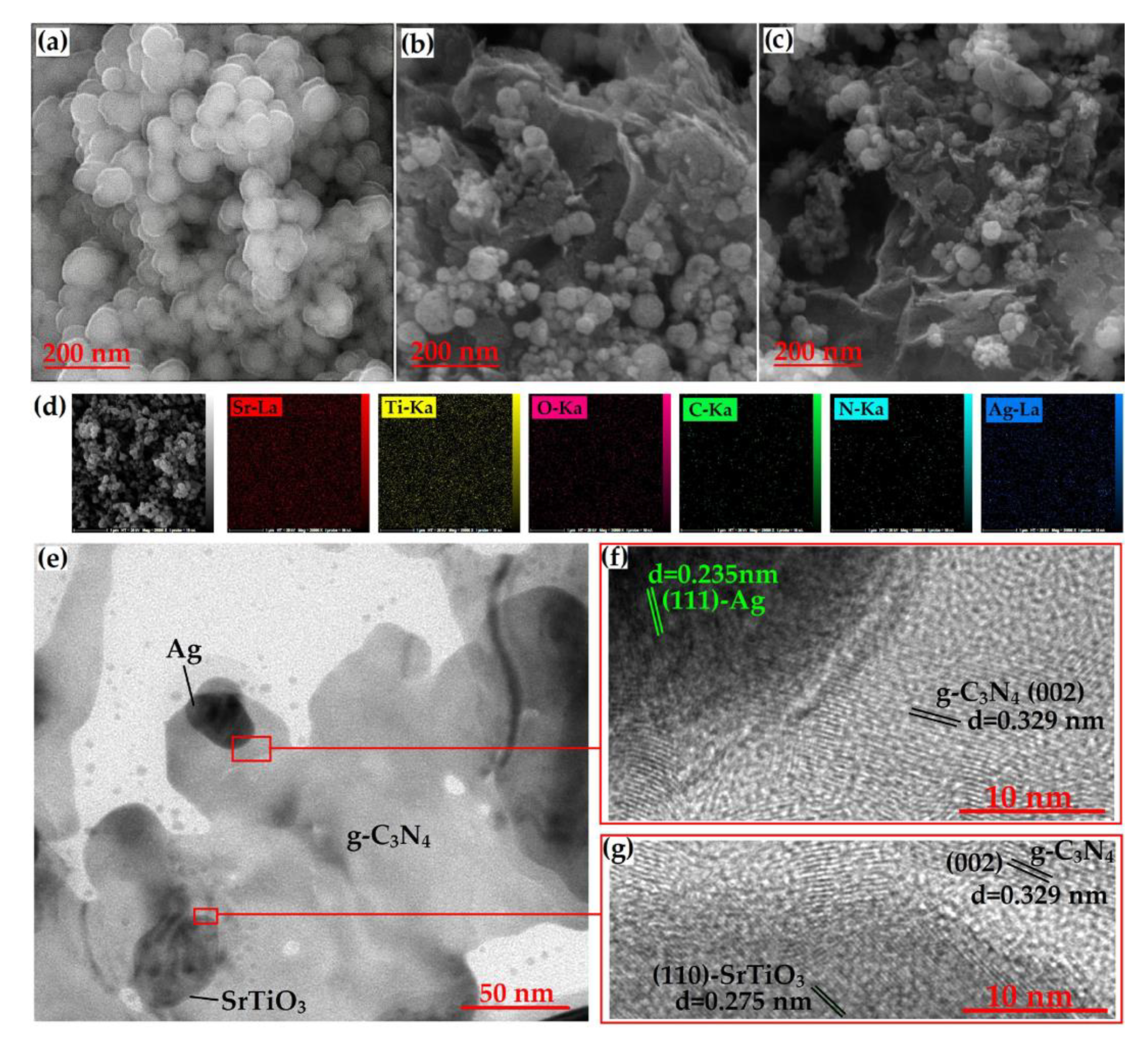
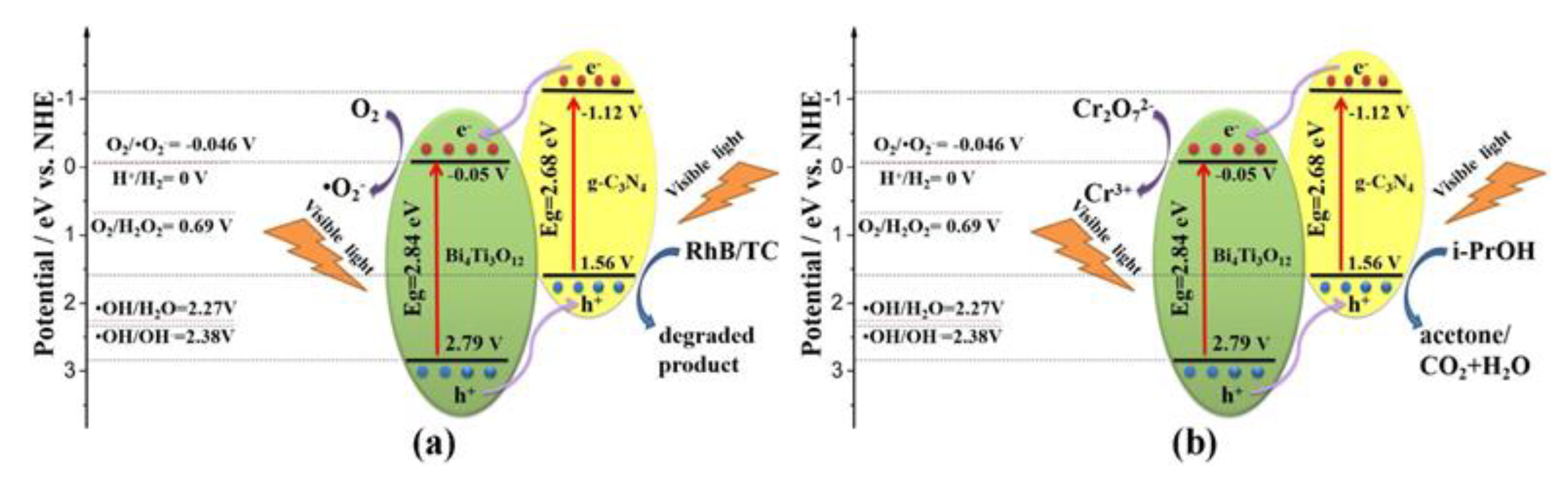
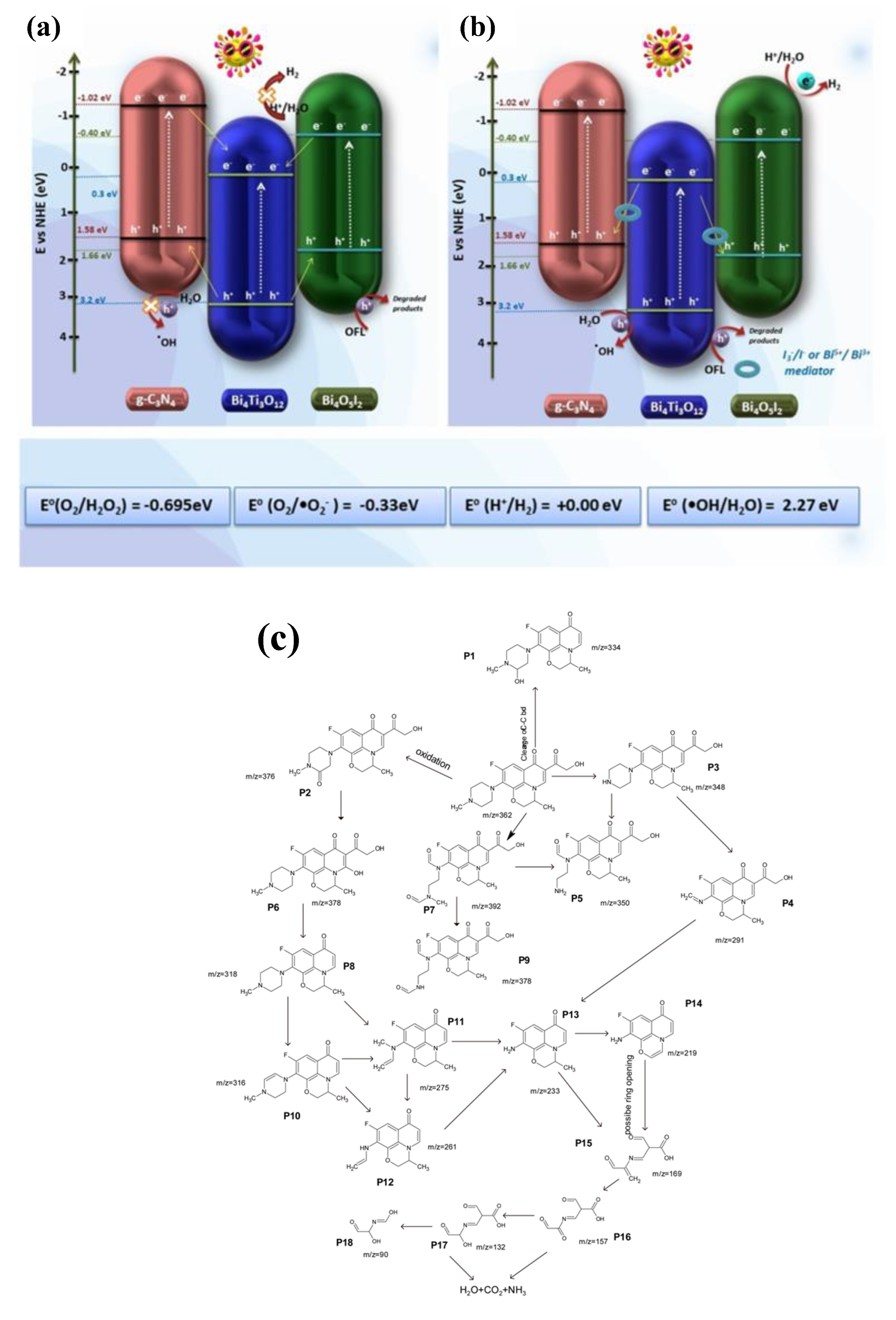

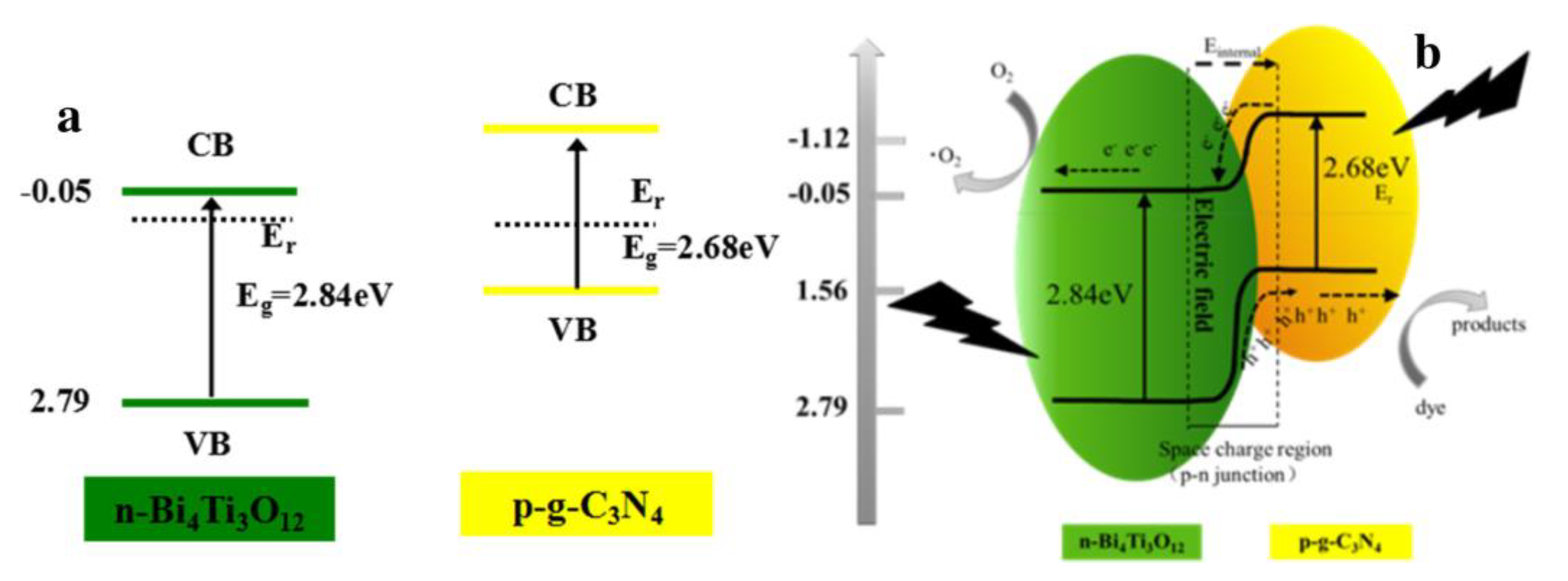
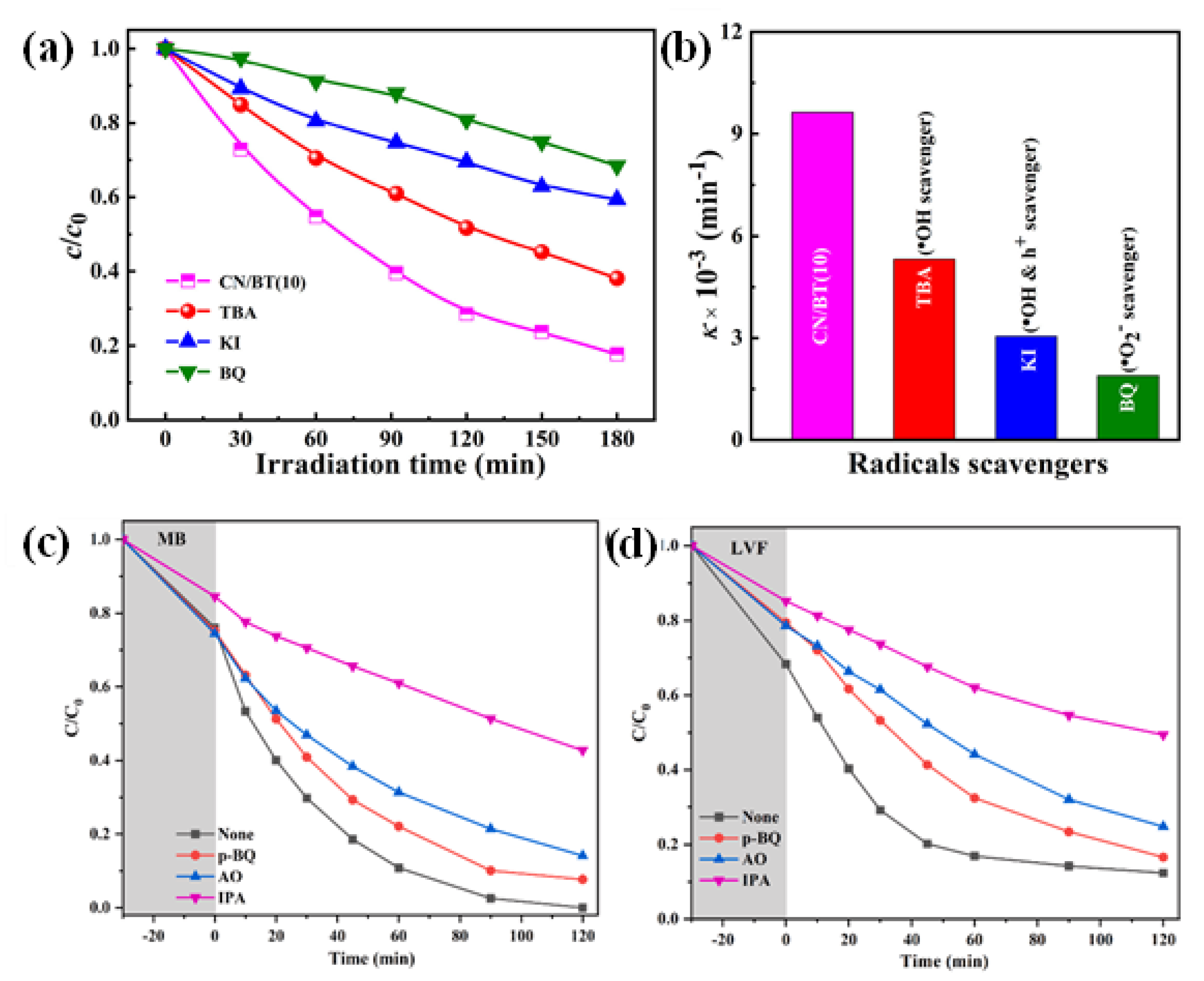
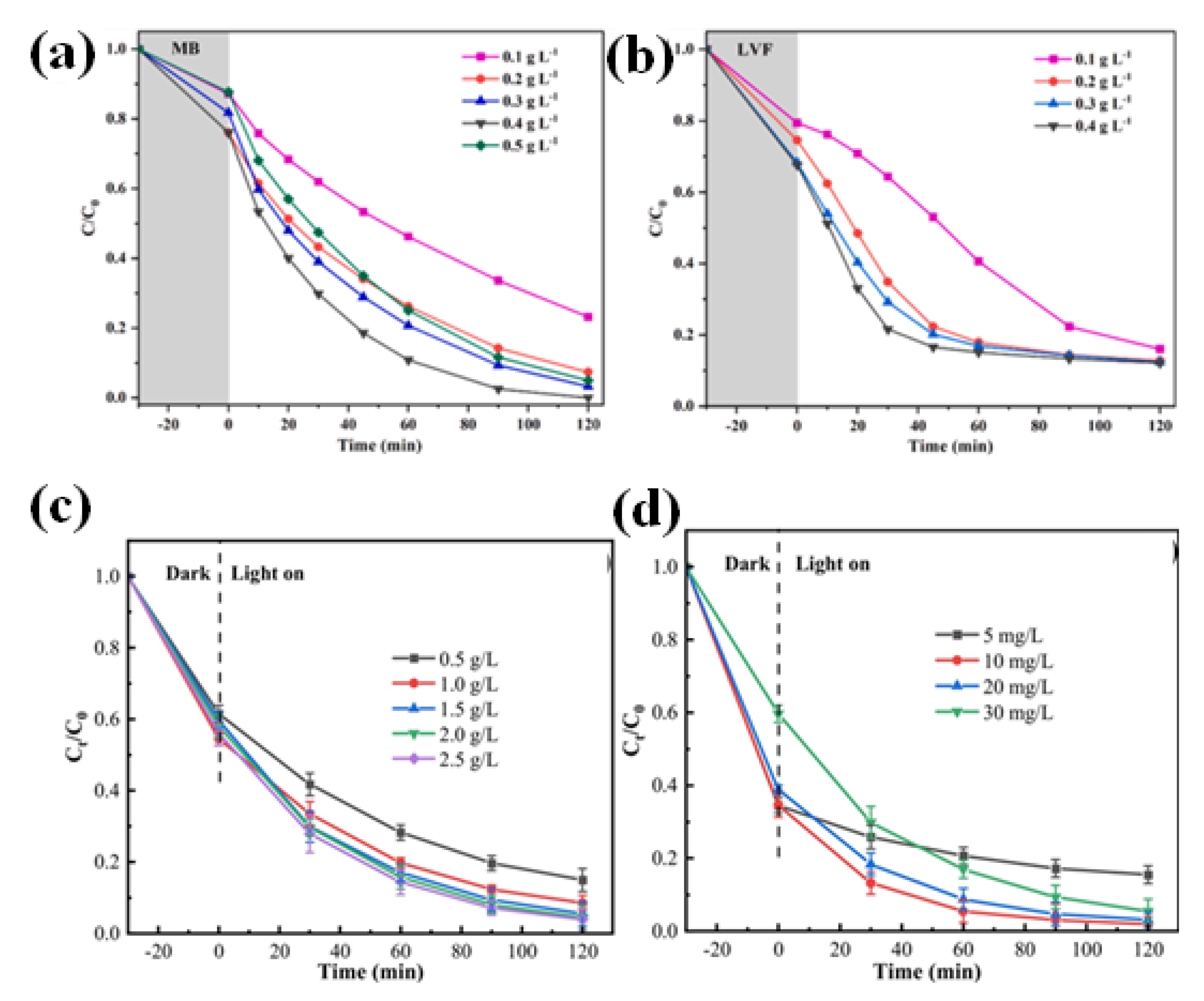
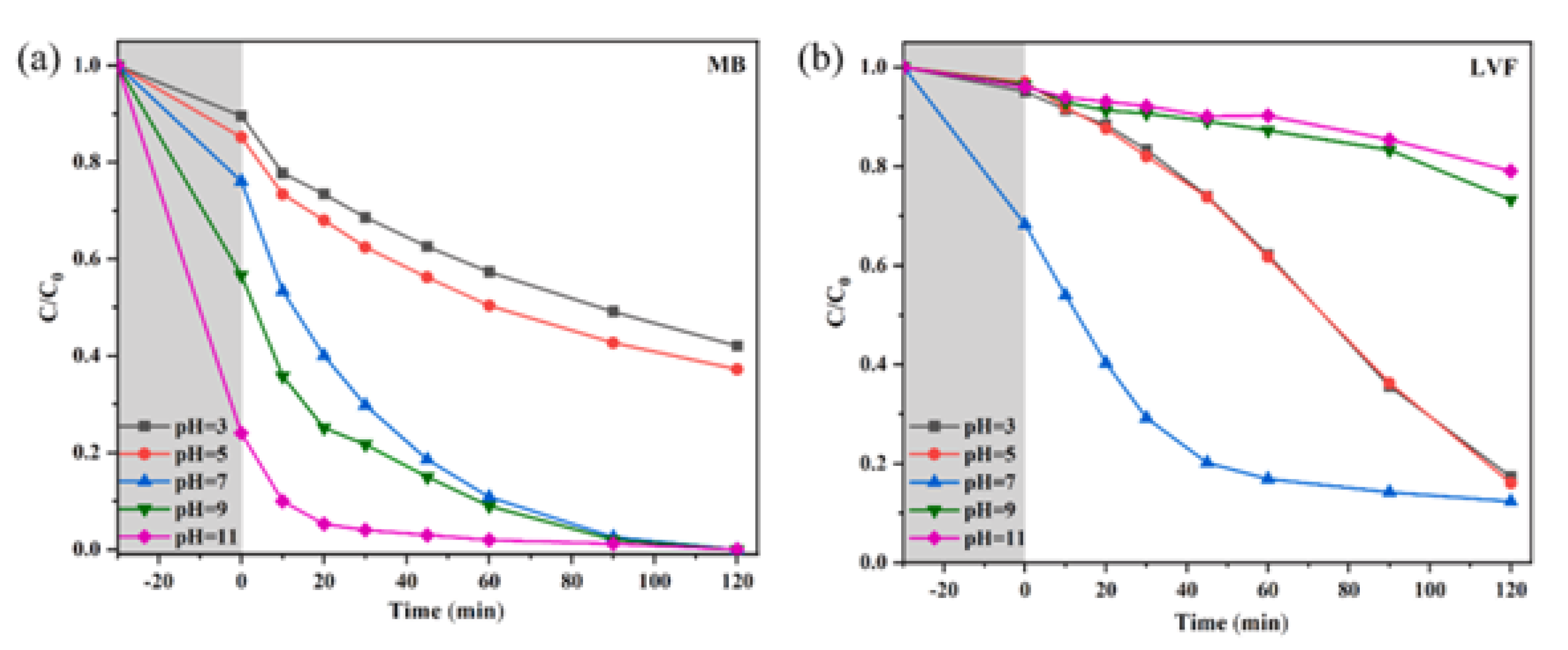
| Precursor | Synthesis Method | Morphology | Bandgap (eV) | Application | Ref. |
|---|---|---|---|---|---|
| Urea | Thermal polymerization | 2D lamellar | NA | Carbamazepine degradation | [46] |
| Melamine, NH4Cl | Thermal polymerization | 2D nanosheets | 2.71 | 2,4-Dichlorophenol degradation | [45] |
| Melamine | Thermal polymerization | NA | 2.75–2.62 | NA | [47] |
| Melamine | Thermal polymerization | 2D nanosheets | 2.6–2.49 | H2 production | [48] |
| Urea | Hydrothermal | Nanosheets | 2.58 | Tetracycline (TC) degradation | [51] |
| Dicyandiamide | Hydrothermal calcination | Laminated hexagonal prisms | 2.62 | Rhodamine B (RhB) degradation, H2 production | [19] |
| Urea | Thermal polymerization | Sheets | 2.8 | CO2 reduction, H2 production | [56] |
| Thiourea | Thermal polymerization/Solvothermal | Sheets | 2.76 | H2 production | [49] |
| Urea, thiourea | Calcination | Nanoflakes | 2.78–2.89 | RhB degradation | [57] |
| 2,4,6-Trichloro-1,3,5-triazine, dicyandiamide, acetonitrile | Solvothermal | Spherical | 2.19 | Tetracycline hydrochloride degradation | [50] |
| Melamine | Hydrothermal | Nanotubes | 2.70 | NO removal | [52] |
| Melamine, ammonium thiosulfate | Hydrothermal | Lamellar | 2.64 | H2 production | [53] |
| Melamine | Thermal polymerization/Hydrothermal | NA | 2.3–2.7 | Methylene blue (MB) degradation | [58] |
| Melamine, calcium cyanamide | Template assisted | Stacked lamellar | 2.66 | H2 production | [54] |
| Urea, glucose, P123 | Template assisted | Curled sheets | NA | Energy storage | [66] |
| Dicyandiamide, NaCl | Template assisted | Honeycomb | 2.6 | H2 production | [55] |
| Melamine, magadiite | Template assisted | Layered | 2.8 | RhB degradation | [67] |
| Melamine, artificial graphite powders | Microwave | Layered | NA | RhB, methyl orange (MO) degradation | [59] |
| Melamine, carbon fiber | Microwave | Nanosheets | 2.88 | Field emission | [68] |
| Urea | Microwave | Nanosheets | NA | White LED | [69] |
| Cyanuric chloride, sodium azide | Microwave | Spherical | 2.41 | NA | [70] |
| Thiourea | Microwave | Nanoplates | 2.7–2.61 | Nitrogen photofixation, RhB degradation | [71] |
| Citric acid, thiourea | Microwave | NA | NA | Fluorescence probe | [72] |
| Photocatalysts | Synthesis Method | Heterojunction Type | Light Source | Photocatalytic Activity/Rate Constant, k (min−1) | Main Active Species | Ref. |
|---|---|---|---|---|---|---|
| Cr/Nb-modified Bi4Ti3O12/g-C3N4 | Hydrothermal | Z-scheme | 300 W Xe lamp | 98.7% of RhB degradation | ●OH, ●O2− | [31] |
| nZVI-doped Al2ZnTiO9/g-C3N4 | Hydrothermal | NA | 70 W Xe arc lamp | 88%, 87%, 80%, 72%, and 90% degradation for methyl orange anion dye, methylene blue cation dye, nitrate, carbon dioxide, and toxic heavy metals, respectively | ●O2−, ●OH | [119] |
| g-C3N4/Bi4Ti3O12/Bi4O5I2 | In situ hydrothermal | Z-scheme | 500 W Xe lamp | 87.1% ofloxacin removal | h+, ●OH | [106] |
| g-C3N4/Bi4Ti3O12 | Thermal polymerization | Heterostructure | 300 W Xe lamp | 96.99% of RhB. 84.20% of TC, 69.64% Cr(iv) reduction | h+, ●O2− | [109] |
| SrTiO3/g-C3N4/Ag | Co-precipitation | Z-scheme | 400 W OSRAM lamp | 100% MB degradation | h+,●O2−, ●OH | [35] |
| g-C3N4/Fe2TiO5/Fe2O3 | Hydrothermal | Z-scheme | Sunlight | 96.1% MB degradation (k = 0.009) | ●O2−, ●OH | [37] |
| g-C3N4/BaTiO3 | Mixing–calcining | Heterostructure | 100 mW/cm2 xenon lamp equipped | MO degradation | ●O2− | [103] |
| g-C3N4/h′ZnTiO3-a′TiO2 | In situ | Z-scheme | 350 W Xe arc lamp | 99.8% MB degradation | ●O2−, ●OH | [105] |
| Cr-SrTiO3/g-C3N4 | Solid state | Z-scheme | 500 W Xe lamp | 97% of RhB degradation | h+, ●O2− | [104] |
| g-C3N4/SrTiO3 | Sonication mixing | Z-scheme | 2.2 kW Xe lamp, LED flood lamps | MB degradation (k = 0.0220) | ●OH, ●O2− | [121] |
| CaTiO3/g-C3N4 | Solid state | Z-scheme | 500W mercury lamp | 92.7% MB, 87.7% levofloxacin degradation | ●OH | [120] |
| g-C3N4/Bi4Ti3O12 | Ball milling | p–n junction | 500 W Xe lamp | 87.2% AO-7 degradation | h+, ●O2− | [117] |
| CoFe2O4/g-C3N4/Bi4Ti3O12 | Ultrasonic-assisted heat treatment | Z-scheme | 45 W energy-saving lamp | 98.05% degradation of MG | h+, ●O2− | [122] |
| Bi4Ti3O12/g-C3N4/BiO5Br | Thermal polymerization | Z-scheme | 65 W energy-saving lamp | 89.84% TC degradation | ●O2− | [123] |
| g-C3N4/N-doped LaTiO3 | Solid state | Heterostructure | 100W halogen lamp | 90% of RhB degradation | ●O2−, ●OH | [124] |
| CaTiO3/g-C3N4/AgBr | Mixing | Z-scheme | 200 W Xe lamp | 99.6% of RhB degradation | ●O2− | [125] |
| g-C3N4/La2Ti2O7 | Wet impregnation | Heterostructure | 400 W Xe lamp | MB degradation | h+, ●O2− | [126] |
| SrTiO3/g-C3N4 | Thermal treatment | Heterostructure | Six fluorescent lamps | MB degradation (k = 1.30 × 10−3), amiloride (AML) degradation (k = 1.82 × 10−3) | ●OH | [127] |
| Pt/g-C3N4/SrTiO3 | Low-temperature calcination | Z-scheme | 500 W Xe lamp | 93% acid red 1 (AR1) dye degradation | ●O2− | [128] |
| FeTiO3/g-C3N4 | self-assembly | S-scheme | 300 W Xe lamp | 92.6% tetracycline hydrochloride degradation | h+, ●O2−, ●OH | [116] |
| 2D/1D g-C3N4/CaTiO3 | Solvothermal | Heterostructure | 300 W Xe lamp | 99.76% crystal violet (CV) and 95.02% MG degradation | h+, ●O2− | [112] |
| g-C3N4/Bi12TiO20 | Annelation | Heterostructure | 500 W Xe lamp | 96.9% of RhB degradation | h+, ●O2− | [113] |
| g-C3N4/Bi4Ti3O12 | Mixing–calcining | p–n heterojunction | 200 W Xe lamp | 85.4% RhB degradation | h+, ●O2− | [118] |
| SrZnTiO3/g-C3N4 | Mixing–calcining | Z-scheme | 500 W halogen lamp 400 W high pressure mercury lamp | 93.1 and 82.2% removal of indigo carmine (IC) and RhB, respectively | h+,●O2−, ●OH | [129] |
| PbTiO3/g-C3N4 | Mixing–calcining | Heterostructure | 300 W UV Xe lamp | RhB degradation (k = 0.1357) | NA | [130] |
| g-C3N4/BaTiO3 | Hydrothermal | Heterostructure | 75 W–220 V lamp | 98.72% MB degradation | ●O2−, ●OH | [110] |
| NiTiO3@g-C3N4 | Ultrasonic-assisted wet-impregnation | Heterostructure | Direct sunlight | 95−98% photoreduction of toluene to benzoic acid | ●OH | [131] |
| La2Ti2O7/C3N4+xHy | Hydrothermal | Heterostructure | 240 W mercury lamp | Degradation of 99, 95 and 93% for RhB, MB, and MO, respectively | h+, ●O2− | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patra, R.; Dash, P.; Panda, P.K.; Yang, P.-C. A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites. Nanomaterials 2023, 13, 2173. https://doi.org/10.3390/nano13152173
Patra R, Dash P, Panda PK, Yang P-C. A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites. Nanomaterials. 2023; 13(15):2173. https://doi.org/10.3390/nano13152173
Chicago/Turabian StylePatra, Rashmiranjan, Pranjyan Dash, Pradeep Kumar Panda, and Po-Chih Yang. 2023. "A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites" Nanomaterials 13, no. 15: 2173. https://doi.org/10.3390/nano13152173
APA StylePatra, R., Dash, P., Panda, P. K., & Yang, P.-C. (2023). A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites. Nanomaterials, 13(15), 2173. https://doi.org/10.3390/nano13152173







